Abstract
Background
The purpose of this research was to investigate the effects of hesperidin on hydrogen peroxide (H2O2)-induced chondrocytes injury and cartilage degeneration in a rat model of osteoarthritis (OA).
Material/Methods
Chondrocytes were isolated from rat knee joints and treated with hesperidin alone or combined with H2O2. Then, Cell Counting Kit-8 (CCK-8) assay was used to assess cell viability. Activity of reactive oxygen species (ROS) and levels of malondialdehyde (MDA) were estimated. Cell apoptosis was assessed by flow cytometry assay. In addition, gene expression levels were measured for caspase 3, tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), collagen type II (Col2a1), aggrecan, (sex-determining region Y)-box 9 (SOX9), matrix metalloproteinase (MMP)-13, and inducible nitric oxide synthase (iNOS) through quantitative real-time polymerase chain reaction (qPCR). To examine the effects on cartilage destruction in vivo, hesperidin or vehicle control were orally administrated in a surgically-induced osteoarthritis (OA) model.
Results
The results indicated that hesperidin pretreatment of chondrocytes reduce H2O2-induced cytotoxicity and apoptosis. Hesperidin pretreatment decreased the formation of MDA and intracellular ROS, including chondrocyte apoptosis. Hesperidin also reversed the activity of H2O2 on inhibiting the Col2a1, aggrecan, and SOX9 gene expression and increasing the gene expression of caspase 3, IL-1β, TNFα, iNOS, and MMP13. In addition, hesperidin administration markedly attenuated cartilage destruction and reduced IL-1β and TNF-α levels in a surgically-induced OA model.
Conclusions
Our study suggests that hesperidin can prevent H2O2-induced chondrocytes injury through its antioxidant effects in vitro and reduce cartilage damage in a rat model of OA.
MeSH Keywords: Chondrocytes, Hesperidin, Osteoarthritis, Oxidative Stress
Background
Osteoarthritis (OA) is a degenerative disease characterized by chronic pain and movement disorders in the joints. The main pathological manifestations of OA include the progressive destruction of articular cartilage, osteophyte formation, subchondral bone sclerosis, and synovitis. OA is associated with many factors, such as obesity, inflammation, aging, genetic factors, and injuries [1]. Previous studies have shown that the essential change in OA is loss of the articular cartilage matrix, which is induced by various factors, such as abnormal immune, genetic, and oxidative stress. It has been generally recognized that overexpression of reactive oxygen species (ROS) plays a key role in the degeneration of articular cartilage function [2].
Oxidative stress is produced by a variety of ROS, including peroxynitrite, superoxide anions, nitric oxide (NO), and hydrogen peroxide (H2O2) [3]. Excess hydrogen peroxide is known as a key mediator in the processes that damage cells, including chondrocytes [4]. Sub-millimolar concentrations of H2O2 can induce inhibition of the extracellular matrix (ECM) synthesis, chondrocyte apoptosis, lipid peroxidation, and inflammatory cytokines overproduction, which further lead to the matrix metalloproteinase (MMPs) formation [5–7]. Thus, H2O2-induced oxidative stress may be helpful to study the occurrence and development of OA and evaluate the therapeutic strategies.
Previous studies have shown that hesperidin, a flavanone glycoside containing the flavanone hesperetin and the disaccharide rutinose, has anti-rheumatic activity in adjuvant arthritis (AA) or collagen-induced arthritis (CIA) rats [8–11]. In addition, several studies have shown that hesperidin has a definite anti-oxidative stress effect. For instance, Maekawa [12] found that hesperidin can protect the retina against damage by suppressing oxidative stress and excessive calpain activation. Li [13] confirmed that hesperidin ameliorates UV radiation-induced skin damage by abrogation of oxidative stress and inflammation in HaCaT cells.
Hesperidin also has a protective role against oxidative stress, injury, and apoptosis induced by γ-radiation in testes [14]. However, it is not clear whether the anti-oxidative activity of hesperidin has a therapeutic effect on osteoarthritis. In the present study, we explored the protective effect of hesperidin on rat chondrocytes and its underlying mechanisms.
Material and Methods
Reagents
Hesperidin was obtained from Sigma-Aldrich (St. Louis, MO, USA). The purity of hesperidin was greater than 80%. Dulbecco’s modified Eagle’s medium/nutrient F-12 Ham (DMEM/F12) medium, 0.25% trypsin, and type II collagenase were from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). CCK-8, RIPA lysis buffer, lipid peroxidation malondialdehyde (MDA) assay kit, and reactive oxygen species (ROS) assay kit were supplied by Beyotime Biotechnology (Nantong, China). Fetal bovine serum (FBS) was from GE Healthcare Life Sciences Hyclone Laboratories (Logan, UT, USA). The FITC Annexin V Apoptosis Detection Kit was supplied by BD Pharmingen (San Diego, CA, USA). RNAiso plus regent, PrimeScript RT Master Mix kit, and SYBR Premix Ex Taq II kit were from Takara Bio, Inc. (Shiga, Japan). Anti-IL-1β antibody (cat. no. ab9722), Anti-TNF-α antibody (cat. no. ab6671), and HRP/DAB detection IHC detection kit (cat. no. ab236469) were from Abcam (Cambridge, MA, USA). Dimethyl sulfoxide (DMSO) was obtained from MP Biomedicals (Santa Ana, CA, USA). Hesperidin was dissolved in DMSO to achieve the storage concentration of 10 ug/ul and diluted with DMEM/F12 medium to get the various working concentrations. DMEM/F12 medium was used to dissolve collagenase II to the concentration 2 mg/ml. The experiments were approved by the Ethics Committee of Nanjing Medical University.
