Abstract
Objective
The present study aimed at evaluating the efficacy of an improved phage lysate marker vaccine for haemorrhagic septicaemia in mice and rabbit model and development of a DIVA ELISA based on iron restricted outer membrane protein (IROMP).
Method
The experimental vaccine was prepared by lysing P. multocida B:2 grown under iron restricted conditions with a Pasteurella bacteriophage and addition of an alum adjuvant to enhance the immunogenicity. The vaccine was administered in mice and rabbits divided into two group each. Phage lysate vaccine (PL-VacI) was administered to group I mice and rabbits whereas group II mice and rabbits received alum precipitated HS vaccine (HS-VacII). Antibody titres were monitored 0, 30, 60, 90, 210 and 240 dpv. An IROMP (130 kDa) based indirect ELISA was also developed to differentiate between infected and vaccinated animals. The Pasteurella phage isolated in present study was sequenced at Georgia Genomic Facilty, Georgia.
Result
The sequence of PMP-GAD-IND (Pasteurella bacteriophage) was deposited in GenBank under no KY203335. The group I mice and rabbits vaccinated with Phage lysate vaccine (PL-VacI) group revealed significantly higher antibody titres than group II mice and rabbits receiving alum-precipitated bacterin (HS-VacII) by MAT, IHA and ELISA (P < 0.05 and P < 0.001). The peak log 10 values (3.46) in case of group I mice by ELISA were attained at 90DPI whereas in group II mice the peak values at 90DPI were 2.82. Mean log10 titres by ELISA in group I and II rabbits were 2.43 and 2.35 respectively at 30DPI whereas at 120DPI the titres were 3.29 and 2.75, respectively. The DIVA ELISA detected presence of a novel 137 kDa IROMP/siderophore antibody in sera of group I mice and rabbits (PL-VacI) absent in sera of mice and rabbits given HS-VacII.
Conclusion
The bacteriophage based marker vaccine (PL-VacI) had a more effective and longer immune response against HS in mice and rabbit in comparison to the widely used alum precipitated HS vaccine (HS-VacII). Moreover, the development of a recombinant IROMP based indirect ELISA could serve as an excellent tool to differentiate between infected and vaccinated cattle and buffaloes for effective control of HS.
Keywords: Haemorrhagic septicaemia, P. multocida, Mice, Rabbits bacteriophage, IROMP(s), DIVA, Marker vaccine, Alum precipitated HS vaccine
1. Introduction
Haemorrhagic Septicaemia (HS) caused by Pasteurella multocida serotypes B:2 is an acute, fatal disease of cattle and buffaloes with high morbidity and mortality rates in cattle and buffaloes in India and other Asian countries. In India, losses of USD 130,000 due to haemorrhagic septicaemia have been reported between 2007 and 2011 (Singh et al., 2014). The annual losses to the North American cattle industry due to the disease have been estimated to the tune of U.S$ 800 million and $1.4 million in Laos (FAO, 1991), US$ 400–6000 in Indonesia, US$ l.0 million in Malaysia (Drummond et al., 1981), US$2.3 million in Bangladesh (Ahmed, 1996) and US$6 million in Srilanka (De Alwis and Vipulasiri, 1981).
Vaccination against haemorrhagic septicaemia is widely practiced in most of the countries experiencing the disease (Ahmad et al., 2014). The commercially used formalin-killed alum-precipitated vaccine and oil emulsion vaccines for HS control, suffer from poor potency, efficacy, safety and other problems (Rahman et al., 2016, Ray and Singh, 2013). Inspite, of use of multivalent vaccines and annual vaccinations in endemic areas outbreaks of disease are recorded every year (Gowrakkal et al., 2014).
In recent decade vaccines with improved immunity like intranasal live attenuated vaccine, P. multocida B:3,4 isolated from a fallow deer, attenuated live aroA gene mutant vaccine derived from a virulent P. multocida B:2 isolate (strain 85020) have been reported (Hodgson et al., 2005, Myint et al., 2005, Rafidah et al., 2011, Saleem et al., 2014). However, these vaccines are unable to differentiate infected from vaccinated animals owing to which HS control programme cannot be monitored effectively (Ray and Singh, 2013). For effective implementation of HS control program in India, a safe and efficacious vaccine to provide long lasting protection and at the same time allows differentiation of infected from vaccinated animals (DIVA) needs to be developed (Ray and Singh, 2015).
Mice models have been used as suitable tools to study HS particularly passive protection tests, defining protective antibody response in cattle and buffaloes, avian pasteurellosis and fowl cholera (Ramdani et al., 1990).
