Abstract
Background
Calcitriol (1 alpha, 25-dihydroxy vitamin D3) is a good vitamin D supplement but can cause hypercalcemia. Whereas, 22-oxa-1 alpha, 25-dihydroxy vitamin D3 (22-oxa-calcitriol) has less hypercalcemic activity than calcitriol and is reported to be effective for cell-proliferative diseases. The objective of the study was to compare renal function and blood tests of arthritis patients receiving calcitriol supplements with those receiving 22-oxa-calcitriol supplements.
Material/Methods
A total of 369 patients with clinically confirmed rheumatoid arthritis were included in this phase II trial. Patients received lactose powder (the placebo group, n=123), 50 000 IU/week of 22-oxa-calcitriol (the treatment group, n=123), or 50 000 IU/week of calcitriol (the control group, n=123) for 6 weeks. At the time of enrollment and after 6 weeks of supplementation, renal function tests, blood tests, and secondary outcome measures were evaluated. One-way ANOVA and the chi-squared test for independence were performed for continuous data and constant data at a 95% of confidence level.
Results
Both 22-oxa-calcitriol and calcitriol successfully decreased swollen joints in patients with rheumatoid arthritis, and both improved Health Assessment Questionnaire Disease Activity Index scores and serum vitamin D levels. The intensity of improvement of serum vitamin D levels in both groups was the same (P<0.0001, q=0.24); however, calcitriol caused hypercalcemia (P<0.0001, q=12.59).
Conclusions
This study found that 22-oxa-calcitriol was a good option for vitamin D supplementation in rheumatoid arthritis patients.
MeSH Keywords: Blood Sedimentation, Calcitriol, Felty Syndrome, Hypercalcemia, Vascular Stiffness, Vitamin D Deficiency
Background
Rheumatoid arthritis (RA) is a chronic autoimmune disorder which has the main characteristic of synovial inflammation in the joints and loss of bone [1]. Vitamin D, as a steroid hormone, plays an important role in intestinal absorption of phosphorus and calcium [2] and it decreases the damage to bone and cartilage in patients with RA [1]. Vitamin D also increases interleukin (IL)-4 production, which might suppress inflammation [3].
Vitamin D deficiency in Chinese people is likely the result of living in an industrialized society, people not willing to spend time in sunlight (due to air pollution), use sunscreen lotions, umbrellas, or hats when outdoors, as well as limitations in macronutrient intakes as food and beverages in the Chinese diet are not rich in vitamin D [4]. T-helper (Th) 17 cells are pathogenic in patients with RA [5]. In China, an inverse association was found between vitamin D levels and RA disease activity [6].
Vitamin D supplementation is considered an additional option in the management of RA patients [7]. Some clinical trials have reported that vitamin D deficiency might increase RA disease activity, flare rates, and/or recurrence; however, other trials have reported no such relationship. Although some studies reported a synergistic effect between vitamin D and analgesic drugs resulting in reduced musculoskeletal pain, other trails have reported no role for vitamin D in relief of musculoskeletal pain [8]. Calcitriol (1 alpha, 25-dihydroxy vitamin D3) increases the IL-4 level and reduces IL-17A and interferon-γ levels in patients with RA, but calcitriol might decrease levels of immunoreactive parathyroid hormone and induce hypercalciuria, hypercalcemia, and nephrolithiasis, which then requires supplementation with steroid therapy. Moreover, hypercalciuria decreases absorption of calcium by the intestine [9]. Thus, the importance of vitamin D supplementation in RA continues to be debatable.
The other derivative of vitamin D3, i.e., 22-oxa-1 alpha, 25-dihydroxy vitamin D3 (22-oxa-calcitriol) has less hypercalcemic activity than calcitriol and has been reported to be effective for cell-proliferative diseases [10]. These reported effects of vitamin D analogs are irrespective of their activity to induce hypercalcemia in terms of structure [11]. To the best of our knowledge, no study has evaluated the efficacy and safety of 22-oxa-calcitriol in RA patients until this current study.
The objective of this study was to compare renal function and blood tests of arthritis patients receiving calcitriol supplementation with those receiving 22-oxa-calcitriol supplementation.
Material and Methods
Drugs
Calcitriol (Rocaltrol) was purchased from Validus Pharmaceuticals, USA; 22-oxa-calcitriol was provided by the Affiliated Hospital of Xuzhou Medical University, China for research purposes. Lactose powder was purchased from Valio Ltd., Helsinki, Finland.
Ethical considerations and consent to participate
This study was registered with the research registry (www.researchregistry.com), UID No. researchregistry4170 dated February 1, 2017. The protocol (AXMU/PH-II/H201627/13/17 dated January 27, 2017) was approved by the Affiliated Hospital of Xuzhou Medical University review board. This study adhered to the law of China, the consolidated standards of reporting trials (CONSORT) guidelines, and the 2013 Declarations of Helsinki [12]. An informed consent form regarding interventions, pathology, and publication of the study in all formats (hard and/or electronics) irrespective of time and language was signed by participating patients or their relatives (legally authorized person).
