Abstract
The presented experiments evaluated the symbiotic performance of soybean genotypes with contrasting salt stress tolerance to arbuscular mycorrhizal fungi (AMF) inoculation. In addition, the physiological stress tolerance mechanisms in plants derived from mutualistic interactions between AMF and the host plants were evaluated. Plant growth, nodulation, nitrogenase activity and levels of endogenous growth hormones, such as indole acetic acid and indole butyric acid, of salt-tolerant and salt-sensitive soybean genotypes significantly decreased at 200 mM NaCl. The inoculation of soybean with AMF improved the symbiotic performance of both soybean genotypes by improving nodule formation, leghemoglobin content, nitrogenase activity and auxin synthesis. AMF colonization also protected soybean genotypes from salt-induced membrane damage and reduced the production of hydrogen peroxide, subsequently reducing the production of TBARS and reducing lipid peroxidation. In conclusion, the results of the present investigation indicate that AMF improve the symbiotic performance of soybean genotypes regardless of their salt stress tolerance ability by mitigating the negative effect of salt stress and stimulating endogenous level of auxins that contribute to an improved root system and nutrient acquisition under salt stress.
Keywords: Arbuscular mycorrhizal fungi, Glycine max, Auxins, Lipid peroxidation, Nodulation
1. Introduction
Plants in nature are confronted by a variety of environmental factors, resulting in adverse changes to growth and developmental patterns. Among several environmental factors, salinity is one of the most prevalent constraints hindering normal growth and metabolism, especially under arid conditions (Alqarawi et al., 2014). Increased salt levels in the soil solution or irrigation waters cause osmotic and ionic stress in plants, resulting in an adverse negative impact on plant growth by hampering several important physiological and biochemical processes (Abd_Allah et al., 2015a, Abd_Allah et al., 2015b, Hashem et al., 2015a). One of the most harmful impacts of high salinity is the disturbance of important cell structures, including membranes, proteins, and lipids, as well as nucleic acids. Membrane peroxidation due to salinity results in the loss of membrane integrity and causes the leakage of important cellular constituents, thereby causing problems in plant growth (Alqarawi et al., 2014). The rapid production of toxic radicals, such as superoxide, hydrogen peroxide, and peroxides, causes alterations in normal plant growth by triggering chain reactions and causing an excess production of peroxyl radicals. It has also been documented that the toxic effects of salt stress on plant growth and metabolism are also related to changes in the endogenous levels of phytohormones (Khan et al., 2014). Previous studies have shown that the inhibition of endogenous levels of phytohormones by salinity stress resulted in decreases root growth and development (Egamberdieva, 2009). Auxins, such as indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA), are the most important hormones involved in growth processes and root development (Epstein and Ludwig-Müller, 1993, Egamberdieva et al., 2015a, Egamberdieva et al., 2015b).
Arbuscular mycorrhizal fungi (AMF) usually form beneficial symbiosis with many plants to modulate the growth of host plants grown under biotic and abiotic conditions by direct and indirect mechanisms, such as the mitigation of reactive oxygen species (ROS) and oxidative damage via the mediation of phytohormone synthesis (Rabie and Almadini, 2005), enhancing the enzymatic and non-enzymatic antioxidant defense system (Wu et al., 2014, Ahmad et al., 2015), the induction of an acquired systemic tolerance (Hashem et al., 2016) and lipid peroxidation (Abd_Allah et al., 2015c). Moreover, AMF improve nutrient uptake, especially P (Tong et al., 2009, Meng et al., 2015) because the mycelium can grow and extend outside the rhizosphere, connecting roots with the surrounding soil microhabitats and enlarging the root area to absorb more nutrients. Thus, water and nutrients can be transported by the huge hyphae network to be absorbed by plants (Liu et al., 2016).
Mutualistic interactions among AMF and host plants were reported to increase plant growth and nutrient uptake of Medicago truncatula (de Varennes and Goss, 2007). The combined inoculation of legumes with AMF and rhizobia also showed improved salt stress tolerance, plant growth, nodulation and nitrogen fixation in soybean under salinity conditions (Elsheikh and Wood, 1995; Younesi et al., 2013). Despite the beneficial association of AMF, studies concerning the response of plant hormonal balance to the symbiotic interaction of AMF with plants in hostile environmental conditions are limited.
Soybean (Glycine max) is an important grain legume in many countries in the world that is sensitive to salinity stress, which reduces plant growth, nodulation, nitrogen fixation, number of pods, and yield (Egamberdieva et al., 2015a). In our study, an experiment was carried out to (i) elucidate the alteration of the auxin balance in soybean genotypes with contrasting salt stress tolerance after AMF colonization and (ii) to evaluate the mechanisms of physiological stress tolerance in plants by mutualistic interactions of AMF and soybean.
2. Materials and methods
Seeds of soybean (Glycine max L. Merrill), with genotypes Clark (salt tolerant) and Kint (salt sensitive), were obtained from the Agricultural Research Center, Giza, Egypt.
2.1. Seed germination test
Seeds were surface sterilized with sodium hypochlorite (0.5% v/v) for 5 min and washed with distilled water. Nine sterilized seeds were placed in a Petri dish (9 cm diameter) containing 10 ml of 1.5% (w/v) autoclaved water agar medium (Sigma-Aldrich Co., St. Louis, MO) supplemented with NaCl to get a concentration of 100, 200 and 300 mM. Plates with distilled water were used as the control. Petri plates were arranged in a randomized complete block design and incubated at 27 °C with 8 h light (40 mMol m−2 S−1) and 16 h dark photocycle at a relative humidity of 70–75%. A Parafilm strip was wrapped around the edge of each Petri dish to prevent evaporation. Germination percentage and rate were recorded daily for 10 days. A seed was considered germinated when its emerged shoot was visible under twofold magnification (McCarty and Dudeck, 1993), and the rate of germination (% per day) was calculated according to an index of germination velocity (Timson, 1965).
2.2. Soil and microorganisms
The experimental soil used in the current study was a sandy soil collected from the Derab Agricultural Research Station, Riyadh, Saudi Arabia, with the following properties (%): sand, 73.6; moisture content, 4.15; organic carbon, 0.14; and total nitrogen 0.006. The electrical conductivity (EC) was 7.12 dS m−1 and the soil pH was 7.8. The soil was autoclaved for 3 h at 121 °C, cooled and then divided among plastic pots (1 kg capacity). The mycorrhizal fungi Funneliformis mosseae (syn. Glomus mosseae), Rhizophagus intraradices (syn. Glomus intraradices) and Claroideoglomus etunicatum (syn. Glomus etunicatum) were previously isolated from a salt marsh habitat in Alserw, Dakahlia, Egypt (Alqarawi et al., 2014). The mycorrhizal inoculum was prepared as described by Hashem et al. (2014b). The mycorrhizal inoculum was added to the experimental soil as 10 g of trap soil culture (approx. 100 spores/g trap soil, M = 80%) per pot (1 kg). Control pots were kept without mycorrhizal inoculum. The soybean seeds were coated with rhizobial inoculants at a rate of 2 g of inoculant/200 g seeds and sown into the pots filled with soil.