2. Materials and methods
2.1. Pasteurella multocida serotype B:2 strains
The standard vaccine strain Pasteurella multocida serotype B:2 (P52) from the Biological Standardization Division, IVRI, Izatnagar was used for bacteriophage isolation. Three P. multocida field isolates were recovered from nasopharyngeal, tracheal swabs and tissue samples of buffalo and cattle from field/veterinary clinics of GADVASU, Ludhiana exhibiting different pathological conditions. These isolates were characterized on the basis of cultural, morphological (Gram’s and bipolarity) and biochemical characteristics as described by Quinn et al. (1994). Multiplex-PCR of the isolates was carried out using PM-specific primers-(KMT1T7 & KMT1SP6) and HSB:2 specific primers (KTSP61 & KTT72) as described by Townsend et al. (1998). PCR was carried out in a final reaction volume of 25 μl (1X PCR buffer, 1.5 mM MgCl2, 200 μM each dNTP, 20 pmol of each primer, 1 U of Taq DNA polymerase and 5 µl of template DNA) at an initial denaturation of 95 °C-1 min, followed by denaturation, annealing and extension at 95 °C-1 min, 55 °C-1 min, 72 °C-1 min (30 cycles) and final extension of 72 °C-6 min. The primer sequence use in the study are given in Table1.
Table1.
Primer sequence used for detection of P. multocida.
| Primers | Sequence | Size of amplified product | Reference |
|---|---|---|---|
| KMT1T7 | 5′-GCTGTAAACGAACTCGCCAC-3′ | 460 bp | Townsend et al. (1998) |
| KMT1SP6 | 5′-ACTCGCTATTTACCCAGTGG-3′ | ||
| KTSP61 | 5′-ATCCGCTAA CACACTCTC-3′ | 590 bp | Townsend et al. (1998) |
| KTT72 | 5′-AGGCTCGTT TGGATTATGAAG-3′ | ||
2.2. Bacteriophage isolation and characterization
Pasteurella bacteriophage was isolated from samples of sewage and liquid manure of animal sheds of GADVASU, Ludhiana using double agar over lay procedure as described by Santos et al. (2009). The phage preparation was amplified to 200 ml master lot using the liquid culture method as described by Rawat and Verma (2007). To calculate the MOI of phage, 100 µl of a suitable dilution of the phage was added aseptically to four tubes having ∼109 viable cells/ml of log culture of P. multocida B:2, so that the phage-bacteria ratio of 1:104, 1:103, 1: 5 × 102, and 1:102 was achieved. No phage was added to the 5th tube which served as the control.
TEM microscopy of the Pasteurella phage was done using Morgagni 268D, Fei Electron Optics, Electron Microscope, (200KV, 29000× magnification) at Department of Anatomy, AIIMS, New Delhi. India. Whole genomic sequencing of Pasteurella bacteriophage was carried out using HiSeq 4000 Systems (DNF-488-33) at Georgia Genomics Facility, University of Georgia.
2.3. Preparation of marker vaccine
For marker vaccine preparation the viable count of a 14–16 h broth culture of P. multocida B:2 field isolate grown under iron restricted conditions (160 µM, 2,2 dipyridyl) in Brain Heart Infusion broth (BHI) was adjusted to ∼2 × 109 cfu/ml following incubation at 37 °C overnight at 120 rpm. An optimized antigenic biomass was generated by adjusting the opacity (OD) of the broth to 0.6 in a nanodrop spectrophotometer (0.6). The dry weight per 100 ml of the organisms was adjusted in the range of 50–60 mg. Based on the multiplicity of infection (MOI,1:100) Pasteurella phage was added in the culture followed by incubation for 7 h at 37 °C till complete lysis and clearance of turbidity (Rawat and Verma, 2007). The phage lysate was passed through a 0.1 µm filter (Pall Life Science) to separate out phage particles from the lysate and the filtrate was stored in sterilized vials at 4 °C. Sterile alum @ 10%(w/v) was added to the lysate as adjuvant. The protein concentration of the phage lysate was determined by nanodrop spectrophotometer and Lowry assay (1951).
2.4. Sterility, safety testing and challenge experiments in mice
The sterility and safety testing in mice of phage lysate marker vaccine was done as per the recommendations of Indian Pharmacopoeia (2010) and OIE (2000). A loopful of the lysate was suspended in 5 ml of BHI broth as well as streaked on BHI and blood agar plates followed by incubated at 37 °C. The broth and the plates were examined up to 48hours of incubation for any microbial growth. Swiss Albino mice (n = 15 each) were divided into two groups. Group I mice previously immunized with 0.1 ml of phage lysate (60 µg protein) vaccine were challenged with 100 mice MLD of P52 organisms 21 days post immunization. Group II control mice (n = 15) without any immunization were challenged directly 100 mice MLD of field strain of P. multocida B:2 were observed for 7 days and protection levels were determined for each group.
2.5. Experiment with the phage lysate marker vaccine (PL-HSvac) in mice and rabbits
Swiss Albino mice and Soviet Chinchilla rabbits used to study the antibody response to phage lysate HS vaccine (PL-VacI), were maintained in Small Animal House, Department of Microbiology, GADVASU, Ludhiana, India. Approval for small animal experimentation was sought from the Institutional Animal Ethics committee (IEAC) authorized by CPCSEA (committee for the purpose of control and supervision of experiments on animals). Approval for small animal experimentation was granted in XII meet of IAEC, GADVASU, dated: 2-03-2012. The alum precipitated HS vaccine was procured from Punjab Veterinary Vaccine Institute, Ludhiana.