Inclusion criteria
Patients of the Department of Orthopedics of the Affiliated Hospital of Xuzhou Medical University, Xuzhou, China, who had clinically confirmed RA (as per ACR/EULAR criteria [13]) during at least the past 3 months [3]) from February 3, 2017 to January 1, 2018 were included in this study. Patients aged 18 years and older were included in this study. Active RA was defined as at least 4 swollen joints (out of 70 examined) [13]. Patients who had erosions in radiographic images, morning stiffness (at least half an hour in duration) were included in this study. The demographic parameters of the patients are presented in Table 1.
Table 1.
The demographic parameters of the enrolled patients.
| Parameters | Groups | Comparisons between groups | |||
|---|---|---|---|---|---|
| Interventions | Placebo | Treatment | Control | ||
| Lactose | 22-oxa-1 alpha, 25-dihydroxyvitamin D3 | 25-dihydroxyvitamin D3 | P-value | ||
| Sample size | 123 | 123 | 123 | - | |
| Age (years) | Minimum | 41 | 37 | 38 | 0.148 |
| Maximum | 62 | 61 | 62 | ||
| Mean ±SD | 51.12±10.23 | 48.47±11.12 | 49.58±10.57 | ||
| Gender | Male | 23 (19) | 25 (20) | 21 (17) | 0.647 |
| Female | 120 (81) | 98 (80) | 102 (83) | ||
| Duration of rheumatoid arthritis (months) | 83±12 | 78±16 | 79±13 | 0.062 | |
| Weight (kg) | 54.22±3.15 | 53.47±4.18 | 56.88±5.47 | 0.082 | |
| Height (cm) | 155.12±5.23 | 154.18±4.89 | 153.23±6.22 | 0.065 | |
| Smoking | No-smokers | 112 (91) | 109 (88) | 111 (90) | 0.726 |
| Former smokers | 6 (5) | 7 (6) | 8 (7) | ||
| Current-smokers | 5 (4) | 7 (6) | 4 (3) | ||
| C-reactive protein (mg/dL) | 1.81±0.71 | 1.91±0.09 | 1.72±0.1 | 0.235 | |
| Visual analog scale score for pain* | 5.91±0.51 | 6.12±0.59 | 5.82±0.62 | 0.689 | |
| Duration of morning stiffness (min) | 135±15 | 146±13 | 141±12 | 0.092 | |
| Erythrocyte sedimentation rate (mm/h) | 38.12±3.15 | 37.15±3.57 | 36.47±3.12 | 0.083 | |
| Random protein in urine (mg/dL) | 38.12±2.89 | 39.15±3.18 | 38.45±4.45 | 0.071 | |
| Protein in urine/day (mg/dl) | 99.45±4.52 | 98.61±5.12 | 97.89±5.67 | 0.059 | |
| Swollen joints | 4–8 | 13 (11) | 15 (13) | 17 (14) | 0.891 |
| 9–13 | 65 (53) | 61 (49) | 65 (53) | ||
| ≥14 | 45 (37) | 47 (38) | 41 (33) | ||
| Serum vitamin D (ng/mL) | 15.45±1.53 | 15.72±1.89 | 16.01±1.98 | 0.054 | |
| Serum calcium level (mg/dL) | 7.67±0.59 | 7.81±0.61 | 7.85±0.82 | 0.095 | |
| Urine albumin level (mg/mL) | 7.71±1.25 | 7.92±1.85 | 7.53±1.01 | 0.098 | |
| Urine Creatinine (mg/dL) | 0.75±0.06 | 0.77±0.09 | 0.76±0.04 | 0.064 | |
| HAQ-DI | 1.31±0.75 | 1.33±0.77 | 1.34±0.79 | 0.953 | |
Constant data were represented as a number (percentage) and continuous data were represented as mean ±SD. Calculations for IU for the treatment group was developed as from control group. All patients were of PR. China origin.
0: Absent pain and 10: the worst possible pain. One-way ANOVA for continuous data and chi-squared test for independence for constant data were performed for statistical analysis. A P<0.01 considered as significant. Numbers of swollen joints counts in each group were 70/patient. HAQ-DI – The Health Assessment Questionnaire Disease Activity Index (0–3 score).
Exclusion criteria
Patients aged younger than 18 years and patients who did not signed informed consent forms were excluded from this study. Patients who were rheumatoid factor negative were excluded from this study. Patients who were already on methotrexate, anti-tumor necrosis factor-α, anakinra, alefacept, infliximab, etanercept, efalizumab, adalimumab, pain-killers, or systemic corticosteroids therapies were excluded from this study.
Sample size calculation
A total of 369 patients were included in this randomized study design. The sample size was 123 for each group. The hypothesized% frequency of outcome was 80%, the confidence level was 95% (α=0.05), the design effect for cluster surveys was 1.
Design of this study
Patients were subjected to simple randomization (1: 1: 1), double-blind study [14]. The software to generate a random sequence was OpenEpi 3.01-English (Open Source Epidemiologic Statistics for Public Health, USA). Blinding was performed by prefilled envelopes.