2.3. Plant growth condition
Plants were grown for 60 days under greenhouse conditions with an 11 h light (750 μmol m−2 S−1) and 6 h dark photocycle at 27 °C. Pots were arranged in randomized complete block design with five replications. The relative air humidity was adjusted to 70%. After plant emergence, plants were watered as needed and salinity conditions were established by adding NaCl (200 mM) to the irrigation water. At harvest, plant shoots were separated from the roots, washed with distilled water and used for further analysis.
2.4. Nodulation and nitrogenase activity
The number of nodules was recorded using a Leica MZF LIII stereomicroscope. The fresh weight of the nodules was taken instantly after harvesting, whereas the dry weight was determined after oven drying (70 °C) for two successive weights. To estimate leghemoglobin, nodules (2.0 g fresh weight) were macerated in liquid N2 and then 50 mM of KPO4 (pH 7.4) buffer containing 1 mM EDTA was added. The mixture was stirred until it thawed to a homogenate at temperature 2 °C and was subsequently centrifuged at 10,000g for 10 min at 4 °C. The supernatant was collected and mixed with 50 mM of KPO4 (pH 7.4) buffer containing 1 mM EDTA, and the color intensity was recorded spectrophotometrically at 710 nm (Keilin and Wang, 1945). Nitrogenase activity was extracted from fresh nodules and measured using the method described by Bergersen and Turner (1967). The concentration of NH4+ was measured calorimetrically according to the methods of Bergersen (1980). In this method, one milliliter of 5% phenol containing 25 mg sodium nitroprusside was added to a known volume of ammonia produced by nitrogenase and followed by 2% (v/v) sodium hypochlorite dissolved in 0.13 N sodium hydroxide. The mixture was incubated at 37 °C for 15 min and then diluted to a known volume, and the optical density was recorded at 630 nm. A standard curve of ammonium chloride (10–70 μg/ml) was used as a reference.
2.5. Determination of arbuscular mycorrhizal colonization
A wet sieving and decanting procedure was employed for the determination of mycorrhizal spores (Daniels and Skipper, 1982, Utobo et al., 2011). Plant roots were washed carefully in ice-cold water (4 °C) to remove the soil and were subsequently cleaned with KOH (10%) and followed by staining with trypan blue in lactophenol as described by Phillips and Hayman (1970). Stained root segments were then examined under light microscope at a 400× magnification. Fungal infection (mycelium, vesicles and arbuscules) and development within the infected soybean roots were calculated according to the following formula:
2.6. Extraction and quantification of plant growth regulators
The endogenous levels of growth regulators were determined by extracting plant tissue in 80% aqueous acetone (4:1, v/v) containing butylated hydroxytoluene (10 mg/l) and were purified using EtOAc and NaHCO3, as described by Kusaba et al. (1998). For quantifying indole acetic acid (IAA) and indole butyric acid (IBA), the purified extract residue was subjected to HPLC using a PEGASIL ODS column (6 mm i.d. × 150 mm, Senshu Kagaku, Tokyo, Japan), according to the method of Kelen et al. (2004). The quantifications were done from the standard curves of IBA ranging from 10 up to 200 ng/ml.
2.7. Plant physiological parameters
2.7.1. Determination of photosynthetic pigments
To estimate photosynthetic pigments (chlorophyll a, chlorophyll b, chlorophyll a + b, carotenoids, and total pigments), leaves were extracted in dimethyl sulfoxide (DMSO) as described by Hiscox and Israelstam (1979). The absorbance of the homogenate was determined spectrophotometrically at 480, 510, 645, 663 nm (T80 UV/VIS Spectrometer, PG Instruments Ltd., USA). DMSO was used as a blank.
2.7.2. Determination of thiobarbituric acid reactive substances (TBARS)
Thiobarbituric acid reactive substances (TBARS) were estimated using the method of Dhindsa et al. (1981). Fresh leaf tissue (200 mg) was macerated in 0.25% 2-thiobarbituric acid (TBA) in 10% trichloroacetic acid (TCA) using a mortar and pestle. After heating at 95 °C for 30 min, the mixture was quickly cooled in an ice bath and centrifuged at 10,000g for 10 min. The absorbance of the supernatant was read at 532 nm and corrected for non-specific turbidity by subtracting the absorbance at 600 nm. The blank was 0.25% TBA in 10% TCA. An extinction coefficient of 155 mM−1 cm−1 was used for calculating TBARS.
2.7.3. Determination of hydrogen peroxide (H2O2)
The method described by Okuda et al. (1991) was used to measure hydrogen peroxide (H2O2) content. Leaf tissue was macerated in ice cold perchloric acid and was centrifuged at 1200g for 10 min, and the supernatant was neutralized with 4 M KOH. The insoluble potassium perchlorate was eliminated by centrifugation at 500g for 3 min. The reaction was started with the addition of peroxidase, and the increase in the absorbance was recorded at 590 nm for 3 min.
2.7.4. Determination of malondialdehyde (MDA)
Fresh leaves were extracted in 1% TCA and the homogenate was centrifuged at 10,000 rpm for 5 min. Next, 1.0 ml of supernatant was taken and 4.0 ml of 0.5% (w/v) thiobarbituric acid (TBA) was added and heated at 95 °C for 30 min. Subsequently, samples were cooled on ice and centrifuged at 5000 rpm for 5 min for clarification. The supernatants were read at 532 and 600 nm (Heath and Packer, 1968). The calculation of MDA was done using the following equation:
2.8. Statistical analysis
The experimental design was a completely randomized design. The data were subjected to Duncan’s Multiple Range Tests and one-way ANOVA using SPSS-21 software. Differences in means were determined by the least significant difference (LSD) (p = 0.05) test.
3. Results
3.1. Effect of salinity on seed germination
The effects of salinity on germination of Glycine max genotypes are shown in Table 1. The results indicated that the soybean genotypes differed in their response to different salinity levels. The reduction in germination of the seeds, compared to the control, was 1.02%, 4.1%, 10.9% for the salt-tolerant variety Clark and 6.8%, 11.1%, 19.47% for the salt-sensitive variety Kint at 100 mM, 200 mM and 300 mM NaCl, respectively (Table 1). The germination rate decreased with increasing salinity levels for Clark by 6.8% and Kint by 1.4%.
Table 1.