Cell culture
We obtained 1-week-old male Sprague-Dawley rats from the Animal Center of Nanjing Medical University (Nanjing, China). Rat chondrocytes were obtained as described previously [7,15]. Briefly, cartilage from the knees of rats was minced into small pieces, then sequentially digested in 0.25% trypsin for 30 min and placed on 2 mg/ml collagenase II-containing medium for 4–5 h at 37°C. The solution was washed using phosphate-buffered saline (PBS) and filtered through a 200-μm cell strainer. The cells collected by centrifugation were cultured in DMEM/F12 medium containing 10% FBS at 37°C in a humidified 5% CO2 atmosphere. Chondrocytes at passage 2 were selected for the subsequent processes.
Cell viability assay
CCK-8 assay was used to measure the cell viability. Briefly, cells were cultured in 96-well plates at a density of 1×104 cells/well. After overnight adhesion, cells were appropriately treated according to the experimental grouping. Then, 10 μl CCK-8 solution/well was added before further incubation of cells for 1 h in a BB5060 incubator. A microplate reader was used to measure the absorbance of each well.
Cell apoptosis assay
Sample cells were washed twice using cold PBS and adjusted to a concentration of 1×106 cells/ml using binding buffer. We transferred 100 μl of the solution (1×105 cells) to a 5-ml culture tube. Then, we added 5 μl FITC Annexin V and propidium iodide and incubated the mixture for 15 min in the dark at room temperature. The chondrocytes were analyzed using a FACScan flow cytometer.
Lipid peroxidation MDA assay
Treated cells were collected after washing with cold PBS. Then, RIPA lysis buffer was added to extract proteins for 5 min. Total cell lysate was centrifuged at 14 000×g for 5 min. BCA assay was used to determine the protein concentrations of samples. According to the kit’s instructions, MDA levels were evaluated by thiobarbituric acid (TBA) test.
Reactive oxygen species detection
According to the kit’s instructions, cells were collected and washed with serum-free DMEM/F12 medium after appropriate treatment. The fluorescent probe DCFH-DA was added to cell suspension at a concentration of 10 μM. After incubation for 30 min in the BB5060 incubator, the cells were collected and washed with serum-free medium 3 times. The samples were analyzed by a FACScan flow cytometer.
Quantitative real-time PCR (qPCR) analysis
RNAiso plus regent was used to extract total RNA from samples, which was then transcribed into cDNA using a PrimeScript RT Master Mix kit. The primers for caspase 3, Col2a1, aggrecan, SOX9, MMP-13, iNOS, IL-1β, TNF-α, and β-actin were amplified using a SYBR Premix Ex Taq II kit in a Step One Plus Real-Time PCR system. The primer sequences are shown in Table 1. The primers were synthesized by Shanghai GenePharma Co., Ltd. All the results were analyzed using the 2−ΔΔCt method [16].
Table 1.
Sequences of primers for the quantitative RT-PCR experiments.
| Gene | Sense | Sequence 5′→3′ |
|---|---|---|
| β-actin | F | GCAGAAGGAGATTACTGCCCT |
| R | GCTGATCCACATCTGCTGGAA | |
| iNOS | F | CCCTTCCGAAGTTTCTGGCAGCAG |
| R | GGGCTCCTCCAAGGTGTTGCCC | |
| MMP-13 | F | AGGCCTTCAGAAAAGCCTTC |
| R | GAGCTGCTTGTCCAGGTTTC | |
| Aggrecan | F | CCCTACCCTTGCTTCTCCA |
| R | CTTGAGAGGCACTCATCAATGT | |
| Col2A1 | F | GGCTCCCAGAACATCACCTA |
| R | GCCCTCATCTCCACATCATT | |
| SOX9 | F | AGAGCGTTGCTCGGAACTGT |
| R | TCCTGGACCGAAACTGGTAAA | |
| IL-1β | F | CTGTGACTCGTGGGATGATG |
| R | GGGATTTTGTCGTTGCTTGT | |
| TNFα | F | CTCCCAGAAAAGCAAGCAAC |
| R | CGAGCAGGAATGAGAAGAGG | |
| Caspase 3 | F | GCTGGACTGCGGTATTGAGA |
| R | CCATGACCCGTCCCTTGA |
Surgical Induction of OA
Male Sprague-Dawley rats (6 weeks old) were divided into 2 groups: a hesperidin group and a control group (n=5 each). All rats were anesthetized with 10% chloral hydrate and the left knee underwent surgery. As previously reported [17], the left knee joint was exposed through a medial capsular incision. Then, the medial collateral ligament was transected and the medial meniscus was removed. All of the rats received penicillin to prevent wound infection after the operation. None of the joints were infected during the experimental period. Two weeks after surgery, hesperidin (200 mg/kg body weight) or vehicle control was administered orally once a 118 day for 28 days [18].