Interventions
Patients in the placebo group, the treatment group, and the control group received lactose powder 5 g/week, 50 000 IU/week, 22-oxa-calcitriol (1 IU of 22-oxa-calcitriol is equivalent to 25 ng of 22-oxa-calcitriol), and 50 000 IU/week calcitriol (1 IU of calcitriol is equivalent to 25 ng of calcitriol) [15], respectively. All supplementations were continued for 6 weeks [16].
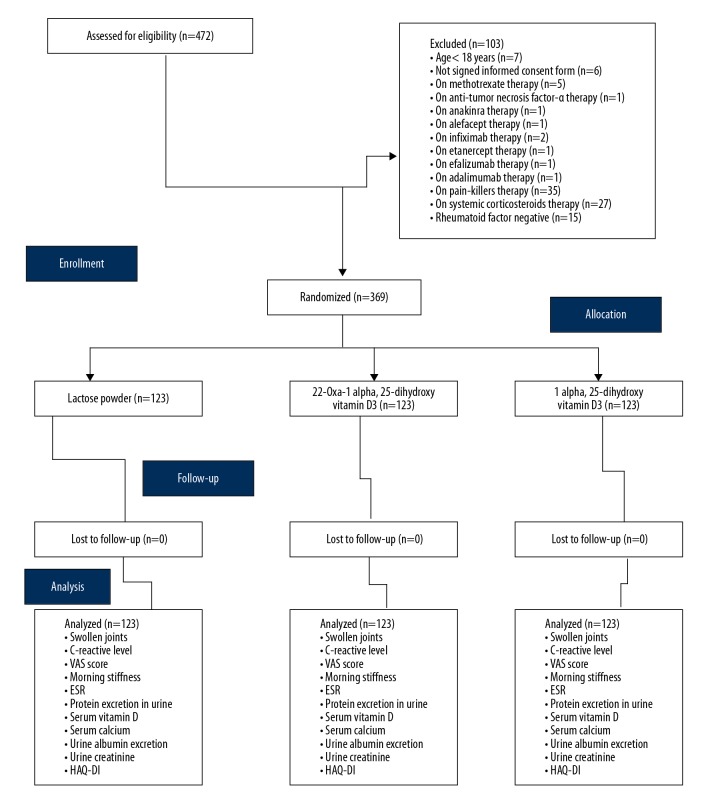
The CONSORT flow diagram of the trial is represented in Figure 1.
Figure 1.
CONSORT flow diagram of the trial. Hypothesized% frequency of outcome was 80%, the confidence level was 95%, the design effect for cluster surveys was 1. ESR – erythrocyte sedimentation rate; HAQ-DI – The Health Assessment Questionnaire disease activity index.
The functional status or disability of each patient was evaluated by the Health Assessment Questionnaire Disease Activity Index (HAQ-DI). HAQ-DI includes 20 questions regarding the performance of the daily physical activity. Every question has 4 scores: 0, perform the activity without any difficulty; 1, perform an activity with moderate difficulty; 2, perform an activity with moderate difficulty; and 3, unable to perform an activity [17].
Renal function tests
Lower renal function might be associated with vitamin D and its metabolites [3]. Therefore, at the time of enrollment and after 6 weeks of supplementation, random urine samples were collected for all study patients. A dip stick (Dipstick, Melson Medical Corporation Limited Shanghai, China) was dipped in the urine, and the change in color of the stick indicated the level of protein in the urine (0–20 mg/dL is considered normal for random protein levels [18]). For urine albumin excretion tests, at enrollment and after 6 months, urine samples of patients were collected in the early morning and stored at −80°C. The kinetic alkaline picrate method was performed to measure urine albumin concentrations [19].
Blood tests
Plasma samples were collected at baseline and after 6 weeks of supplementation. Serum vitamin D levels were measured by radioimmunoassay method using γ-B, 25-hydroxy vitamin D RIA (IDS, Boldon, UK) [20]. The remaining sample was subjected to erythrocyte sedimentation rate [21], C-reactive protein [17], and serum calcium testing by the modified Arsenazo method (Biobase Biotech (Jinan) Co., Ltd., China) [22].
Secondary outcome measures
After 6 weeks of supplementation, all the enrolled patients were evaluated using counts of swollen joints [14], duration of morning stiffness (difficulty or slowness in moving the joints) [23], and visual analog scale (VAS) score for pain [14]. The nursing staff who evaluated the patient outcome measures were blind to the form of supplementation.
Statistical analysis
One-way analysis of variance (ANOVA) [14] and the chi-squared test for independence [21] was performed for continuous data and constant data, respectively. For the demographic parameters of the enrolled patient, results were considered significant at 99% of confidence level and results related to the trial were considered significant at 95% of confidence level. Tukey test (considering critical value (q) >3.329 as significant) was performed for post-hoc analysis. Intention-to-treat analysis method was adopted [14]. InStat (GraphPad, USA) was used for statistical analysis.