Effects of NaCl concentrations on seed germination of two soybean genotypes with different salt stress tolerance. Values denoted by different letters are significantly different at P < 0.05.
| Salt concentration (mM NaCl) | Clark (salt-tolerant) |
Kint (salt-sensitive) |
||
|---|---|---|---|---|
| Final germination (%) | Rate of germination/day | Final germination (%) | Rate of germination/day | |
| Control | 97.2 a ± 1.92 | 47.8 a ± 1.81 | 98.6 a ± 2.14 | 36.2 b ± 1.64 |
| 100 mM NaCl | 96.2 a ± 1.30 | 34.4 b ± 1.58 | 91.8 b ± 1.87 | 26.4 c ± 1.67 |
| 200 mM NaCl | 93.2 b ± 0.83 | 23.0 c ± 1.42 | 87.6 c ± 1.04 | 16.4 d ± 1.34 |
| 300 mM NaCl | 68.6 e ± 2.40 | 6.81 e ± 0.37 | 79.4 d ± 0.89 | 1.47 f ± 0.54 |
| LSD at: 0.05 | 0.78 | 1.41 | 1.68 | 1.00 |
3.2. Nodulation, leghemoglobin content and nitrogenase activity
The results given in Table 1 show that the number and fresh and dry weight of nodules of both soybean genotypes with contrasting salt stress tolerance significantly decreased at 200 mM NaCl. The number and fresh and dry weight of nodules were reduced by 45.2%, 37.8%, and 34.5% in the salt-tolerant Clark variety and by 69.1%, 58.8%, and 43.8% in the salt-sensitive Kint variety, respectively (Table 2). The inoculation of the soybean genotypes with AMF significantly (p < 0.5) increased the number and fresh and dry weight of nodules by 33.2%, 90.6%, and 43.6% for the salt-tolerant Clark genotype and by 39.1%, 78.9%, and 47.9% for the salt-sensitive Kint genotype, respectively. An increased content of leghemoglobin (72.9–73.5%) in the nodules and nitrogenase activity (45.9–54.9%) was recorded in the Clark and Kint genotypes, respectively. In the salt-tolerant Clark genotype, AMF treatment enhanced the number, fresh and dry weight of nodules, and leghemoglobin content by 33.2%, 90.6%, 43.6%, and 54.4%, respectively, while in the Kint genotype, the percent increase was 39.1%, 78.9%, 47.9%, and 53.1%, respectively. The nitrogenase activity was higher (48.2%) in the salt-sensitive genotype Kint inoculated with AMF compared to the salt-tolerant genotype Clark (65.9%) (Table 2).
Table 2.
Nodule number, nodule fresh and dry weight, leghemoglobin content and nitrogenase activity of soybean genotypes with contrasting salt stress tolerance inoculated with AMF under salt stress. Values denoted by different letters are significantly different at P < 0.05.
| Treatments | Clark (salt-tolerant) |
Kint (salt-sensitive) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NN | NFW | NDW | LG | NA | NN | NFW | NDW | LG | NA | ||
| 0 mM NaCl | Control | 50.5 d ± 2.04 | 202.2 d ± 10.3 | 29.8 c ± 1.76 | 13.4 b ± 0.71 | 0.87 b ± 0.04 | 58.0 c ± 1.75 | 237.8 c ± 7.35 | 24.4 d ± 1.27 | 7.0 d ± 0.24 | 0.47 c ± 0.06 |
| AMF | 67.4 ± 2.06 b | 385.1 ± 14.6 b | 42.8 a ± 1.56 | 20.7 a ± 0.69 | 1.29 a ± 0.14 | 80.7 a ± 2.73 | 424.0 a ± 11.63 | 36.1 b ± 2.68 | 10.8 c ± 0.92 | 0.78 b ± 0.07 | |
| 200 mM NaCl | Control | 27.4 e ± 1.43 | 125.6 f ± 6.84 | 19.5 e ± 1.07 | 3.6 f ± 0.15 | 0.47 c ± 0.06 | 17.9 f ± 0.96 | 97.6 g ± 2.04 | 13.7 f ± 1.22 | 1.8 g ± 0.23 | 0.21 d ± 0.01 |
| AMF | 41.6 d ± 1.87 | 209.6 d ± 4.69 | 29.5 c ± 1.28 | 11.4 c ± 0.47 | 0.72 b ± 0.05 | 27.7 e ± 1.25 | 188.2 e ± 6.41 | 24.8 d ± 1.78 | 6.9 e ± 0.58 | 0.42 c ± 0.07 | |
| LSD at: 0.05 | 1.92 | 7.28 | 1.09 | 0.78 | 0.04 | 1.97 | 7.27 | 1.09 | 0.78 | 0.03 | |
NN: nodule number; NFW: nodule fresh weight (g); NDW: nodule dry weight (g); LG: leghemoglobin (mg/g nodule fresh weight); NA: nitrogenase activity (mg ammonia/g nodule fresh weight/h).
3.3. AMF colonization
Salinity reduced the AMF colonization efficiency in the soybean genotypes. In the Clark genotype, mycelia, vesicles and arbuscules were reduced by salt stress by 23.7%, 44.8% and 40.6%, respectively (Table 3). In contrast, the Kint genotype showed a 69.8%, 70.2% and 84.5% reduction in mycelia, vesicles and arbuscules, respectively (Table 3). Under the 200 mM NaCl stress treatment, AMF colonization was higher in the salt-tolerant compared to the salt-sensitive genotypes.
Table 3.
Mycorrhizal colonization (%) of soybean genotypes with contrasting salt stress tolerance under salt stress. Values denoted by different letters are significantly different at P < 0.05.
| Treatments | Clark (salt-tolerant) |
Kint (salt-sensitive) |
||||
|---|---|---|---|---|---|---|
| Mycelia | Vesicles | Arbuscules | Mycelia | Vesicles | Arbuscules | |
| 0 mM NaCl | 72.1 a ± 3.74 | 43.7 a ± 2.01 | 30.7 a ± 2.63 | 49.9 b ± 3.74 | 8.1 c ± 0.37 | 7.0 c ± 0.24 |
| 200 mM NaCl | 55.0 b ± 2.7 | 24.1 b ± 0.81 | 18.2 b ± 1.21 | 15.0 c ± 1.73 | 5.4 d ± 0.14 | 1.1 d ± 0.04 |
| LSD at: 0.05 | 12.4 | 9.3 | 8.0 | 17.6 | 8.5 | 4.3 |
3.4. The correlation between AMF and nodulation under salt stress
The results in Table 4 showed that salinity had a strong negative correlation for genotype Clark with nodule number (−0.9907), nodule fresh weight (−0.9961), nodule dry weight (−0.9752), leghemoglobin (−0.9961), nitrogen activity (−0.9875) and mycelial growth (−0.9118). However, arbuscules showed a strong positive correlation with salinity (0.99133), and vesicles activity was improved due to the increase in salt stress conditions and showed a strong correlation at the value (0.99915). The soybean genotype Clark showed resistance against salinity and withstood the harsh conditions and grew well. The vesicles and arbuscules activity improved with the increasing trend of salinity. Moreover, the results showed that salinity had a significant correlation with vesicles and arbuscules. The number of nodules had a strong significant effect on NFW (0.987), NDW (0.987), LG (0.95), NA (0.9896) and M (0.9515), while strongly negatively correlated with V and A. The arbuscules activity in soybean genotypes (salt tolerant) showed a negative correlation for NN, NFW, NDW, LG, NA, M, while a positive association was recorded for vesicles. The nodulation activity was decreased with the increase of salinity for soybean genotype in the experiment. The results showed a negative correlation for the number of nodules, nodule fresh weight and nodule dry weight. Nitrogenase activity was also affected by the increase of salinity during the experiment. The vesicles showed a varied trend (increasing or decreasing) during the experiment, and leghemoglobin showed a medium (0.8384) correlation with mycelia.