Histologic and immunohistochemical analysis
Total knee joints from rats were fixed in 4% paraformaldehyde, then decalcified in EDTA and embedded in paraffin. Tissue sections of 5-μm thickness were stained using Safranin O/Fast green to determine proteoglycan depletion and cartilage destruction. To analyze IL-1β and TNF-α distribution in the cartilage, the sections were de-paraffinized and rehydrated in graded ethanol. After respective incubation with primary antibodies of IL-1β and TNF-α, the samples were treated according to the kit’s instruction. Finally, the sections were observed under a microscope. The stained sections were analyzed using ImageJ 1.43 software (imagej.nih.gov).
Statistical analysis
Statistical analyses were performed with SPSS 15.0 software (SPSS Inc., Chicago, IL, USA) or GraphPad Prism (GraphPad Software, San Diego, CA, USA). CCK-8 assay, cell apoptosis assay, MDA assay, ROS detection, and qPCR analysis data were analyzed using one-way ANOVA followed by Dunnett’s multiple comparisons. Histologic and immunohistochemistry analysis data were assessed using the unpaired t test. A p value of <0.05 was considered statistically significant.
Results
Effect of hesperidin on cell viability
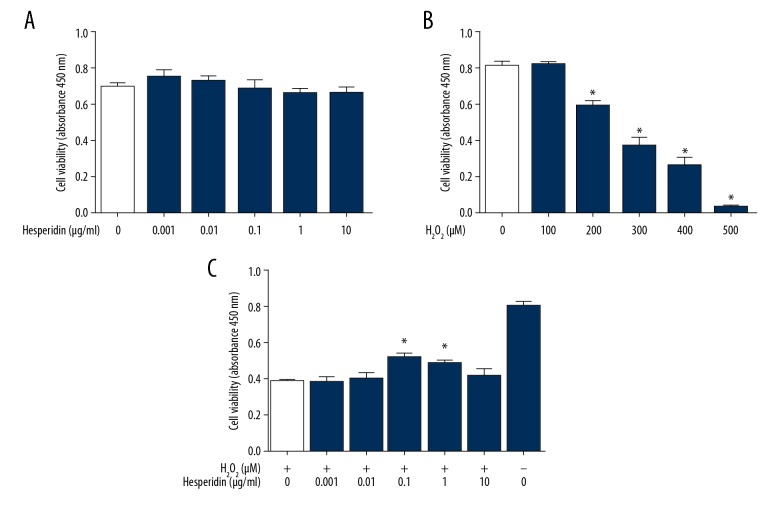
Chondrocytes were incubated with different concentrations of hesperidin (0, 0.001, 0.01, 0.1, 1, and 10 μg/ml) for 24 h. Then, the CCK-8 assay was used to evaluate the effect of hesperidin on cell viability. The data confirmed that concentrations of hesperidin in this range did not significantly affect cell viability (Figure 1A). H2O2 treatment (0, 100, 200, 300, 400, and 500 μM for 24 h) induced a dose-dependent reduction in chondrocytes viability. A 50% inhibition concentration value (IC50) was observed at 230 μM (Figure 1B). Hesperidin (0.1 and 1 μg/mL) attenuated the changes in cell viability induced by 230 μM H2O2 (Figure 1C). The optimal concentration of hesperidin (0.1 μg/mL) was selected for further experiments.
Figure 1.
Effect of hesperidin on rat chondrocytes viability and H2O2-induced reduction. The viability of chondrocytes in different groups was assessed by CCK-8 assay. (A) After adherence, cells were treated with 0, 0.001, 0.01, 0.1, 1, or 10 μg/mL of hesperidin for 2 h. (B) The dose-dependent effects of H2O2 on cell viability after 24 h treatment. The 50% inhibition concentration value (IC50) was found to be 230 μM. *p<0.05, compared with the 0 μM H2O2 group. (C) Hesperidin (0, 0.001, 0.01, 0.1, 1, or 10 μg/mL) was added for 2 h prior to 24 h treatment with 230 μM H2O2. * p<0.05, compared with the H2O2 group. All data are expressed as the mean ± standard deviation.