2.6. Experimental design
Soviet Chinchilla rabbits (n = 14) weighing approx 2–3 kg of either sex were maintained under ideal conditions of feeding and management. The rabbits were divided into two groups (n = 7each) designated as group I (PL-VacI) and group II (HS-vacII). Group I rabbits were immunized with 0.8 ml (approx 480 µg protein) of phage lysate marker vaccine through subcutaneous route whereas group II received 0.8 ml of alum precipitated HS vaccine. Swiss albino mice (n = 14) on similar lines were divided into two groups. Group I mice (n = 7) were immunized with 0.1 ml (60 µg protein) of phage lysate marker vaccine through subcutaneous route whereas group II (n = 7) received 0.1 ml of alum precipitated HS vaccine. Assessment of health and body condition of the experimental animals was done prior to start as well as during entire experimentation period.
2.7. Serological assays
Serum samples were collected pre and post vaccination (0, 30, 60, 90, 120, 210 days) using vacutainer system (Greiner Bio-one, Austria). Sera were heat-inactivated at 56 °C for 30 min and stored at −20 °C until use. Microplate agglutination test (MAT), Indirect Haemagglutination Assay (IHA) and indirect ELISA were used for estimation of antibody titres in sera sample. MAT was done as per the protocol of Williams and Whitemore, 1971. The whole cell killed antigen for MAT was prepared from P. multocida (B:2) vaccine strain P52 at Punjab Veterinary Vaccine Institute, Ludhiana. IHA was done as per the method of Sawada et al. (1982). Monoclonal antibody ELISA kit developed by Department of Veterinary Microbiology, COVS, LUVAS, Hisar, India was used to estimate HS specific IgG antibodies in the sera. The plates were read on a micro-plate ELISA reader (Tecan) at 450 nm.
2.8. DIVA ELISA
2.8.1. Antigen preparation
IROMPs of P. multocida P52 grown under iron restricted conditions (160 µM, 2,2 dipyridyl) extracted as per method of Choi et al. (1991) were run on 12% SDS-PAGE as per the procedure of Laemmli (1970). A major IROMP (137 kDa) based on the stain intensity and band thickness was subjected to in-gel purification as per the protocol of Claudio et al. (1999). The band (137 kDa) was excised out with a sterile razor blade, washed (three times for 5 min) with 2 ml of 250 mM Tris buffer, 250 mM EDTA (pH 7.4), followed by three rinses (5 min each) in distilled water. Gel slices were homogenized in 1.0 ml of 20 mM Tris buffer, pH 7.4, containing 0.1% SDS (in ratio of 2:1) followed by sonication for 3 min (5–6 times for 30 s each), using soniprep sonicator (MSE). 1.5 ml of sample was applied to a desalting column (Genei). Prior to use the column was equilibrated with 20 mM Tris buffer, pH 7.4, containing 0.1% SDS. The protein concentration of the elute determined by nanodrop was 0.726 mg/ml. Aliquots were removed for protein determination using Nanodrop spectrophotometer and stored at −20 °C.
96-well Immunoplates (Maxisorp Nunc) were coated with 100 µl/well antigen (1 µg/ml) prepared above in 1:50 and 1:100 dilutions coating buffer incubated at 4 °C overnight. After proper washing the unbound sites were blocked using 5% skimmed milk in PBST@ 200 µl/ well and incubated at 37 °C for 2hours followed by washing 3 times with PBST. 100 µl (1:100) of test sera (30, 60, 90, 120 dpv sera from mice and rabbits) from marker as well as alum precipitated HS vaccine group of mice and rabbits were added to the wells except antibody control wells and incubated for 1 h at 37 °C. Rabbit anti mouse IgG HRP and Goat anti rabbit HRP (Sigma, 1:5000) were added to the respective wells excluding conjugate control wells and incubated for 1 h at 37 °C After a final wash 100 µl of freshly prepared substrate solution was added . The reaction was stopped and OD was read at 490 nm in an ELISA Reader (Tecan).
3. Results
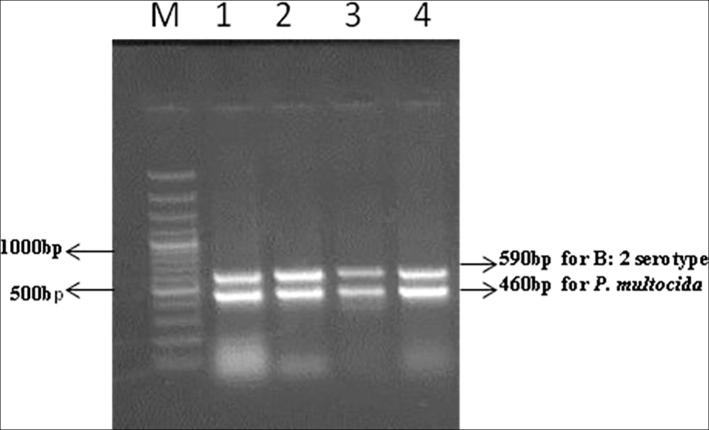
PCR of field isolates revealed amplified products of 460 and 590 bp corresponding to P. multocida and the serotype B: 2, respectively (Fig. 1). PMP-GAD-IND phage isolated in the present study was found to exhibit lytic activity against genus Pasteurella. It exhibited lytic activity against the vaccine strain P52 (B:2), fowl cholera agent (P. multocida type A:1) as well as multidrug resistant field isolates of P. multocida serotype (B:2) isolated in present study. The phage was inactive against Staphlylococcus aureus, Escherichia coli, Brucella, Salmonella Dublin, S. Enteritidis, S. Typhimurium and B. bronchiseptica isolates indicating its genus specificity.