Table 4.
Correlations (r) between salt, mycorrhiza, and bacteria with number of nodules, nodule fresh weight, leghemoglobin nodule fresh weight, nitrate reductase, nitrite reductase, nitrogenase, total nitrogen content, and crude protein in soybean genotype Clark (salt-tolerant).
| Salinity | NN | NFW | NDW | LG | NA | M | V | A | |
|---|---|---|---|---|---|---|---|---|---|
| Salinity | 1.00000 | −0.9907 | −0.9961 | −0.9752 | −0.9600 | −0.9875 | −0.9118 | 0.99915 | 0.99133 |
| NN | 1.00000 | 0.98798 | 0.98701 | 0.95003 | 0.98960 | 0.95151 | −0.9883 | −0.9830 | |
| NFW | 1.00000 | 0.98097 | 0.97653 | 0.99604 | 0.90178 | −0.9958 | −0.9831 | ||
| NDW | 1.00000 | 0.93801 | 0.98765 | 0.93515 | −0.9696 | −0.9552 | |||
| LG | 1.00000 | 0.97944 | 0.83847 | −0.9640 | −0.9445 | ||||
| NA | 1.00000 | 0.91567 | 0.98689 | −0.9732 | |||||
| M | 1.00000 | −0.9104 | −0.9294 | ||||||
| V | 1.00000 | 0.99455 | |||||||
| A | 1.00000 |
NN: nodule number; NFW: nodule fresh weight (g); NDW: nodule dry weight (g); LG: leghemoglobin (mg/g nodule fresh weight); NA: nitrogenase activity (mg ammonia/g nodule fresh weight/h); M: mycelia; V: vesicles; A: arbuscules.
The correlation between salt, mycorrhiza and bacteria for the soybean Kint genotype is presented in Table 5. The results showed that salinity had a strong negative correlation for nodule number (−0.9989), nodule fresh weight (−0.9986), nodule dry weight (−0.9938), leghemoglobin (−0.9874), nitrogenase activity (−0.9882), mycelia growth (−0.992), vesicles (−0.9980) and arbuscules (−0.9983). If we compare Kint with Clark, the vesicles and arbuscules were positive and a significant correlation against salinity was recorded during the experiment. On the other hand, Kint (salt sensitive) behaved differently compared to the Clark genotype; arbuscules were shown, and there was a significant effect with nodule number, nodule fresh weight, nodule dry weight, leghemoglobin, nitrogenase activity, and mycelia. Vesicle activity improved. On the other hand, Clark showed a negative correlation with these characters. Kint behaved as a salt-sensitive genotype and showed a strong positive correlation with nodule number (0.99401), nodule fresh weight (0.99462), nodule dry weight (0.99694), leghemoglobin (0.98005), nitrogenase activity (0.99205) and mycelial growth with vesicles. However, a negative correlation was recorded for the Clark genotype, which showed salt tolerance for these characters.
Table 5.
Correlations (r) between salt, mycorrhiza, and bacteria with number of nodules, nodule fresh weight, leghemoglobin nodule fresh weight, nitrate reductase, nitrite reductase, nitrogenase, total nitrogen content, and crude protein in soybean genotype Kint (salt-sensitive).
| Salinity | NN | NFW | NDW | LG | NA | M | V | A | |
|---|---|---|---|---|---|---|---|---|---|
| Salinity | 1.00000 | −0.9984 | −0.9986 | −0.9938 | −0.9874 | −0.9882 | −0.9992 | −0.9980 | −0.9983 |
| NN | 1.00000 | 0.99967 | 0.98917 | 0.99370 | 0.98489 | 0.99610 | 0.99401 | 0.99887 | |
| NFW | 1.00000 | 0.98875 | 0.99237 | 0.98777 | 0.99623 | 0.99462 | 0.99966 | ||
| NDW | 1.00000 | 0.97120 | 0.98893 | 0.99552 | 0.99694 | 0.98773 | |||
| LG | 1.00000 | 0.96780 | 0.98106 | 0.98005 | 0.98975 | ||||
| NA | 1.00000 | 0.98743 | 0.99205 | 0.98823 | |||||
| M | 1.00000 | 0.99797 | 0.99650 | ||||||
| V | 1.00000 | 0.99420 | |||||||
| A | 1.00000 |
NN: nodule number; NFW: nodule fresh weight (g); NDW: nodule dry weight (g); LG: leghemoglobin (mg/g nodule fresh weight); NA: nitrogenase activity (mg ammonia/g nodule fresh weight/h); M: mycelia; V: vesicles; A: arbuscules.
3.5. Indole acetic acid (IAA) and indole butyric acid (IBA) levels
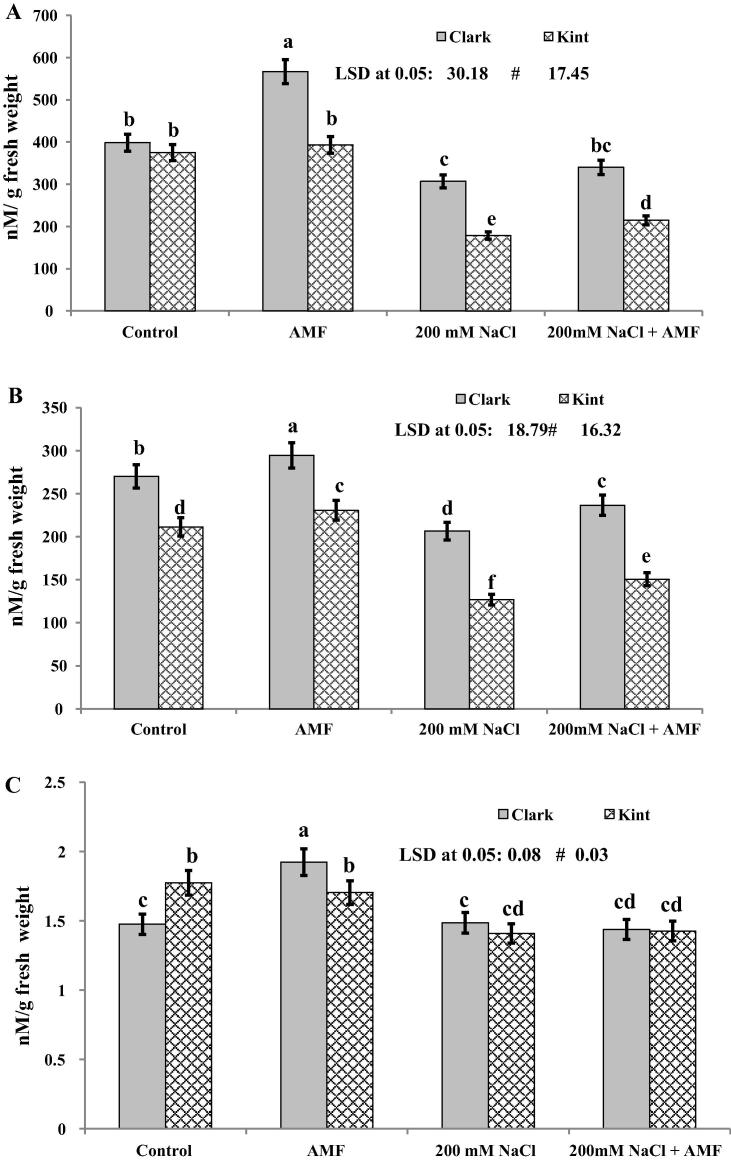
Salinity reduced the endogenous levels of the hormones studied in both genotypes; however, AMF inoculation not only enhanced levels but also alleviated the negative impact of salinity to some extent (Fig. 1a and b). AMF treatment caused an increase of 42.2% and 8.8% in indole acetic acid (IAA) and indole butyric acid (IBA) levels in the Clark genotype, respectively, while in the Kint genotype, the percent increase was 4.8% and 9.0% under non-saline conditions, respectively (Fig.1a and b). Relative to the control, the percent decrease in IAA and IBA levels in the Clark genotype was 22.8% and 23.4% and was 52.5% and 40.2% in the Kint genotype, respectively (Fig.1a and b). However, in plants grown under salt stress and inoculated with AMF, the level of IAA and IBA was reduced by 14.5% and 12.5% in Clark and by 42.33% and 28.9% in the Kint genotype (Fig.1a and b), respectively, compared to the control. The ratio of IAA and IBA was also altered due to salinity (Fig.1c).