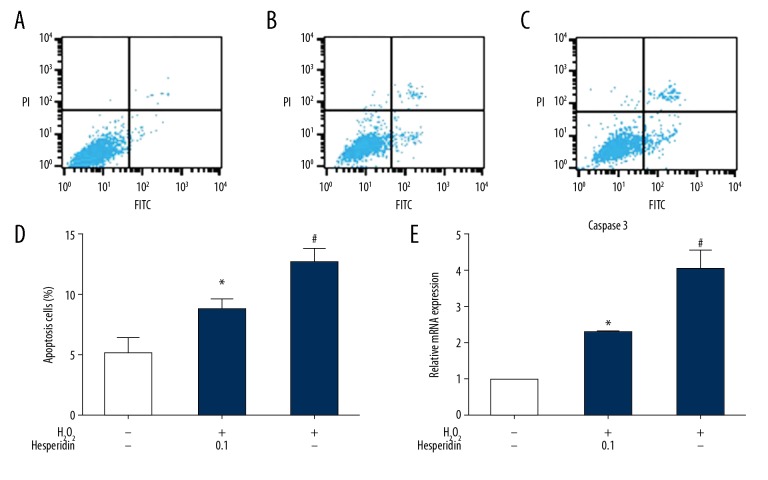
Effect of hesperidin on chondrocytes apoptosis
Flow cytometry assay was used to measure chondrocyte apoptosis (Figure 2A–2D). Cells were treated with 0.1 μg/mL hesperidin for 2 h before 230 μM H2O2 treatment for 24 h. The cells in the negative control group were treated with medium alone for 24 h, and the cells in the positive control group were treated with 230 μM H2O2 alone for 24 h. The results (Figure 2D) showed that the percentage of apoptotic chondrocytes in the hesperidin-pretreated group was significantly decreased compared to that in the 230 μM H2O2 alone group (p<0.05). Stimulation with 230 μM H2O2 led to a significant increase in caspase-3 gene expression, which was alleviated by hesperidin pretreatment (0.1 μg/mL) (p<0.05, Figure 2E).
Figure 2.
Protective effect of hesperidin on chondrocyte apoptosis. FITC Annexin V/PI staining and flow cytometry assays were used to detect cell apoptosis. (A) Chondrocytes were cultured in DMEM/F12 medium for 24 h. (B) Chondrocytes were pretreated with 0.1 μg/ml hesperidin for 2 h and then incubated with 230 μM H2O2 for 24 h. (C) Chondrocytes were treated with 230 μM H2O2 for 24 h. (D) Chondrocyte apoptosis measured by flow cytometry is represented by bar graphs. (E) Effect of hesperidin on H2O2-induced gene expression of caspase 3. All data are expressed as the mean ± standard deviation. # p<0.05, compared with the control group. * P<0.05, compared with the H2O2 group.
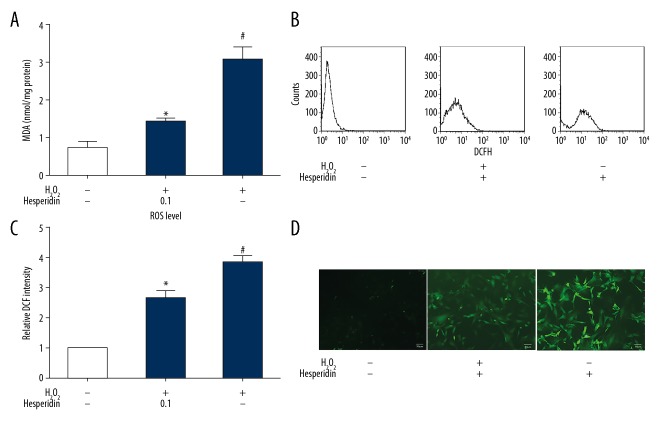
Antioxidant capacity of hesperidin in H2O2-treated chondrocytes
Lipid oxidation and ROS formation occur when cells undergo oxidative stress. Our data show that 230 μM H2O2 significantly increased the MDA levels compared to the medium alone group (p<0.05). However, pretreatment with hesperidin (0.1 μg/mL) significantly reduced the levels of MDA equivalents (Figure 3A). Exposure of chondrocytes to 230 μM H2O2 plus 0.1 μg/mL hesperidin remarkably reduced the intracellular ROS formation compared with 230 μM H2O2 alone (Figure 3B–3D).
Figure 3.
Antioxidant capacity of hesperidin in H2O2-treated chondrocytes. Chondrocytes were pretreated with hesperidin (0.1 μg/mL) for 2 h and incubated with 230 μM H2O2 for 24 h. (A) Total proteins were extracted to detect MDA equivalents. (B) Effect of hesperidin on intracellular reactive oxygen species (ROS) in chondrocytes. Intracellular ROS were measured by flow cytometry using an oxidation-sensitive fluorescent probe, DCFH-DA, which is oxidized to DCF in the presence of ROS. (C) ROS level measured by flow cytometry is represented by the relative DCF intensity. (D) Microscopic images showing the preventive effect of hesperidin against H2O2-induced ROS generation by DCFH-DA staining. H2O2 treatment significantly increased the levels of ROS, while hesperidin pretreatment significantly decreased the levels of ROS. Original magnification ×100. All data are expressed as the mean ± standard deviation. # p<0.05, compared with the control group. * P<0.05, compared with the H2O2 group.