Fig. 1.
Multiplex Pasteurella multocida B:2 PCR. Lane M: 100 bp DNA ladder. Lane 1: P. multocida P52. Lanes 2, 3, 4: Field isolates of P. multocida.
3.1. TEM of PMP-GAD-IND
The negative stain transmission electron microscopy of the phage revealed an isometric head and a well marked long non-contractile tail characteristic of the order Caudovirales, family Siphoviridae suggesting its placement to Group B. The icosahedral head of the phage measured approximately 27 × 24 nm (Fig. 2). The plaques were round, discrete, 3–5 mm in diameter and exhibited a clear zone of lysis (Fig. 3). The Multiplicity of infection (MOI) was 1:100 and PFU/ml of phage was 2.8 × 1010. The genome of the phage PMP-GADVASU-IND consists of 46,335 bp of dsDNA with a GC content of 55.39%. The genome contained 65 predicted genes and 52 of these predicted proteins are hypothetical. The complete genome of the Pasteurella multocida phage PMP-GAD-IND was deposited in GenBank under the accession number KY203335.
Fig. 2.
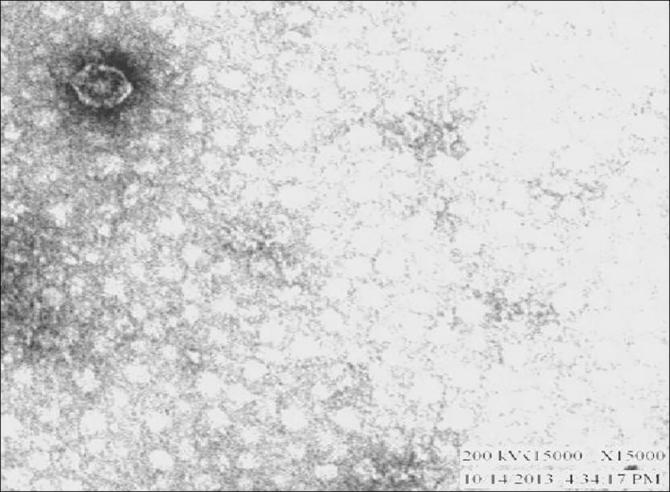
Characteristic hexagonal head of Pasteurella phage under TEM (100 nm).
Fig. 3.

Lytic plaques of lytic Pasteurella phage.
The phage lysate was found to be bacteriologically sterile and free from any fungal contamination. The lysate was found to be stable at 4 °C as well as room temperature. Mice and rabbits inoculated with the phage lysate for safety trials did not reveal any untoward reaction or death during an observation period of 7 days prior to immunization of the experimental animals.
3.2. Antibody response in mice and rabbit
Comparison of antibody response to PL-VacI and HS-VacII (alum precipitated HS vaccine) in mice and rabbits, revealed higher antibody titres in groupI mice and rabbits at all the days post vaccination by MAT, IHA and ELISA. However, antibody response revealed a significant difference between PL-VacI and HS-VacII rabbits groups. The comparison of antibody titres based on ‘t’ test revealed significantly higher titres in group I rabbits (PL-VacI) at 30 dpv by MAT and IHA (1.95; P < 0.05) and at 120 dpv (3.29; P < 0.05) by ELISA. Analysis of variance (ANOVA) revealed that the antibody titres at 30 dpv were significantly higher (P < 0.05) by MAT as well as by IHA than all other days within group I rabbits (PL-VacI). In group II rabbits the titres did not differ significantly (P < 0.05) between various days post vaccination by MAT, IHA and ELISA (Fig. 4).
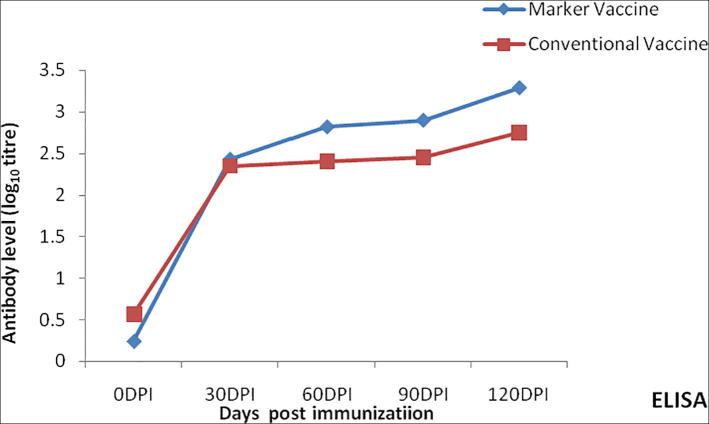
Fig. 4.
Antibody response to Phage lysate marker vaccine (PL.VacI) and alum precipitated vaccine (HS-VacII) vaccine in rabbits by ELISA.