Results
After 6 weeks of intervention, 22-oxa-calcitriol (P<0.0001, q=5.82) and calcitriol (P<0.0001, q=5.81) were successful in decreasing swollen joints in patients with RA. In addition, 22-oxa-calcitriol was found to have the same effect as calcitriol (P<0.0001, q=0.48, Table 2).
Table 2.
Effects of interventions on swollen joints.
| Parameters | Groups | Comparisons between groups at EP | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Placebo (1) | Treatment (2) | Control (3) | ||||||||||||||||
|
| ||||||||||||||||||
| Level | BL | EP | SA bet-ween BL and EP | BL | EP | SA between BL and EP | BL | EP | SA between BL and EP | P-value | q-value 1 vs. 2 | q-value 1 vs. 3 | q-value 2 vs. 3 | |||||
|
| ||||||||||||||||||
| Sample size | 123 | 123 | P | 123 | 123 | P | q | 123 | 123 | P | q | |||||||
| Swollen joints | 4–8 | T | 13(11) | 12(10) | 0.32 | 15(13) | 45(37) | <0.0001 | 8.01 | 17(14) | 50(41) | <0.0001 | 8.55 | <0.0001 | 5.51 | 5.99 | 0.48 | |
|
| ||||||||||||||||||
| M | 1(1) | 1(1) | N/A | 1(1) | 1(1) | N/A | N/A | 1(1) | 2(2) | 0.32 | N/A | |||||||
|
| ||||||||||||||||||
| F | 12(10) | 11(9) | 0.32 | 14(12) | 44(36) | <0.0001 | 8.12 | 16(13) | 48(39) | <0.0001 | 8.74 | |||||||
|
| ||||||||||||||||||
| SA between M and F | P | 0.0007 | 0.0013 | – | 0.0002 | <0.0001 | – | <0.0001 | <0.0001 | – | ||||||||
|
| ||||||||||||||||||
| q | 5.519 | 5.2 | – | 6.13 | 13.71 | – | 6.7 | 14.2 | – | |||||||||
|
| ||||||||||||||||||
| 9–13 | T | 65(53) | 65(53) | N/A | 61(49) | 45(37) | <0.0001 | 3.59 | 65(53) | 39(32) | <0.0001 | 5.7 | ||||||
|
| ||||||||||||||||||
| M | 12(10) | 13(11) | 0.32 | 14(11) | 13(11) | 0.32 | N/A | 11(9) | 10(8) | 0.32 | N/A | |||||||
|
| ||||||||||||||||||
| F | 53(43) | 52(42) | 0.32 | 47(38) | 32(26) | <0.0001 | 3.56 | 54(44) | 29(24) | <0.0001 | 5.95 | |||||||
|
| ||||||||||||||||||
| SA between M and F | P | <0.0001 | <0.0001 | – | <0.0001 | <0.0001 | – | <0.0001 | <0.0001 | – | ||||||||
|
| ||||||||||||||||||
| q | 11.05 | 10.43 | – | 8.84 | 5.52 | – | 11.3 | 5.47 | – | |||||||||
|
| ||||||||||||||||||
| ≥14 | T | 45(37) | 46(37) | 0.32 | 47(38) | 33(26) | 0.0001 | 3.11 | 41(33) | 35(27) | 0.014 | 1.43 | ||||||
|
| ||||||||||||||||||
| M | 10(8) | 9(7) | 0.32 | 10(8) | 11(9) | 0.32 | N/A | 9(7) | 9(7) | N/A | N/A | |||||||
|
| ||||||||||||||||||
| F | 35(29) | 37(30) | 0.16 | 37(30) | 22(17) | <0.0001 | 3.9 | 32(26) | 26(20) | 0.014 | 1.56 | |||||||
|
| ||||||||||||||||||
| SA between M and F | P | <0.0001 | <0.0001 | – | <0.0001 | 0.0007 | – | <0.0001 | <0.0001 | – | ||||||||
|
| ||||||||||||||||||
| q | 7.37 | 8.26 | – | 7.87 | 3.58 | – | 7.01 | 5.46 | – | |||||||||
Data were represented as number (percentage). Chi-squared test for independence following Tukey post-hoc tests was performed for statistical analysis. P<0.05 and q>3.329 were considered significant. BL – baseline; EP – after 6 weeks of supplementation. Numbers of swollen joints counts in each group were 70/patient. T – total; M – Male; F – Female; SA – statistical analysis; N/A – not applicable.
After 6 weeks of treatment, lactose powder did not improved serum vitamin D levels (P=0.28) or serum calcium levels (P=0.9). However, 22-oxa-calcitriol improved serum vitamin D levels (P<0.0001, q=8.09) but did not improve serum calcium levels (P=0.031, q=3.29). Calcitriol improved both serum vitamin D levels (P<0.0001, q=7.17) and serum calcium levels (P<0.0001, q=15.2). The intensity of improvement of vitamin D levels by both vitamin D supplements was the same (P<0.0001, q=0.24); however, calcitriol caused hypercalcemia in patients with RA compared to 22-oxa-calcitriol (P<0.0001, q=12.59, Table 3).