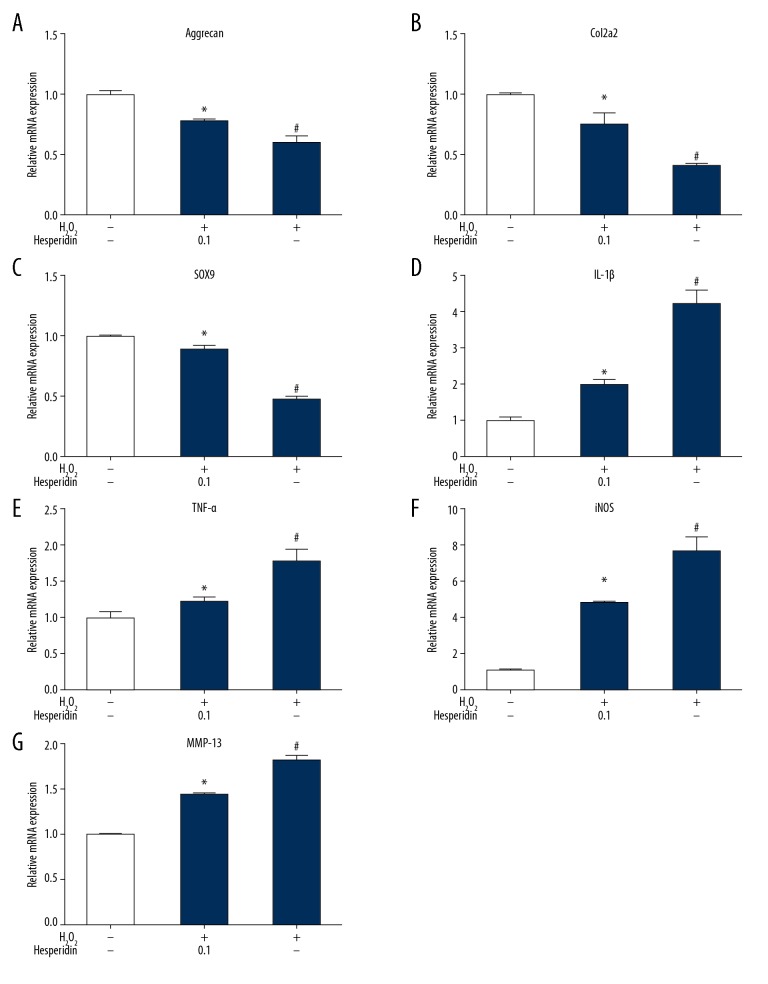
Effect of hesperidin on chondrogenic and inflammatory gene expression
The gene expression of aggrecan, Col2a1, and SOX9 was decreased in chondrocytes treated with 230 μM H2O2 alone (Figure 4A–4C), while the gene expression of IL-1β, TNFα, iNOS, and MMP13 was increased (Figure 4D–4G), compared with normal chondrocytes (p<0.05). Hesperidin alleviated downregulation of aggrecan, Col2a1, and SOX9 induced by H2O2 and suppressed induction of IL-1β, TNFα, iNOS, and MMP13 compared with H2O2 alone (p< 0.05). These results show that hesperidin alleviated H2O2-induced inflammation and improved the recovery of injury in chondrocytes.
Figure 4.
(A–G) Effect of hesperidin on H2O2-induced gene expression of aggrecan, Col2a1, SOX9, IL-1β, TNFα, iNOS, and MMP13 in chondrocytes. Chondrocytes were pretreated with 0.1 μg/ml hesperidin for 2 h and then incubated with 230 μM H2O2 for 24 h or with H2O2 alone. Total RNA was extracted, followed by RT-qPCR for detection of relative gene expression levels. All data are expressed as the mean ± standard deviation. # p<0.05, compared with the control group. * P<0.05, compared with the H2O2 group.
Protective effect of hesperidin on cartilage destruction in a surgically-induced OA model
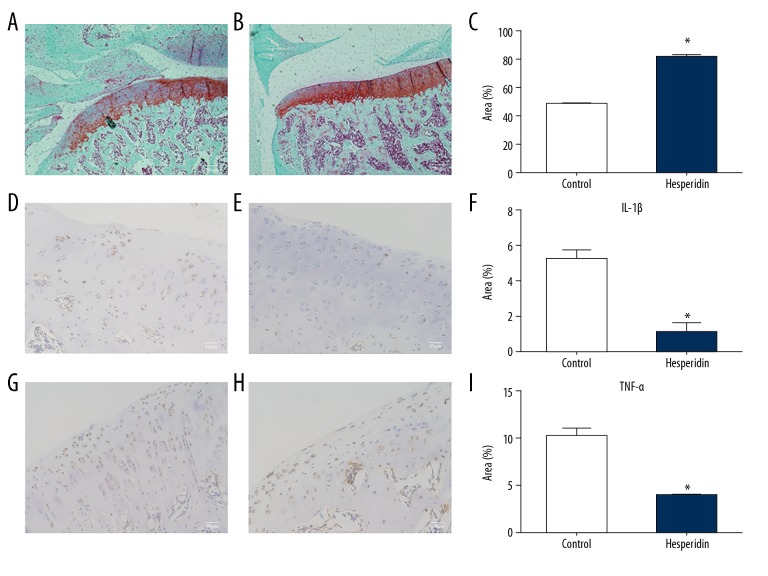
To observe whether hesperidin protects against cartilage destruction, OA was surgically induced in rats. After hesperidin or a vehicle control was orally administered, the sections obtained from rat knee joints were stained using Safranin O/Fast green to observe histological changes. In Figure 5B, the red color of cartilage tissue was obviously darker and the integrity of cartilage structure was better than that in Figure 5A, illustrating increased cartilage proteoglycan depletion in the control group without hesperidin treatment (Figure 5C). IL-1β (Figure 5D, 5E) and TNF-α (Figure 5G, 5H) levels were also analyzed by immunohistochemistry. More IL-1β-positive areas (Figure 5D) and TNF-α-positive areas (Figure 5G) were found in the control group compared to those in the hesperidin group (Figure 5E, 5H), illustrating decreased inflammatory cytokines expression after hesperidin treatment (Figure 5F, 5I).