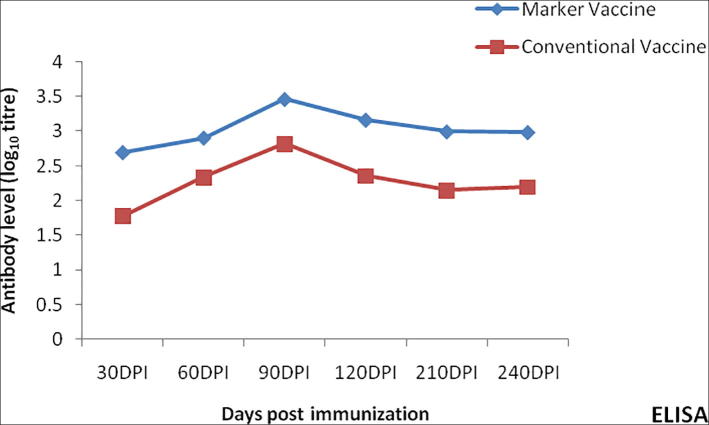
Within the group I mice the titres were significantly higher (P < 0.05) on 90 dpv than rest of the days by all the three techniques as revealed by ANOVA. Comparison between the titres of vaccine group I(PL-VacI) and II(HS-VacII) in mice by ‘t’ test revealed that antibody levels were significantly higher (P < 0.01) in group I mice at 30, 60, 90, 120, 210 and 240 dpv by all the three serological assays. The mean antibody titres in case of group I mice (PL-VacI) ranged from 2.59 to 1.73 during the period between 90 and 240DPI whereas the corresponding titres in group II mice were between 1.39 and 0.42 respectively, by MAT. The IHA titres during 90–240 dpv in group I mice ranged from 2.89 to 1.73 respectively, whereas the corresponding titres in group II mice ranged from 1.17 to 0.42, respectively. The peak (log10) value (3.46) in case of group I mice by ELISA was attained at 90DPI whereas in group II mice the peak value at 90DPI was 2.82 (Fig. 4). At 240 dpv group I mice had a titre of 2.77 while the group II mice a measurable titre was not noticed (see Fig. 5).
Fig. 5.
Antibody response to phage lysate marker vaccine (PL.VacI) and alum precipitated vaccine (HS-VacII) in mice by ELISA.
The antibody levels in the group I mice at 210 and 240 dpv, respectively were higher than those of mice in group II. The antibody titres showed a steep increase with a reduced lag phase and a sharp peak of high titre in rabbits. The antibody titres increased gradually achieving a peak at around 90DPI in marker vaccinated mice. An interesting observation in the study with mice was that titres were exhibited even at 240 dpv in group I mice.
In the present study the immunodominant 137 kDa IROMPs was used to develop a DIVA ELISA for the improved bacteriophage based marker vaccine against HS. Precoated ELISA plates (purified IROMP 137 kDa) were used to screen sera from group I(PL-VacI) and group II mice and rabbits(HS-VacII) for response against the IROMP. It was observed that sera from group I mice and rabbits revealed presence of anti-IROMP antibody indicated by significantly higher O.D. values for the sera in group I mice and rabbits at (P < 0.05) than the group II animals. The IROMP ELISA appears to be a promising and effective DIVA, applicable under field conditions. The ELISA detected anti-IROMP antibody in sera of marker vaccinated mice and rabbits even at 90 and 120 dpv. The mice in phage lysate marker vaccine group (PLvacI) group showed 100% protection on challenge whereas alum precipitated vaccine (HSVac-II) group showed had 10% protection, respectively. All the unvaccinated mice in control group during challenge study succumbed to infection within 24hours post challenge with P. multocida B:2. organism.
4. Discussions
PMP-GAD-IND (KY203335) bacteriophage isolated in present study exhibited lytic activity against multidrug resistant field isolates of P multocida B:2, vaccine strain as well as .P. multocida type A (fowl cholera). Bacteriophages being specific for their target bacterium create no negative effects on the surrounding tissues or the environment (Basdew and Laing, 2011, Golkar et al., 2014). Pasteurella bacteriophage morphology similar to that isolated in present study have been reported earlier (Ackermann and Karaivanov, 1984). Phage therapy and phage based vaccines have received lot of comeback attention due to an increase in the prevalence of antibiotic resistant strains. A numbers of experimental studies have proved the efficacy of phages in treating different infections. Chhibber et al. (2008) reported therapeutic potential of phage SS in treating Klebsiella pneumoniae induced respiratory infection in mice. Sekanikova et al. (1999) used Pseudomonas aeruginosa phage lysate as immunobiological agents and concluded that action of virulent phages on a virulent strain releases an antigen complex with immediate immunomodulating effects. Phage generated bacterial lysate and/or ghost preparation can be promising in veterinary medicine owing to its safety, effectiveness and relatively cost efficient. Phage lysates are simpler to prepare with an added advantage over ghost vaccines that require genetic engineering and optimal expression of e-gene containing constructs for bacterial lysis. The relative simplicity of preparation of phage lysates is advantageous when vaccine against multiple serotypes of a given species of bacteria have to be prepared. Bacteriophages represent a more economical and environment-friendly alternative to the environmentally-damaging use of chemical bacteriocides (Balogh et al., 2010).