Fig. 1.
(A–C) Endogenous levels of (A) indole acetic acid, IAA, (B) indole butyric acid, IBA, and (C) the IAA/IBA ratio in soybean (Glycine max L. Merr) genotypes with different salt stress tolerance inoculated with AMF under salt stress. Columns represent the means for five plants (N = 5) with error bars showing standard deviation. Values denoted by different letters are significantly different at P < 0.05.
3.6. Chlorophyll pigments
The inoculation of non-stressed soybean genotypes with AMF resulted in increased chlorophyll synthesis (Table 6). The percent increase in chlorophyll a, chlorophyll b, total chlorophylls and carotenoids was 39.1%, 42.2%, 45.92% and 90.89% for the Clark genotype and 9.55%, 23.7%, 14.1% and 13.05% for the Kint genotype, respectively, compared to the control plants (Table 6). Under salt stress conditions, soybean exhibited considerable reductions in chlorophyll a, chlorophyll b, total chlorophylls and carotenoids by 32.7%, 34.7%, 35.2% and 49.5% for Clark and 71.5%, 76.5%, 74.1% and 81.3% for Kint, respectively (Table 6). However, AMF treatment showed only a slight decrease of photosynthetic pigments by 12.5%, 11.6%, 12.9%, 17.9% and 48.7%, 55.07%, 53.2%, 69.9% in the Clark and Kint genotypes, respectively (Table 6).
Table 6.
Chlorophyll a, chlorophyll b, chlorophyll a + b, carotenoids and total pigments of soybean genotypes with contrasting salt stress tolerance inoculated with AMF under salt stress. Values denoted by different letters are significantly different at P < 0.05.
| Treatments | Clark (salt-tolerant) |
Kint (salt-sensitive) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chl. a | Chl. b | a + b | a/b | Carot. | Total | Chl. a | Chl. b | a + b | a/b | Carot. | Total | ||
| 0 mM NaCl | Control | 1.04 c ± 0.08 | 0.44 c ± 0.03 | 1.48 ± 0.12 | 2.34 ± 0.17 | 0.19 d ± 0.02 | 1.67 c ± 0.15 | 1.24 b ± 0.08 | 0.63 b ± 0.08 | 1.88 ± 0.16 | 1.96 ± 0.17 | 0.26 c ± 0.07 | 2.15 b ± 0.18 |
| AMF | 1.44 a ± 0.13 | 0.63 b ± 0.07 | 2.08 ± 0.16 | 2.28 ± 0.16 | 0.36 a ± 0.04 | 2.45 a ± 0.17 | 1.36 a ± 0.09 | 0.78 a ± 0.05 | 2.15 ± 0.18 | 1.73 ± 0.14 | 0.30 ab ± 0.02 | 2.45 a ± 0.19 | |
| 200 mM NaCl | Control | 0.69 e ± 0.04 | 0.29 d ± 0.02 | 0.98 ± 0.05 | 2.40 ± 0.18 | 0.09 f ± 0.01 | 1.08 f ± 0.12 | 0.35 f ± 0.03 | 0.14 e ± 0.02 | 0.50 ± 0.07 | 2.44 ± 0.18 | 0.05 g ± 0.02 | 0.55 g ± 0.03 |
| AMF | 0.91 d ± 0.05 | 0.39 c ± 0.03 | 1.30 ± 0.07 | 2.31 ± 0.15 | 0.15 e ± 0.02 | 1.46 cd ± 0.13 | 0.63 e ± 0.07 | 0.28 d ± 0.01 | 0.92 ± 0.04 | 2.23 ± 0.14 | 0.08 f ± 0.03 | 1.00 e ± 0.05 | |
| LSD at: 0.05 | 0.21 | 0.07 | 0.08 | 0.02 | 0.03 | 0.18 | 0.07 | 0.06 | 0.31 | 0.09 | 0.02 | 0.29 | |
Chl. a: chlorophyll a; Chl. b: chlorophyll b; Carot: carotenoids (mg/g fresh weight).