Table 3.
Effects of interventions on serum vitamin D and calcium levels.
| Parameters | Group | Comparisons between groups at EP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (1) | Treatment (2) | Control (3) | ||||||||
| Level | BL | EP | BL | EP | BL | EP | P-value | q-value 1 vs. 2 | q-value 1 vs. 3 | q-value 2 vs. 3 |
| Sample size | 123 | 123 | 123 | 123 | 123 | 123 | ||||
| Serum vitamin D level (ng/mL) | 15.45± 1.53 | 15.92± 4.56 | 15.72± 1.89 | 17.85± 1.09 | 16.01± 1.98 | 17.91± 1.11 | <0.0001 | 7.7 | 7.93 | 0.24 |
| Serum calcium level (mg/dL) | 7.67± 0.59 | 7.68± 0.61 | 7.81± 0.61 | 8.01± 0.82 | 7.85± 0.82 | 8.78± 0.58 | <0.0001 | 5.394 | 17.98 | 12.587 |
Data were represented as mean ±SD. One-way ANOVA following Tukey post-hoc test was performed for statistical analysis. P<0.05 and q>3.329 were considered as significant. BL – baseline; EP – after 6 weeks of supplementation. Radioimmunoassay method was preferred.
After oral supplementation for 6 weeks, the treatment group (1.91±0.09 mg/dL vs. 1.61±0.12 mg/dL, P=0.023, q=3.56) and the control group (1.72±0.1 mg/dL vs. 1.52±0.08, P=0.03, q=3.41) both had reduction in C-reactive protein. Reduction of C-reactive protein by 22-oxa-calcitriol and calcitriol was the same after 6 weeks (P=0.026, q=1.26).
22-oxa-calcitriol (6.12±0.59 vs. 5.15±0.81, P=0.005, q=4.07) and calcitriol (5.82±0.62 vs. 5.04±0.51, P=0.018, q=3.43) both decreased pain (due to inflammation) in patients. However, the intensity of decreased pain of patients were the same (P=0.016, q=0.45).
The enrolled patients suffered from severe morning stiffness. Therefore, the placebo effect was also observed for decrease in morning stiffness (135±15 min at the time of enrollment and 130±17 min after 6 weeks of lactose powder supplementation, P=0.015, q=3.39). The treatment group (146±13 min vs. 115±15 min, P<0.0001, q=22.8) and the control group (141±12 min vs. 105±14 min, P<0.0001, q=27.57) had improved morning stiffness of patients after supplementation. Moreover, 22-oxa-calcitriol was less effective in improving morning stiffness of patients than calcitriol (P<0.0001, q=7.21).
The treatment group using 22-oxa-calcitriol (37.15±3.57 mm/h vs. 32.45±3.11 mm/h, P<0.0001, q=14.8) and the control group using calcitriol (36.47±3.12 mm/h vs. 31.53±3.54 mm/h, P<0.0001, q=15.58) had improved erythrocyte sedimentation rates. However, effects of 22-oxa-calcitriol and calcitriol were equal (P<0.0001, q=2.9).
Supplementation with 22-oxa-calcitriol decreased random protein in urine (39.15±3.18 mg/dL vs. 32.18±2.89 mg/dL, P<0.0001, q=25.05) and protein in urine/day (98.61±5.12 mg/dL vs. 90.12±6.12 mg/dL, P<0.0001, q=16.78). Calcitriol also decreased random protein in urine (38.45±4.45 mg/dL vs. 33.53±3.35 mg/dL, P<0.0001, q=14.74) and protein in urine/day (97.89±5.67 mg/dL vs. 92.56±7.96 mg/dL, P<0.0001, q=9.11). Moreover, 22-oxa-calcitriol more significantly reduced random protein in urine (P<0.0001, q=4.19) and protein in urine/day (P<0.0001, q=4.09) than calcitriol.
Both 22-oxa-calcitriol and calcitriol improved urine albumin excretion, HAQ-DI, and urine creatinine levels. The intensity of the decrease of urine albumin excretion by calcitriol was better than 22-oxa-calcitriol (P<0.0001, q=4.533). The intensity of improvement in HAQ-DI by both vitamin D supplements was the same (P=0.04, q=0.998). The intensity of the decrease in urine creatinine levels by both vitamin D supplements was the same (P=0.451, Table 4).
Table 4.