Durairajan et al. (2012) reported that bacteriophage based phage lysate vaccines are more effective in providing a longer duration of immunity against haemorrhagic septicemia at a reduced antigenic mass (in comparison to killed vaccines) besides having cross protective immunity against fowl cholera as well.
In the present study variations in antibody response to phage lysate marker vaccine were observed between group I mice and rabbits administered phage lysate vaccine. However the antibody responses were persistent in both group I mice and rabbit . The encouraging results of mice models for disease study and development of improved vaccines against HS has lead to recommendation of small animal models to be used for improvement of HS vaccines by FAO (Ramdani et al., 1990) and Animal Production and Health Commission for Asia (APHCA, Bangkok, Thailand, 1987). Mice and rabbit are extremely susceptible to Pasteurella infection and die within 24–48 h following an infection (Chaudhuri et al., 2012).
Group I mice revealed persistence of substantial antibody response even at 240 dpv. Significant differences in antibody titres were observed in the group I and II mice and rabbits by MAT/IHA and ELISA. This can be reconciled with the fact that MAT/IHA being agglutination assays target particulate antigen whereas ELISA detects immune response to soluble antigens. In view of similarity of HS in cattle to the disease which results in mice infected with the P. multocida researchers have supported the usefulness of mice as a model for understanding HS. The correlation between immunity in cattle and the passive mouse protection test has been proposed by several workers to 'indicate a similarity in the mechanism of immunity' to HS occurring in mice, buffalo and cattle. Results of such studies have been sufficiently encouraging to warrant further evaluation and comparison of the disease of livestock with infections in mice.
Rabbits, as experimental model for HS have also been used by many workers (Bain et al., 1982, Yadev and Ahooja, 1983), Chaudhuri et al. (2012) proposed rabbit as a better laboratory animal model for studying immunogenic properties of an antigen against Pasteurella multocida, causing HS. Vaccine containing IROMPS of iron regulated protein of P. heamolytica A:2 have been reported to enhance protection against experimental pasteurellosis in lambs (Gilmour et al., 1991). Antibodies to IROMPs from sera of specific pathogen free lambs against whole cell antigens of P. haemolytica A2 grown under iron-restricted conditions have been reported in the immunoblots of animals immunized with sodium salicylate extract of iron regulated proteins. Study revealed that Mannheimia haemolytica A2 and A7 serotype combination expressing iron regulated outer membrane protein as a vaccine gave significant protection against homologous strains against intra-tracheal challenge exposure to Pasteurella in sheep (Tesfaw et al., 2014)
Due to difficulty in differentiating between infected and vaccinated animals in event of HS outbreaks under field conditions several workers have tried to develop DIVA assay against HS. Ray and Singh (2015) developed a DIVA strategy for HS using aluminium nanoparticles and keyhole limpet hemocyanin (KLH) with formalin inactivated P. multocida B:2 (P52 strain) in mouse model. Anti-KLH antibodies produced in sera of vaccinated animals were detectable by employing indirect-ELISA for a long time as anti-bacterial antibodies indicating the suitability of KLH inclusion for DIVA strategy in HS control programmes.
The fact that mice achieved higher and prolonged titres extending up to 240 dpv besides being protected with improved marker vaccine in the present study augurs well for its future usefulness in evaluating the new improved HS vaccines in bovines. DIVA ELISA assay could help to detect vaccination failures or a new infection.
The 137 kDa IROMP appears to be a promising antigen for development of a diagnostic DIVA assay in association with the novel marker vaccine. Marker vaccines and their companion DIVA tests have been developed for very few bacterial infections (Maas et al., 2006).
A recombinant IROMP based ELISA in conjunction with the new improved phage lysate marker vaccine developed in present study can help to differentiate between vaccination failure and infection in cattle thereby serving as effective tool monitoring the effectiveness of vaccination programmes run by different Government and non-Government agencies. However, results of the present study need to be validated phase II trial in cattle the principal host for HS which was initiated after the completion of small animal study.