Figure 5.
Representative images of Safranin O/Fast green stained and immunohistochemical sections obtained from knee joints after surgically-induced osteoarthritis. Safranin O/Fast green stained sections from rats in control group (A) and hesperidin group (B). (C) Bar graphs represent mean percentage of Safranin O-positive area in each group. * p<0.05 compared with the control group. The cartilage degenerations were ameliorated in hesperidin group compared with those in control group, illustrating increased cartilage proteoglycan depletion in control group. Original magnification×40. Images of immunohistochemical staining for IL-1β in control group (D) and in hesperidin group (E). Images of immunohistochemical staining for TNF-α in control group (G) and in hesperidin group (H). IL-1β and TNF-α positive area are stained brown. Bar graphs represent mean percentage of IL-1β-positive area (F) and TNF-α-positive area (I) in each group. *p<0.05 compared with the control group. Original magnification ×100.
Discussion
With the aging of the population, the prevalence of OA is also rising. In China, epidemiological surveys show that the prevalence of OA in the population is about 4%, while the incidence of knee osteoarthritis in people over 60 years old is as high as 42.8% [19]. Therefore, research on OA is very important and has great social benefits. In this study, we evaluated the antioxidant effect of hesperidin against H2O2-induced rat chondrocytes reactions and confirmed that hesperidin can restore the metabolic balance of OA. H2O2 can be endogenously produced in the pathogenesis of OA and can induce chondrocyte injury [20]. Our study found that inhibition of rat chondrocyte proliferation increased dose-dependently with H2O2 concentration but was significantly decreased by supplementation of hesperidin, and the concentration of 0.1 ug/ml hesperidin had the best effect in this process. Furthermore, FITC Annexin V/PI staining revealed that hesperidin suppressed chondrocyte apoptosis induced by H2O2 in vitro. Meanwhile, the gene expression of caspase-3, a crucial biomarker of apoptosis, also could be reduced after hesperidin treatment under H2O2 induction. Previous studies have shown that chondrocyte apoptosis induced by oxidative stress is responsible for the development of OA [21–23]. This observation may indicate that hesperidin can attenuate the progression of OA through suppression of H2O2-mediated injury. Through the detection of cellular ROS and MDA, we also assessed whether hesperidin has antioxidant effects and inhibits oxidative stress. We assessed ROS flow cytometry assays, and MDA, a natural product of lipid peroxidation, was assessed by TBA test. Lipid peroxidation can be induced by ROS and causes significant tissue damage in degenerative osteoarthritis [24–27]. Our data confirmed that H2O2-mediated oxidative stress can enhance ROS and lipid peroxidation levels in chondrocytes, which was clearly suppressed by hesperidin. NO, an oxygen free radical accumulating in the articular tissue of patients with osteoarthritis, induces cartilage degeneration. In vivo, NO is produced in the process of oxidative oxidation of L-arginine to L-citrulline by iNOS. Thus, the content of iNOS in chondrocytes may play an important role in the course of osteoarthritis. Our results showed that elevated gene expression of iNOS due to hydrogen peroxide can be inhibited by hesperidin, suggesting that hesperidin slows the progression of osteoarthritis.
Inflammatory cytokines play a key role in the pathogenesis of arthritis, and IL-1β and TNF-α are the 2 most important factors [28–30]. Related research has confirmed that oxidative stress and inflammatory responses are closely related in arthritis [31–33]. Our in vitro experimental results showed that oxidative stress can lead to significantly increased gene expression of TNF-α and IL-1β, and hesperidin treatment significantly alleviated this inflammatory response. Chondrocyte ECM mainly contains type 2 collagen and aggrecan. The degradation of ECM is a prominent feature of OA, and MMPs are the major enzymes responsible for the ECM degradation of OA chondrocytes [34,35]. In the MMPs family, MMP-13 has the strongest ability to degrade type 2 collagen [36]. SOX9 is an important transcriptional regulator during chondrogenesis [37]. Col2a1 expression in chondrocytes is positively correlated with SOX9 concentration, confirming that Col2a1 is a SOX9-dependent chondrocyte proliferation gene regulator [38]. In our research, we observed that MMP-13 gene expression in chondrocytes were significantly increased after H2O2 treatment, while chondrogenic genes (Col2a1, aggrecan, and SOX9) expression were clearly depressed. However, the tendency was changed after hesperidin treatment, which suggested that damaged chondrocytes were protected against oxidative stress.
Since in vitro experiments have confirmed that hesperidin inhibits cell apoptosis and inflammatory cytokines expression caused by hydrogen peroxide, we further assessed whether hesperidin has therapeutic effects on OA in vivo. In an experimental rat model of OA, histologic and immunohistochemical analysis showed that hesperidin administration clearly prevented progression of cartilage destruction. Pathological sections showed that cartilage degeneration was significantly improved and inflammatory cytokines expression was reduced after hesperidin treatment.