Primary and secondary outcome measures of the study.
| Parameters | Groups | Comparisons between groups | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Treatment | Control | |||||||||||||
| Level | BL | EP | SA | BL | EP | SA | BL | EP | SA | EP | |||||
| Sample size | 123 | 123 | P | 123 | 123 | P | q | 123 | 123 | P | q | P | q 1 vs. 2 | q 1 vs. 3 | q 2 vs. 3 |
| CRP (mg/dL) | 1.81± 0.71 | 1.79± 0.72 | 0.827 | 1.91± 0.09 | 1.61± 0.12 | 0.023 | 3.56 | 1.72± 0.1 | 1.52± 0.08 | 0.03 | 3.41 | 0.026 | 3.52 | 3.58 | 1.26 |
| #VAS | 5.91± 0.51 | 5.89± 0.53 | 0.95 | 6.12± 0.59 | 5.15± 0.81 | 0.005 | 4.066 | 5.82± 0.62 | 5.04± 0.51 | 0.018 | 3.43 | 0.016 | 3.38 | 3.77 | 0.45 |
| DMS (min) | 135± 15 | 130± 17 | 0.015* | 146± 13 | 115± 15 | <0.0001 | 22.8 | 141± 12 | 105± 14 | <0.0001 | 27.57 | <0.0001 | 10.814 | 18.023 | 7.21 |
| ESR (mm/h) | 38.12± 3.15 | 37.98± 3.85 | 0.755 | 37.15± 3.57 | 32.45± 3.11 | <0.0001 | 14.8 | 36.47± 3.12 | 31.53± 3.54 | <0.0001 | 15.58 | <0.0001 | 17.46 | 20.36 | 2.9 |
| RPU (mg/dL) | 38.13± 2.89 | 37.45± 3.18 | 0.085 | 39.15± 3.18 | 32.18± 2.89 | <0.0001 | 25.05 | 38.45± 4.45 | 33.53± 3.35 | <0.0001 | 14.74 | <0.0001 | 16.37 | 12.17 | 4.19 |
| PUD (mg/dL) | 99.45± 4.52 | 98.36± 5.55 | 0.093 | 98.61± 5.12 | 90.12± 6.12 | <0.0001 | 16.78 | 97.89± 5.67 | 92.56± 7.96 | <0.0001 | 9.11 | <0.0001 | 13.98 | 9.71 | 4.09 |
| AU (mg/mL) | 7.71± 1.25 | 7.65± 1.19 | 0.7 | 7.92± 1.85 | 6.65± 1.01 | <0.0001 | 8.699 | 7.53± 1.01 | 6.21± 1.02 | <0.0001 | 10.829 | <0.0001 | 10.302 | 14.835 | 4.533 |
| UC (mg/dL) | 0.75± 0.06 | 0.74± 0.03 | 0.1 | 0.77± 0.09 | 0.74± 0.08 | 0.0009 | 4.644 | 0.76± 0.04 | 0.73± 0.09 | 0.0004 | 5.597 | 0.451 | N/A | N/A | N/A |
| @HAQ-DI | 1.31± 0.75 | 1.29± 0.71 | 0.83 | 1.33± 0.77 | 1.15± 0.1 | 0.011 | 4.453 | 1.34± 0.79 | 1.19± 0.28 | 0.0483 | 3.438 | 0.04 | 3.494 | 2.5 | 0.998 |
One-way ANOVA following Tukey post-hoc test was performed for statistical analysis. P<0.05 and q>3.329 were considered as significant. Data were represented as mean ±SD.
0: Absent pain and 10: the worst possible pain. BL – baseline; EP – after 6 weeks of supplementation; N/A – napplicable; HAQ-DI – The Health Assessment Questionnaire Disease Activity Index (0–3 score); CRP – C-reactive protein; VAS – visual analog scale score for pain; DMS – duration of morning stiffness; ESR – erythrocyte sedimentation rate; RPU – random protein in urine; PUD – protein in urine/day; AU – albumin in urine; UC – urine creatinine; SA – statistical analysis.
q=3.39.
20 questions for the performance of the daily physical activity. 0: Perform the activity without any difficulty, 1: Perform an activity with moderate difficulty, 2: Perform an activity with moderate difficulty, and 3: Unable to perform an activity.
Younger age patients had significant improvements compared to older patients. In the same way, women had significant improvements compared to men in the follow-up period.
Discussion
In this study, patients treated with 22-oxa-calcitriol or calcitriol (as a control group) were followed prospectively for 6 weeks and the effects of 22-oxa-calcitriol on some aspects of disease activity of RA patients was reported. Calcitriol supplementation is mostly used in RA patients [8] because the prevalence of osteoporosis and fracture might increase in women and older patients with RA compared to healthy persons [24]. Vitamin D inhibits the Th17 and Th1 proinflammatory responses and also promotes Th2 and Treg (regulatory T) cell immunomodulating responses. These responses might result in a decrease of the immune response of effector T-cells [23]. The consideration of supplementation adopted by rheumatologists in clinically confirmed RA, is generally to protect patients against high bone turnover and progression of disease activity.
In this study, 2-oxa-calcitriol showed a significant reduction in proteinuria, urine albumin excretion, VAS score for pain (P=0.005, q=4.07), and HAQ-DI. Other experimental studies have shown that 2-oxa-calcitriol is effective and safe in autoimmune disorders [25,26]. The results of this study, the first phase II trial on a synthetic analog of vitamin D3, support the usefulness of 2-oxa-calcitriol supplementation in patients with RA.