5. Conclusion
The development of a field applicable DIVA ELISA is feasible as indicated with the results of present study in mice and rabbits. Such as DIVA in combination with the vaccine could go a long way towards better control and eradication of HS. However, recombinant IROMPs would be required for developing a commercial penside DIVA kit. HS phage lysate vaccine can also prove to be a better alternative to the currently available vaccines suffering from short term immunity or other drawbacks.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgement
The authors thank the funding agency University Grants Commission, India. We are thankful to Dr. Mayank Rawat, Division of Biological Standardization, IVRI, Izatnagar as well as Dr Arvind Kumar Department of Microbiology, LUVAS for help. The financial support from Dr David Donavani, USDA Agricultural Research Service (ARS), Baltimore, USA for sequencing PMP-GAD-IND bacteriophage and bioinformatic analysis by Dr Xianghe Yan, Environmental Microbial and Food Safety Laboratory, USDA related to PMP-GAD-IND phage genome is acknowledged.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ackermann H.W., Karaivanov L. Morphology of Pasteurella multocida bacteriophages. Can. J. Microbiol. 1984;30:1141–1148. doi: 10.1139/m84-179. [DOI] [PubMed] [Google Scholar]
- Ahmed S. Status of some bacterial diseases of animals in Bangladesh. Asian Livest. 1996;21:112–114. [Google Scholar]
- Ahmad T.A., Rammah S.S., Shewelta S.A., Haroun M., El-Sayed L.H. Development of immunization trials against Pasteurella multocida. Vaccine. 2014;32:909–917. doi: 10.1016/j.vaccine.2013.11.068. [DOI] [PubMed] [Google Scholar]
- Bain R.V.S., De Alwis M.C.L., Carter G.R., Carter G.R., Gupta B.K. vol. 33. Food and Agricultural Organisation; United Nations, Rome: 1982. pp. 11–33. (Haemorrhagic Septicaemia). [Google Scholar]
- Balogh B., Jones J.B., Iriarte F.B., Momol M.T. Phage therapy for plant disease control. Curr. Pharm. Biotechnol. 2010;11:48–57. doi: 10.2174/138920110790725302. [DOI] [PubMed] [Google Scholar]
- Basdew, I.H., Laing, M.D., 2011. Biological control of bovine mastitis using bacteriophage therapy, in: Mendez-Villas, A. (Ed.), Science against Microbial Pathogens: Communicating Current Research and Technological Advances. Scottsville 3209, Pietermaritzburg, Republic of South Africa, Formatex, pp. 386–393.
- Chaudhuri P., Singh V.P., Thamizharasan A., Lalsiamthara J. Pasteurella multocida P52 aroA mutant conferred protection to rabbits and mice against haemorrhagic septicaemia. DHR. Int. J. Biomed Life Sci. 2012;3:127–136. [Google Scholar]
- Chhibber S., Kaur S., Kumari S. Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J. Med. Microbiol. 2008;57:1508–1513. doi: 10.1099/jmm.0.2008/002873-0. [DOI] [PubMed] [Google Scholar]
- Choi K.H., Maheswaran S.K., Felice L.J., Molitor T.W. Relationship between the iron regulated outer membrane proteins and the outer membrane proteins of in vivo grown Pasteurella multocida and Molitor TW. Vet. Microbiol. 1991;28:75–92. doi: 10.1016/0378-1135(91)90100-t. [DOI] [PubMed] [Google Scholar]
- Claudio A.R., Thiebaut P., Alves E.W. Protein purification from polyacrylamide gels by sonication extraction. Anal. Biochem. 1999;268:15–20. doi: 10.1006/abio.1998.2977. [DOI] [PubMed] [Google Scholar]
- De Alwis M.C.L., Vipulasiri A.A. An epizootological study of haemorrhagic septicaemia in Srilanka. Ceylon Vet. J. 1981;28:24–35. [Google Scholar]
- Drummond R.O., Lambert G., Smallfy H.E., Trrilli C.E. Estimated losses of livestock in pets. In: Pimental D., editor. CRC Handbook of Pest Management in Agriculture. CRC Press; Boca Raton: 1981. pp. 111–127. [Google Scholar]
- Durairajan R., Verma H., Prajapati A., Abbas M., Rawat M. Application of bacteriophage lysate for treatment of fowl cholera in poultry. Indian J. Poult. Sci. 2012;47:260–261. [Google Scholar]
- FAO, 1991. Proceedings of the FAO/APHCA Workshop on Haemorrhagic Septicaemia. February 1991, Kandy, Sri Lanka.
- Gilmour N.J., Donachie W., Sutherland A.D., Gilmour J.S., Jones G.E., Quirie M. Vaccine containing iron-regulated proteins of Pasteurella haemolytica A:2 enhances protection against experimental pasteurellosis in lambs. Vaccine. 1991;9:137–140. doi: 10.1016/0264-410x(91)90271-7. [DOI] [PubMed] [Google Scholar]
- Golkar Z., Bagasra O., Gene Pace D. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J. Infect. Dev. Ctries. 2014;8:129–136. doi: 10.3855/jidc.3573. [DOI] [PubMed] [Google Scholar]
- Gowrakkal M., Chandrashekar M., Bhajantri S., Satav J., Chandakala G.C., Mayanna A. Evaluation of immuno efficacy of hemorrhagic septicemia vaccine (vaccine seed) Asian Pac. J. Trop. Biomed. 2014;4:S263–S267. doi: 10.12980/APJTB.4.2014C554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J.C., Finucane A., Dagleish M.P., Ataei S., Parton R., Coote J.G. Efficacy of vaccination of calves against hemorrhagic septicemia with a live aroA derivative of Pasteurella multocida B: 2 by two different routes of administration. Infect. Immun. 2005;73:1475–1481. doi: 10.1128/IAI.73.3.1475-1481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indian Pharmacopoeia Volume III. Indian Pharmacopoeia Commission, 2010. Government of India, Ministry of Health and Family Welfare, Ghaziabad.