Conclusions
These findings suggest that hesperidin can prevent H2O2-induced chondrocytes injury through its antioxidant effects in vitro and reduced cartilage damage in a rat model of OA. These results support the potential therapeutic applications of hesperidin as a supplementation in human OA treatment.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
Ethical approval All applicable international, national, and institutional guidelines for the care and use of animals were followed. Procedures involving animals were performed with approval and under the guidance of the Ethics Committee of Nanjing Medical University.
References
- 1.Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107:152–62. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandl A, Hartmann A, Bechmann V, et al. Oxidative stress induces senescence in chondrocytes. J Orthop Res. 2011;29:1114–20. doi: 10.1002/jor.21348. [DOI] [PubMed] [Google Scholar]
- 3.Díaz-Gallego L, Prieto JG, Coronel P, et al. Apoptosis and nitric oxide in an experimental model of osteoarthritis in rabbit after hyaluronic acid treatment. J Orthop Res. 2005;23:1370–76. doi: 10.1016/j.orthres.2005.05.003.1100230619. [DOI] [PubMed] [Google Scholar]
- 4.Yin W, Park JI, Loeser RF. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling pathways. J Biol Chem. 2009;284:31972–81. doi: 10.1074/jbc.M109.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates EJ, Johnson CC, Lowther DA. Inhibition of proteoglycan synthesis by hydrogen peroxide in cultured bovine articular cartilage. Biochim Biophys Acta. 1985;838:221–42. doi: 10.1016/0304-4165(85)90082-0. [DOI] [PubMed] [Google Scholar]
- 6.Tiku ML, Gupta S, Deshmukh DR. Aggrecan degradation in chondrocytes is mediated by reactive oxygen species and protected by antioxidants. Free Radic Res. 1999;30:395–405. doi: 10.1080/10715769900300431. [DOI] [PubMed] [Google Scholar]
- 7.Zhuang C, Xu NW, Gao GM, et al. Polysaccharide from Angelica sinensis protects chondrocytes from H2O2-induced apoptosis through its antioxidant effects in vitro. Int J Biol Macromol. 2016;87:322–28. doi: 10.1016/j.ijbiomac.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad S, Alam K, Hossain MM, et al. Anti-arthritogenic and cardioprotective action of hesperidin and daidzein in collagen-induced rheumatoid arthritis. Mol Cell Biochem. 2016;423:115–27. doi: 10.1007/s11010-016-2830-y. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi K, Maruyama H, Kometani T, Kumazawa Y. Suppression of collagen-induced arthritis by oral administration of the citrus flavonoid hesperidin. Planta Med. 2006;72:477–79. doi: 10.1055/s-2005-916254. [DOI] [PubMed] [Google Scholar]
- 10.Li R, Cai L, Xie XF, et al. Hesperidin suppresses adjuvant arthritis in rats by inhibiting synoviocyte activity. Phytother. 2010;24(Suppl 1):S71–76. doi: 10.1002/ptr.2906. [DOI] [PubMed] [Google Scholar]
- 11.Li R, Li J, Cai L, et al. Suppression of adjuvant arthritis by hesperidin in rats and its mechanisms. J Pharm Pharmacol. 2008;60:221–28. doi: 10.1211/jpp.60.2.0011. [DOI] [PubMed] [Google Scholar]
- 12.Maekawa S, Sato K, Fujita K, et al. The neuroprotective effect of hesperidin in NMDA-induced retinal injury acts by suppressing oxidative stress and excessive calpain activation. Sci Rep. 2017;7:6885. doi: 10.1038/s41598-017-06969-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Lin XF, Lu J, et al. Hesperidin ameliorates UV radiation-induced skin damage by abrogation of oxidative stress and inflammatory in HaCaT cells. J Photochem Photobiol B. 2016;165:240–45. doi: 10.1016/j.jphotobiol.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Shaban NZ, Ahmed ZAM, El-Rashidy FH, Abdo KAS. Protective role of hesperidin against γ-radiation-induced oxidative stress and apoptosis in rat testis. J Biol Res (Thessalon) 2017;24:5. doi: 10.1186/s40709-017-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Wu D, Fan W. Protection of ginsenoside Rg1 on chondrocyte from IL-1beta-induced mitochondria-activated apoptosis through PI3K/Akt signaling. Mol Cell Biochem. 2014;392:249–57. doi: 10.1007/s11010-014-2035-1. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Kamekura S, Kawasaki Y, Hoshi K, et al. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006;54:2462–70. doi: 10.1002/art.22041. [DOI] [PubMed] [Google Scholar]
- 18.Elshazly SM, Mahmoud AA. Antifibrotic activity of hesperidin against dimethylnitrosamine-induced liver fibrosis in rats. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:559–67. doi: 10.1007/s00210-014-0968-2. [DOI] [PubMed] [Google Scholar]