During this trial, calcitriol induced hypercalcemia (P<0.0001, q=15.2), but 2-oxa-calcitriol did not induce hypercalcemia (P=0.031, q=3.29). This finding supports other reports that 2-oxa- calcitriol as a good vitamin D supplement that does not induce hypercalcemia [10,27]. With respect to the results of our study, additional trials are required to justify the role of calcitriol in RA patients.
Our study patients had low levels of serum vitamin D, and it was confirmed that all participants had vitamin D deficiency due to RA disease activity [17]. Calcitriol and 2-oxa-calcitriol both reduced C-reactive protein and urine creatinine levels and improved serum vitamin D levels (P<0.0001, q=8.09). For the renal function tests, our study showed that 2-oxa-calcitriol improved renal functions of RA patients.
Calcitriol was more effective in improving morning stiffness of RA patients than 22-oxa-calcitriol (P<0.0001, q=7.21). Morning stiffness is a recognized symptom of RA but is difficult to measure and poorly understood [28]. Effects of oral supplementations in RA patients with less than 60 min of morning stiffness compared to more than 60 min were not analyzed separately in our study. Such data might have provided a different result [29]. Our study provided useful information regarding an important characteristic of RA. Further study is required to understand the mechanism of action of 22-oxa-calcitriol on morning stiffness in RA patients.
Limitations of this study included the lack of a dose oscillation component for 22-oxa-calcitriol; and all patients were of Chinese heritage only. In addition, results were significant in a very narrow range, and limited information regarding RA was gathered for the trial. Only a random serum vitamin D level was measured during the trial.
Conclusions
This phase II trial concluded that 22-oxa-calcitriol was a good option for vitamin D supplementation for RA patients. However, a dose oscillation study is required to demonstrate metabolism, tissue distribution, and a clear mechanism of action.
Acknowledgments
Authors are thankful for the medical and non-medical staff of the Affiliated Hospital of Xuzhou Medical University, Xuzhou, China for their contributions to this study.
Footnotes
Source of support: The study was supported by Jiangsu Provincial Health and Family Planning Commission (No: H201627)
Conflict of interest
None.
References
- 1.Luo J, Wen H, Guo H, et al. 1,25-dihydroxy vitamin D3 inhibits the RANKL pathway and impacts on the production of pathway-associated cytokines in early rheumatoid arthritis. Biomed Res Int. 2013;2013 doi: 10.1155/2013/101805. 101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Stoecklin E, Eggersdorfer M. A glimpse of vitamin D status in Mainland China. Nutrition. 2013;29:953–57. doi: 10.1016/j.nut.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Baker JF, Baker DG, Toedter G, et al. Associations between vitamin D, disease activity, and clinical response to therapy in rheumatoid arthritis. Clin Exp Rheumatol. 2012;30:658–64. [PubMed] [Google Scholar]
- 4.Li LM, Rao KQ, Kong LZ, et al. Technical Working Group of China National Nutrition and Health Survey. [A description on the Chinese national nutrition and health survey in 2002]. Chinese J Epidemiol. 2005;26:478–84. [in Chinese] [PubMed] [Google Scholar]
- 5.Fawaz L, Mrad MF, Kazan JM, et al. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin Immunol. 2016;166–67:59–71. doi: 10.1016/j.clim.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Sahebari M, Mirfeizi Z, Rezaieyazdi Z, et al. 25(OH) vitamin D serum values and rheumatoid arthritis disease activity (DA S28 ESR) Caspian J Intern Med. 2014;5:148–55. [PMC free article] [PubMed] [Google Scholar]
- 7.Di Franco M, Barchetta I, Iannuccelli C, et al. Hypovitaminosis D in recent onset rheumatoid arthritis is predictive of reduced response to treatment and increased disease activity: A 12-month follow-up study. BMC Musculoskelet Disord. 2015;16:53. doi: 10.1186/s12891-015-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bragazzi NL, Watad A, Neumann SG, et al. Vitamin D and rheumatoid arthritis: An ongoing mystery. Curr Opin Rheumatol. 2017;29:378–88. doi: 10.1097/BOR.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 9.Gates S, Shary J, Turner RT, et al. Abnormal calcium metabolism caused by increased circulating 1,25-dihydroxyvitamin D in a patient with rheumatoid arthritis. J Bone Miner Res. 1986;1:221–26. doi: 10.1002/jbmr.5650010209. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto K, Iwagami M, Seki T, et al. Dual antiplasmodial activity of vitamin D3 and its analog, 22-Oxacalcitriol, by direct and indirect mechanisms. Parasitol Int. 2017;66:89–99. doi: 10.1016/j.parint.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto R, Gery S, Kuwayama Y, et al. Novel Gemini vitamin D3 analogs: Large structure/function analysis and ability to induce antimicrobial peptide. Int J Cancer. 2014;134:207–17. doi: 10.1002/ijc.28328. [DOI] [PubMed] [Google Scholar]