3.7. Thiobarbituric acid reactive substances (TBARS), hydrogen peroxide (H2O2) and lipid peroxidation
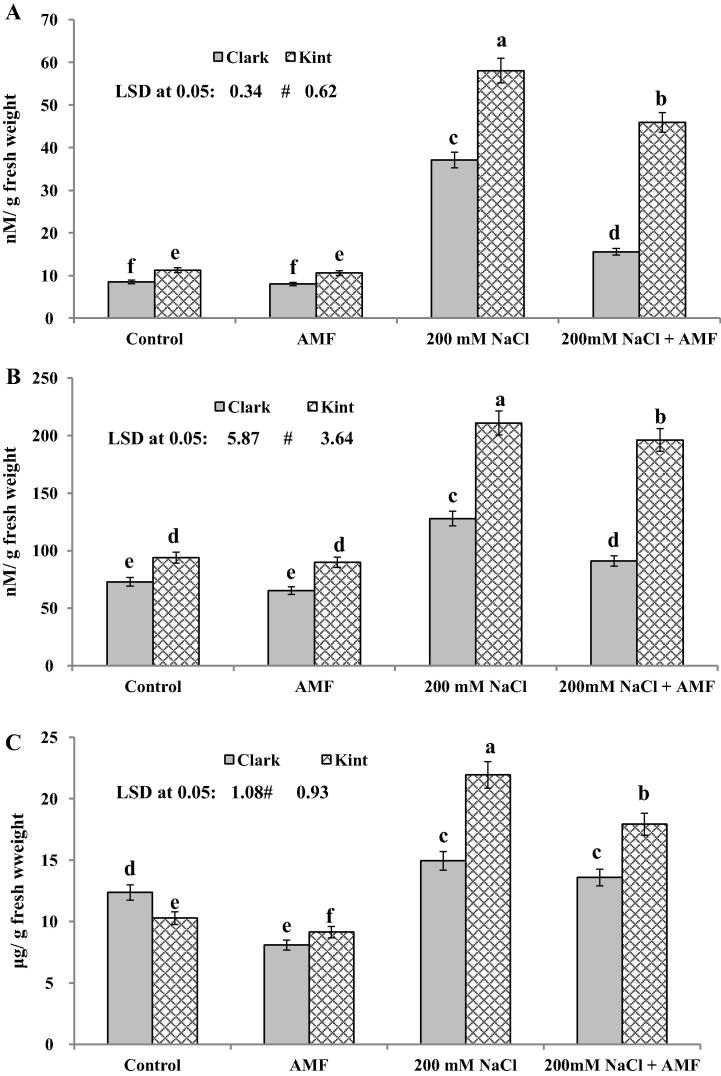
The effects of AMF on the production of TBARS, H2O2 and lipid peroxidation were measured in terms of MDA content and are presented in Fig.2a–c. The production of TBRAS, H2O2 and MDA in both genotypes inoculated with AMF was reduced in comparison with the control plants. AMF induced a reduction in the TBRAS, H2O2 and MDA contents by 5.9%, 10.5%, 34.5% in Clark and 6.25%, 4.3% and 11.2% in Kint, respectively (Fig.2a–c). Salinity stress enhanced TBRAS, H2O2 and MDA production by 336.5%, 75.2% and 20.7% in Clark and by 418.4%, 124.6% and 113.3% in Kint, respectively (Fig.2a–c). However, the increase in TBRAS, H2O2 and MDA in AMF-inoculated, salt-stressed seedlings was lower in comparison with the control plants (Fig.2a–c).
Fig. 2.
(A–C) Thiobarbituric acid reactive substances (TBARS) (A), H2O2 (B) and malondialdehyde (MDA) (C) in soybean (Glycine max L. Merr) genotypes with different salt stress tolerance inoculated with AMF under salt stress. Columns represent the means for five plants (N = 5) with error bars showing standard deviation. Values denoted by different letters are significantly different at P < 0.05.
4. Discussion
Previous studies have already shown that germination and seedling growth of leguminous plants were reduced in saline soils, with varying responses due to different cultivars (Egamberdieva et al., 2013). Genotypic variation in leguminous crops for traits affecting nodulation and N2 fixation was also found (Montealegre et al., 1995). We have also observed a negative effect of salinity (200 mM NaCl) on the germination of two soybean genotypes with contrasting salt stress tolerance; however, the germination of the salt-tolerant genotype was less affected by salt stress. Abiotic stresses, such as salinity and drought, decrease rhizobial colonization, inhibit the infection processes and nodule development, and reduce N2 fixation and nitrogenase activity in legumes (Severin et al., 2012, Abd_Allah et al., 2015a, Egamberdieva et al., 2013, Egamberdieva et al., 2015a). In our study, salt stress inhibited nodulation and nitrogenase activity of inoculated soybean. However, AMF improved the symbiotic performance of soybean and increased nitrogenase activity. Our results corroborate the findings of Abd_Allah et al. (2015a) for Sesbania sesban and Hashem et al. (2015a) for Panicum turgidum. Furthermore, Zarea et al. (2011) demonstrated that AMF inoculation to Trifolium alexandrinum L. and Trifolium resupinatum L. resulted in enhanced growth associated with increased nitrogenase activity, as well as other attributes, such as nodule growth and number. The colonization ability of AMF in soybean, such as mycelium, arbuscules and vesicles, was also inhibited by salt stress. Hashem et al. (2014a) observed that higher salt levels reduce the development of mycelium, arbuscules and vesicles in Vicia faba plants, resulting in restricted growth. Stressful environmental conditions reduce spore viability and affect root colonization (Ahanger et al., 2014). However, the salt-tolerant genotype maintained higher colonization characteristics compared to the salt-sensitive genotype.
AMF resulted in a considerable increase in the uptake of mineral nutrients by plants, which ultimately promotes synthesis of metabolically important metabolites and enzymes (Yuan et al., 2010). Among these essential plant metabolites, plant hormones have an intriguing role in plant growth maintenance. Auxins play a major role in the signaling events between AMF and host plants (Fernandez et al., 2014). Auxins also play an important role in the development of nodule vasculature in leguminous plants (Mathesius, 2008).
The salt tolerance in soybean is modulated by a single dominant allele of the tolerance-associated gene GmSALT3, which is the dominant locus on soybean chromosome 3 in both cultivated and wild soybean (Guan et al., 2014, Liu et al., 2016). In the present study, a highly significant negative correlation between salinity and nodulation (growth and activities of nodules) as well as for all AMF incidences was detected in the soybean genotype Kint (salt-sensitive); however, in the soybean genotype Clark (salt tolerant), such a negative correlation was less related, especially with mycorrhizal mycelia (M). Nevertheless, a significant correlation with the resting stages of vesicles (V) and arbuscules (A) of AMF was observed, and consequently, the soybean genotype showed resistance against salinity and could withstand the harsh conditions and grow well. A significant correlation was reported in our study between the structural colonization of AMF and nodulation and was directly proportional with salt resistance and the incidence of AMF. In this context, plants associated with AMF had a greater phosphorus content and derived more nitrogen from the atmosphere by rhizobium (Tong et al., 2009) than plants grown in soil without AMF, which is generally considered beneficial for nitrogen uptake (Younesi et al., 2013, Meng et al., 2015).
Salt-stressed soybean genotypes exhibited a drastic decline in the endogenous levels of growth regulators, such as indole acetic acid (IAA) and indole butyric acid (IBA), while the inoculation of AMF promoted the synthesis of these growth regulators. Endophytic fungi inhabiting plants are known to cause an increase in the endogenous levels of IAA (Waqas et al., 2012). In salt-stressed Sesbania sesban, Abd_Allah et al. (2015a) observed a reduction in the endogenous levels of growth regulators, such as IAA, and the ameliorative role of AMF in reversing the effect of salinity with the levels of growth hormones. Maintaining the endogenous levels of growth regulators is important for several physiological and biochemical key functions, such as stomatal closure, growth regulation and maintenance of developmental events in both normal and stressed conditions (Wasilewska et al., 2008).