- 19.Yuan PW, Kang WL, Li XQ, et al. [Pathogenesis of osteoarthritis and related cytokines]. Orthopedic Journal of China. 2016;24:1010–14. [in Chinese] [Google Scholar]
- 20.Li B, Jiang TM, Liu H, et al. Andrographolide protects chondrocytes from oxidative stress injury by activation of the Keap1-Nrf2 – are signaling pathway. J Cell Physiol. 2018;234:561–71. doi: 10.1002/jcp.26769. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Wei X, Lu Y, et al. Glutaredoxin 1 (GRX1) inhibits oxidative stress and apoptosis of chondrocytes by regulating CREB/HO-1 in osteoarthritis. Mol Immunol. 2017;90:211–18. doi: 10.1016/j.molimm.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Pan Y, Chen D, Lu Q, et al. Baicalin prevents the apoptosis of endplate chondrocytes by inhibiting the oxidative stress induced by H2O2. Mol Med Rep. 2017;16:2985–91. doi: 10.3892/mmr.2017.6904. [DOI] [PubMed] [Google Scholar]
- 23.Sakata S, Hayashi S, Fujishiro T, et al. Oxidative stress-induced apoptosis and matrix loss of chondrocytes is inhibited by eicosapentaenoic acid. J Orthop Res. 2015;33:359–65. doi: 10.1002/jor.22767. [DOI] [PubMed] [Google Scholar]
- 24.Karakurum G, Karakok M, Tarakcioglu M, et al. Comparative effect of intra-articular administration of hyaluronan and/or cortisone with evaluation of malondialdehyde on degenerative osteoarthritis of the rabbit’s knee. Tohoku J Exp Med. 2003;199:127–34. doi: 10.1620/tjem.199.127. [DOI] [PubMed] [Google Scholar]
- 25.Abusarah J, Bentz M, Benabdoune H, et al. An overview of the role of lipid peroxidation-derived 4-hydroxynonenal in osteoarthritis. Inflamm Res. 2017;66:637–51. doi: 10.1007/s00011-017-1044-4. [DOI] [PubMed] [Google Scholar]
- 26.Aydogan NH, Baydar ML, Atay T, et al. The effect of arthroscopic surgery and intraarticular drug injection to the antioxidation system and lipid peroxidation at osteoarthritis of knee. Saudi Med J. 2008;29:397–402. [PubMed] [Google Scholar]
- 27.Ostalowska A, Birkner E, Wiecha M, et al. Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. Osteoarthritis Cartilage. 2006;14:139–45. doi: 10.1016/j.joca.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang Z, Ye G, Huang B. Kaempferol alleviates the tnterleukin-1β-induced inflammation in rat osteoarthritis chondrocytes via suppression of NF-κB. Med Sci Monit. 2017;23:3925–31. doi: 10.12659/MSM.902491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruggeri R, Pulsatelli L, Melchiorri C, et al. Differential expression of IL-1 and TNF receptors in inflammatory arthritis and osteoarthritis. Boll Soc Ital Biol Sper. 1996;72:15–20. [PubMed] [Google Scholar]
- 30.Killock D. Osteoimmunology: Could inhibition of IL-1 and TNF improve healing of meniscal lesions and prevent the development of osteoarthritis. Nat Rev Rheumatol. 2011;8:4. doi: 10.1038/nrrheum.2011.191. [DOI] [PubMed] [Google Scholar]
- 31.Khadem HM, Alipoor B, Malek MA, et al. Effects of sesame seed supplementation on inflammatory factors and oxidative stress biomarkers in patients with knee osteoarthritis. Acta Med Iran. 2015;53:207–13. [PubMed] [Google Scholar]
- 32.Maghsoumi-Norouzabad L, Alipoor B, Abed R, et al. Effects of Arctium lappa L. (Burdock) root tea on inflammatory status and oxidative stress in patients with knee osteoarthritis. Int J Rheum Dis. 2016;19:255–61. doi: 10.1111/1756-185X.12477. [DOI] [PubMed] [Google Scholar]
- 33.Germanou EI, Chatzinikolaou A, Malliou P, et al. Oxidative stress and inflammatory responses following an acute bout of isokinetic exercise in obese women with knee osteoarthritis. Knee. 2013;20:581–90. doi: 10.1016/j.knee.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Hou SM, Hou CH, Liu JFL. CX3CL1 promotes MMP-3 production via the CX3CR1, c-Raf, MEK, ERK, and NF-κB signaling pathway in osteoarthritis synovial fibroblasts. Arthritis Res Ther. 2017;19:282. doi: 10.1186/s13075-017-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19:248. doi: 10.1186/s13075-017-1454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perotto JH, Camejo FA, Doetzer AD, et al. Expression of MMP-13 in human temporomandibular joint disc derangement and osteoarthritis. Cranio. 2018;36:161–66. doi: 10.1080/08869634.2017.1315511. [DOI] [PubMed] [Google Scholar]
- 37.Jiang X, Huang X, Jiang T, et al. The role of Sox9 in collagen hydrogel-mediated chondrogenic differentiation of adult mesenchymal stem cells (MSCs) Biomater Sci. 2018;6:1556–68. doi: 10.1039/c8bm00317c. [DOI] [PubMed] [Google Scholar]
- 38.Huang W, Zhou X, Lefebvre V, de Crombrugghe B. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9’s ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol Cell Biol. 2000;20:4149–58. doi: 10.1128/mcb.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]