- 12.World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–94. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Cho SK, Han M, et al. The role of bone scintigraphy in the diagnosis of rheumatoid arthritis according to the 2010 ACR/EULAR classification criteria. J Korean Med Sci. 2014;29:204–9. doi: 10.3346/jkms.2014.29.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kremer J, Ritchlin C, Mendelsohn A, et al. Golimumab, a new human anti-tumor necrosis factor alpha antibody, administered intravenously in patients with active rheumatoid arthritis: Forty-eight-week efficacy and safety results of a phase III randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2010;62:917–28. doi: 10.1002/art.27348. [DOI] [PubMed] [Google Scholar]
- 15.Salesi M, Farajzadegan Z. Efficacy of vitamin D in patients with active rheumatoid arthritis receiving methotrexate therapy. Rheumatol Int. 2012;32:2129–33. doi: 10.1007/s00296-011-1944-5. [DOI] [PubMed] [Google Scholar]
- 16.Chandrashekara S, Patted A. Role of vitamin D supplementation in improving disease activity in rheumatoid arthritis: An exploratory study. Int J Rheum Dis. 2017;20:825–31. doi: 10.1111/1756-185X.12770. [DOI] [PubMed] [Google Scholar]
- 17.Buondonno I, Rovera G, Sassi F, et al. Vitamin D and immunomodulation in early rheumatoid arthritis: A randomized double-blind placebo-controlled study. PLoS One. 2017;12:e0178463. doi: 10.1371/journal.pone.0178463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makihara N, Yamasaki M, Morita H, Yamada H. A dipstick test combined with urine specific gravity improved the accuracy of proteinuria determination in pregnancy screening. Kobe J Med Sci. 2010;56:E165–72. [PubMed] [Google Scholar]
- 19.Becetti K, Oeser A, Ormseth MJ, et al. Urinary albumin excretion is increased in patients with rheumatoid arthritis and associated with arterial stiffness. J Rheumatol. 2015;42:593–98. doi: 10.3899/jrheum.141295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadat-Ali M, Al-Elq AH, Al-Shaikh IH, et al. Assessment of low vitamin D among Saudi Arabians. Did we overshoot the runway? Saudi Med J. 2014;35:1243–49. [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima T, Yabe Y, Kaneko A, et al. Monitoring C-reactive protein levels to predict favourable clinical outcomes from tocilizumab treatment in patients with rheumatoid arthritis. Mod Rheumatol. 2013;23:977–85. doi: 10.1007/s10165-012-0782-y. [DOI] [PubMed] [Google Scholar]
- 22.Chavan VU, Ramavataram D, Patel PA, Rupani MP. Evaluation of serum magnesium, lipid profile and various biochemical parameters as risk factors of cardiovascular diseases in patients with rheumatoid arthritis. J Clin Diagn Res. 2015;9:C01–5. doi: 10.7860/JCDR/2015/12206.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abourazzak FE, Talbi S, Aradoini N, et al. 25-hydroxy vitamin D and its relationship with clinical and laboratory parameters in patients with rheumatoid arthritis. Clin Rheumatol. 2015;34:353–57. doi: 10.1007/s10067-014-2713-0. [DOI] [PubMed] [Google Scholar]
- 24.Avouac J, Koumakis E, Toth E, et al. Increased risk of osteoporosis and fracture in women with systemic sclerosis: A comparative study with rheumatoid arthritis. Arthritis Care Res. 2012;64:1871–78. doi: 10.1002/acr.21761. [DOI] [PubMed] [Google Scholar]
- 25.Abe J, Nakamura K, Takita Y, et al. Prevention of immunological disorders in MRL/l mice by a new synthetic analogue of vitamin D3: 22-oxa-1 alpha,25-dihydroxy Vitamin D3. J Nutr Sci Vitaminol (Tokyo) 1990;36:21–31. doi: 10.3177/jnsv.36.21. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa K, Sasaki Y, Kato S, et al. 22-oxa-1alpha,25-dihydroxy vitamin D3 inhibits metastasis and angiogenesis in lung cancer. Carcinogenesis. 2005;26:1044–54. doi: 10.1093/carcin/bgi049. [DOI] [PubMed] [Google Scholar]
- 27.Somjen D, Katzburg S, Grafi-Cohen M, et al. Vitamin D metabolites and analogs induce lipoxygenase mRNA expression and activity as well as reactive oxygen species (ROS) production in human bone cell line. J Steroid Biochem Mol Biol. 2011;123:85–89. doi: 10.1016/j.jsbmb.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Halls S, Dures E, Kirwan J, et al. Stiffness is more than just duration and severity: A qualitative exploration in people with rheumatoid arthritis. Rheumatology. 2015;54:615–22. doi: 10.1093/rheumatology/keu379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zakeri Z, Sandoughi M, Mashhadi MA, et al. Serum vitamin D level and disease activity in patients with recent onset rheumatoid arthritis. Int J Rheum Dis. 2016;19:343–47. doi: 10.1111/1756-185X.12181. [DOI] [PubMed] [Google Scholar]