In the present study, both soybean genotypes subjected to salinity stress exhibited a considerable reduction in the synthesis of photosynthetic pigments, thereby resulting in the reduced synthesis of photosynthetic products. AMF were able to mitigate the harmful impacts of salinity on chlorophyll content, and similar results were observed by Alqarawi et al. (2014) for Ephedra aphylla and by Hashem et al. (2014a) for Vicia faba. Recently, Khan et al. (2015) demonstrated in chickpea genotypes subjected to saline stress that tolerant genotypes maintain a relatively better photosynthetic efficiency compared to sensitive genotypes. Under salt stress, normal functioning of the oxygen evolving complex is also altered, as reflected in the reductions of metabolic rates.
Tolerant soybean genotypes exposed to high salt concentrations showed a lower production of thiobarbituric acid reactive substances (TBARS), lower rates of lipid peroxidation measured in terms of malondialdehyde (MDA) formation, and a lower production of hydrogen peroxide (H2O2) compared to the sensitive genotype. An increased production of free radicals, such as H2O2, induced the peroxidation of lipids and resulted in the excessive formation of TBARS and MDA in salt-stressed seedlings. During stressed conditions, the rapid peroxidation of membrane lipids is an increasingly observed effect of oxidative damage. Early reports published showing increased production of H2O2 and MDA and a subsequent amelioration by AMF inoculation include Alqarawi et al. (2014) for Ephedra aphylla, Hashem et al. (2014a) for Vicia faba, and Abd_Allah et al. (2015a) for Sesbania sesban. The combined inoculation of soybean with AMF and rhizobium reduced the production of H2O2 and MDA, thereby strengthening the positive role of AMF in protecting biological membranes from the toxic effects of free radicals. A reduction in the peroxidation of membrane lipids and a lower accumulation of free radicals in AMF of inoculated plants have been correlated with increased activities of antioxidant enzymes and improved production of non-enzymatic antioxidants, hence mediating the quick removal of free radicals and enhancing the protection of the stability of membranes (Alqarawi et al., 2014, Wu et al., 2014, Hashem et al., 2015b).
5. Conclusion
The results of the present investigation indicate that salt stress significantly decreased the growth and physiological parameters of soybean; however, the salt-tolerant Clark genotype showed a minimum reduction in all parameters compared with the salt-sensitive Kint genotype. We observed that AMF improved plant growth and symbiotic performance in both soybean genotypes by stimulating the endogenous level of auxins that contribute to improved root systems and nutrient acquisition under salt stress. We found variable physiological responses among the two genotypes, showing stress tolerance and sensitive properties to AMF inoculation.
Acknowledgment
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for the funding of this research through the Research Group Project no. RGP-271.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd_Allah, E.F., Hashem, A., Alqarawi, A.A., Bahkali, A.H., Alwhibi Mona, S., 2015a. Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 22(3), 274–283. [DOI] [PMC free article] [PubMed]
- Abd_Allah E.F., Hashem A., Alqarawi A.A., Alwhibi M.S. Alleviation of adverse impact of salt in Phaseolus vulgaris L. by arbuscular mycorrhizal fungi. Pak. J. Bot. 2015;47:167–176. [Google Scholar]
- Abd_Allah, E.F., Hashem, A., Alqarawi, A.A., Alwathnani Hend, A., 2015c. Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 47(2), 785–795.
- Ahmad P., Hashem A., Abd_Allah E.F., Alqarawi A.A., John R., Egamberdieva D., Gucel S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015;6:1–15. doi: 10.3389/fpls.2015.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahanger, M.A., Hashem, A., Abd_Allah, E.F., Ahmad, P., 2014. Arbuscular mycorrhiza in crop improvement under environmental stress. In: Ahmad, P. (Ed.), Emerging Technologies and Management of Crop Stress Tolerance, vol. 2. Elsevier Inc.
- Alqarawi A.A., Abd-Allah E.F., Hashem A. Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J. Plant Int. 2014;9:802–810. [Google Scholar]
- Epstein E., Ludwig-Müller J. Indole-3-butyric acid in plants: occurrence, synthesis, metabolism and transport. Physiol. Plant. 1993;88:382–389. [Google Scholar]
- Bergersen F.J., Turner G.L. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim. Biophys. Acta. 1967;141:507–515. doi: 10.1016/0304-4165(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Bergersen F.J. Measurement of nitrogen fixation by direct means. In: Bergersen F.J., editor. Methods of Evaluating Biological Nitrogen Fixation. John Wile and Sons; New York, USA: 1980. [Google Scholar]
- Daniels, B.A., Skipper, H.D., 1982. Methods for the recovery and quantitative estimation of propagules from soil. In: Schenck, N.C. (Ed.), Methods and Principles of Mycorrhizal Research. The American Phytopathological Society, pp. 29–36.
- de Varennes A., Goss M.J. The tripartite symbiosis between legumes, rhizobia and indigenous mycorrhizal fungi is more efficient in undisturbed soil. Soil Biol. Biochem. 2007;39:2603–2607. [Google Scholar]
- Dhindsa R.S., Plumb-Dhindsa P., Thorpe T.A. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981;32:93–101. [Google Scholar]
- Egamberdieva D., Jabborova D., Berg G. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, nodulation and nutrition of soybean under salt stress. Plant Soil. 2015:1–11. [Google Scholar]
- Egamberdieva, D., Li, L., Lindström, K., Räsänen, L., 2015b. A synergistic interaction between salt tolerant Pseudomonas and Mezorhizobium strains improves growth and symbiotic performance of liquorice (Glycyrrhiza uralensis Fish.) under salt stress. Appl. Microbiol. Biotechnol. http://dx.doi.org/10.1007/s00253-015-7147-3. [DOI] [PubMed]
- Egamberdieva D., Berg G., Lindström K., Räsänen L.A. Alleviation of salt stress of symbiotic Galega officinalis L. (goat’s rue) by co-inoculation of rhizobium with root colonising Pseudomonas. Plant Soil. 2013;369(1):453–465. [Google Scholar]
- Egamberdieva D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009;31:861–864. [Google Scholar]
- Elsheikh E.A.E., Wood M. Nodulation and N2 fixation by soybean inoculated with salt-tolerant rhizobia or salt-sensitive Bradyrhizobia in saline soil. Soil Biol. Biochem. 1995;27(4):657–661. [Google Scholar]
- Fernandez I., Merlos M., López-Ráez J.A., Martínez-Medina A., Ferrol N., Azcón C., Bonfante P., Flors V., Pozo M.J. Defense related phytohormones regulation in arbuscular mycorrhizal symbioses depends on the partner genotypes. J. Chem. Ecol. 2014;40(7):791–803. doi: 10.1007/s10886-014-0473-6. [DOI] [PubMed] [Google Scholar]
- Guan R., Qu Y., Guo Y., Yu L., Liu Y., Jiang J., Chen J., Ren Y., Liu G., Tian L., Jin L., Liu Z., Hong H., Chang R., Gilliham M., Qiu L. Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J. 2014;80:937–950. doi: 10.1111/tpj.12695. [DOI] [PubMed] [Google Scholar]
- Hashem A., Abd_Allah E.F., Alqarawi A.A., Al-Didamony G., Al-Whibi M., Egamberdieva D., Ahmad P. Alleviation of adverse impact of salinity on faba bean (Vicia faba L.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 2014;46:2003–2013. [Google Scholar]
- Hashem A., Abd_Allah E.F., Alqarawi A.A., Al-Huqail A.A., Egamberdieva D. Alleviation of abiotic salt stress in Ochradenus baccatus (Del.) by Trichoderma hamatum (Bonord.) Bainier. J. Plant Int. 2014;9:857–868. [Google Scholar]
- Hashem A., Abd_Allah E.F., Alqarawi A.A., Aldubise A., Egamberdieva D. Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J. Plant Int. 2015;10(1):230–242. [Google Scholar]
- Hashem, A., Abd_Allah, E.F., Alqarawi, A.A., Egamberdieva, D., 2015b. Induction of salt stress tolerance in cowpea [Vigna unguiculata (L.) Walp.] by arbuscular mycorrhizal fungi. Legume Res. 38(5), 579–588.