- Laemmli U.K. Cleavage of the structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with folin-phenol reagent. J. Biol. Chem. 1951;193:265. [PubMed] [Google Scholar]
- Maas A., Meens J., Baltes N., Hennig-Pauka I., Gerlach G.F. Development of a DIVA subunit vaccine against Actinobacillus pleuropneumoniae infection. Vaccine. 2006;24:7226–7232. doi: 10.1016/j.vaccine.2006.06.047. [DOI] [PubMed] [Google Scholar]
- Myint A., Jones T.O., Nyunt H.H. Safety, efficacy and crossprotectivity of a live intranasal aerosol hemorrhagic septicemia vaccine. Vet. Rec. 2005;156:41–45. doi: 10.1136/vr.156.2.41. [DOI] [PubMed] [Google Scholar]
- OIE . Hamorrhagic septicaemia. fourth ed. OIE; Paris: 2000. (Manual of standards for Diagnostic Tests and Vaccines). [Google Scholar]
- Quinn P.J., Carter M.E., Markey B.K., Carter G.R. Wolfe Publication; London, U K: 1994. Clinical Veterinary Microbiology; pp. 254–258. [Google Scholar]
- Rafidah O., Zamri-Saad M., Nasip E., Saharee A.A. Herd immunity in buffaloes after intranasal live gdhA derivative P. multocida B:2 vaccine. Online J. Vet. Res. 2011;15:283–290. [Google Scholar]
- Rahman M.H., Shahiduzzamman A.N.M., Haque M.E., Nazir K.H.M.N.H., Rahman M.B. Development of experimental oil based inactivated HS vaccine from field Isolates of Pasteurella multocida from Cattle in Bangladesh. Int. J. Vaccines. 2016;2:27. [Google Scholar]
- Ramdani H.J.S., Dawkins R.B., Johnson T.L.S. Pasteurella multocida infections in mice with reference to haemorrhagic septicaemia in cattle and buffalo. Immunol. Cell Biol. 1990;68:57–61. doi: 10.1038/icb.1990.8. [DOI] [PubMed] [Google Scholar]
- Rawat, M., Verma, R., 2007. Isolation, cauterization, preservation and therapeutic use of bacteriophage against Staphylococcus aureus with ruminant mastitis. Progress Report (BT/PR4194/AAQ/158/2003), Department of Biotechnology, New Delhi.
- Ray, S.M., Singh, A., 2013. Improvement in bovine haemorrhagic septicaemia vaccine potency and efficacy by aluminium nanoparticles and keyhole limpet hemocyanin, and an approach to develop a DIVA strategy. Front. Immunol. Conference Abstract: 15th International Congress of Immunology (ICI).
- Ray S.M., Singh A. Aluminum oxide nanoparticle-adjuvanted Pasteurella multocida B:2 vaccine is potent and efficacious and is able with keyhole limpet hemocyanin as a marker to differentiate infected from vaccinated animals (DIVA) Res. Rev.: A J.l Immunol. 2015;5(2):8–16. [Google Scholar]
- Saleem L., Munir R., Ferrari G., Afral M., Chaudhry F.R. Efficacy and cross-protection of live intranasal aerosol hemorrhagic septicemia vaccine in buffalo calves. Int. J. Curr. Microb. Appl. Sci. 2014;3:300–307. [Google Scholar]
- Santos S.B., Carvalho C.M., Sillankorva S. The use of antibiotics to improve phage detection and enumeration by the double-layer agar technique. BMC Microbiol. 2009;9:148. doi: 10.1186/1471-2180-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada T., Rimler R.B., Rhoades K.R. Indirect haemagglutination test that uses glutaraldehyde fixed sheep erythrocytes sensitized with extract antigen for detection of Pasteurella antibody. J. Clin. Microbiol. 1982;15:752–756. doi: 10.1128/jcm.15.5.752-756.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekanikova G., Kolarova M., Pillich J., Seménka H., Slavíková D., Kubíčková V..Zajícová. Pseudomonas aeruginosa phage lysate as an immunobiological agent. Folia Microbiol. 1999;44:93–97. doi: 10.1007/BF02816229. [DOI] [PubMed] [Google Scholar]
- Singh B., Prasad S., Verma M.R., Sinha D.K. Estimation of economic losses due to hemorrhagic septicemia in cattle and buffaloes in India. Agric. Econ. Res. Rev. 2014;27:217–279. [Google Scholar]
- Tesfaw L., Jenberie S., Sori H., Sisay T., Negussie H. Efficacy of Mannheimia haemolytica A2, A7, and A2 and A7 combined expressing iron regulated outer membrane protein as a vaccine against intratracheal challenge exposure in sheep. Afr. J. Microbiol. Res. 2014;8:1237–1244. [Google Scholar]
- Townsend K.M., Frost A.J., Lee C.W., Papadimitriou J.M., Dawkins H.J.S. Development of PCR assays for species and type specific identification of Pasteurella multocida isolates. J. Clin. Microbiol. 1998;36:1096–1100. doi: 10.1128/jcm.36.4.1096-1100.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.E., Whitemore A.D. Serological diagnosis of pullorum disease with the microagglutination system. Appl. Microbiol. 1971;21:392–399. doi: 10.1128/am.21.3.394-399.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadev M.S., Ahooja M.L. Immunity trials in mice, rabbit and calves with oil adjuvant and multi emulsion oil adjuvant vaccines against haemorrhagic septicaemia. Indian J. Anim. Sci. 1983;53:705–708. [Google Scholar]