- Hashem, A., Abd_Allah, E.F., Alqarawi, A.A., Al Huqail Asma, A., Egamberdieva, D., Wirth, S., 2016. Alleviation of cadmium stress in Solanum lycopersicum L. by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J. Biol. Sci. 23(2), 271–281. [DOI] [PMC free article] [PubMed]
- Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hiscox, J.D., Israelstam, G.F., 1979. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 57, 1332–1334.
- Keilin D., Wang Y.L. Hemoglobin in the root nodules of leguminous plants. Nature. 1945;155:227–229. doi: 10.1038/159692a0. [DOI] [PubMed] [Google Scholar]
- Kelen M.E., Çubek Demiralay S., Sen Ozkan G. Separation of abscisic acid, indole-3-acetic acid, gibberellic acid in 99 R (Vitis berlandieri × Vitis rupestris) and rose oil (Rosa damascena Mill.) by reversed phase liquid chromatography. Turk. J. Chem. 2004;28:603–610. [Google Scholar]
- Khan H.A., Siddique K.H.M., Munir R., Colmer T.D. Salt sensitivity in chickpea: growth, photosynthesis, seed yield components and tissue ion regulation in contrasting genotypes. J. Plant Physiol. 2015;182:1–12. doi: 10.1016/j.jplph.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Khan A.L., Waqas M., Lee I.J. Resilience of Penicillium resedanum LK6 and exogenous gibberellin in improving Capsicum annuum growth under abiotic stresses. J. Plant Res. 2014;128(2):259–268. doi: 10.1007/s10265-014-0688-1. [DOI] [PubMed] [Google Scholar]
- McCarty L.B., Dudeck A.E. Salinity effects on bent grass germination. Hort. Sci. 1993;28:15–17. [Google Scholar]
- Kusaba S., Kano-Murakami Y., Matsuoka M., Tamaoki M., Sakamoto T., Yamaguchi I., Fukumoto M. Alteration of hormone levels in transgenic tobacco plants over expressing a rice homeobox gene OSH1. Plant Physiol. 1998;116:471–476. doi: 10.1104/pp.116.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yu L., Qu Y., Chen J., Liu X., Hong H., Liu Z., Chang R., Gilliham M., Qiu L., Guan R. GmSALT3, which confers improved soybean salt tolerance in the field, increases leaf Cl− exclusion prior to Na+ exclusion but does not improve early vigor under salinity. Front. Plant Sci. 2016;2016(7):1485. doi: 10.3389/fpls.2016.01485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U. Auxin: at the root of nodule development? Funct. Plant Biol. 2008;35(8):651–668. doi: 10.1071/FP08177. [DOI] [PubMed] [Google Scholar]
- Meng L., Zhang A., Wang F., Han X., Wang D., Li S. Arbuscular mycorrhizal fungi and rhizobium facilitate nitrogen uptake and transfer in soybean/maize intercropping system. Front. Plant Sci. 2015;2015(6):339. doi: 10.3389/fpls.2015.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montealegre C., Graham P.H., Kipe-Nolt J.A. Preference in the nodulation of Phaseolus oulgais cultivar RAB39. Can. J. Microbiol. 1995;47:992–998. [Google Scholar]
- Okuda T., Matsuda Y., Yamanaka A., Sagisaka S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991;97:1265–1267. doi: 10.1104/pp.97.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J.M., Hayman D.S. Improved produces for clearing roots and staining parasitic and VAM fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc. 1970;55:158–161. [Google Scholar]
- Rabie G.H., Almadini A.M. Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr. J. Biotech. 2005;4:210–222. [Google Scholar]
- Severin I., Confurius-Guns V., Stal L.J. Effect of salinity on nitrogenase activity and composition of the active diazotrophic community in intertidal microbial mats. Arch. Microbiol. 2012;194:483–491. doi: 10.1007/s00203-011-0787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timson J. New method of recording germination data. Nature. 1965;207:216–217. [Google Scholar]
- Tong L.N., Li S.M., Meng L.B. Effect of inoculating arbuscular mycorrhizal fungi and rhizobium on soybean by utilizating organic phosphorus source. J. Northeast Agric. Univ. 2009;40:37–47. (in Chinese) [Google Scholar]
- Utobo E.B., Ogbodo E.N., Nwogbaga A.C. Techniques for extraction and quantification of arbuscular mycorrhizal fungi. Libyan Agric. Res. Center Int. 2011;2:68–78. [Google Scholar]
- Waqas M., Khan A.L., Kamran M., Hamayun M., Kang S.M., Kim Y.H., Lee I.J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules. 2012;17:10754–10773. doi: 10.3390/molecules170910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilewska A., Vlad F., Sirichandra C., Redko Y., Jammes F., Valon C., Freidit Frey N., Leung J. An update on abscisic acid signaling in plants and more. Mol. Plant. 2008;1:198–217. doi: 10.1093/mp/ssm022. [DOI] [PubMed] [Google Scholar]
- Wu, Q., Zou, Y.N., Abd_Allah, E.F., 2014. Mycorrhizal association and ROS in plants. In: Ahmad, P. (Ed.), Oxidative Damage to Plants. Elsevier Inc. http://dx.doi.org/10.1016/B978-0-12-799963-0.00015-0.
- Zarea M.J., Karimi N., Goltapeh E.M., Ghalavand A. Effect of cropping systems and arbuscular mycorrhizal fungi on soil microbial activity and root nodule nitrogenase. J. Saudi Soc. Agric. Sci. 2011;10:109–120. [Google Scholar]
- Younesi O., Moradi A., Namdari A. Influence of arbuscular mycorrhiza on osmotic adjustment compounds and antioxidant enzyme activity in nodules of salt-stressed soybean (Glycine max) Acta Agric. Slovenica. 2013;101(2):219–230. [Google Scholar]
- Yuan Z.L., Zhang C.L., Lin F.C. Role of diverse non-systemic fungal endophytes in plant performance and response to stress: progress and approaches. J. Plant Growth Regul. 2010;29:116–126. [Google Scholar]