Abstract
Binding of a BMP to its cognate cell surface receptors is the initiating step in the BMP signaling cascade. Thus, knowing which BMP–receptor complexes form is vital for understanding the physiological activities of a particular BMP. Here, we describe a surface plasmon resonance (SPR)-based, high-throughput approach that allows fast identification and evaluation of BMP–receptor complexes. Briefly, the extracellular, BMP-binding domains of receptors are produced as human IgG1-Fc-fusion proteins. The Fc moiety enables simple capture of the Fc-receptor-fusion protein on the sensor chip, supports a highly reproducible, uniform approach of surface regeneration, and ensures full activity of the receptor moiety. BMPs are injected over the captured receptors at one concentration (approximately 60–100 nM), permitting stratification of high-affinity, medium-affinity, and low-affinity binders. Using this concentration range, equilibrium dissociation constants for high-affinity and medium-affinity binders can be estimated with good accuracy and with great precision from the single injection binding curves.
Keywords: Surface plasmon resonance, Bone morphogenetic protein, Activin, TGF-β, Protein–protein interactions
1. Introduction
The transforming growth factor (TGF)-β family plays a central role in animal cell physiology. Members of the family have vital functions controlling cell growth, differentiation, homeostasis, and cell death [1, 2]. They also play critical roles in many diseases, including inflammation, cancers, and fibrosis [3–5]. In mammals, the family consists of 33 genes encoding TGF-βs, bone morphogenetic proteins (BMPs), growth and differentiation factors (GDFs), activins, and nodal.
The basic mechanism of TGF-β family action is well established: a growth factor (TGF-β, BMP, GDF, activin, or nodal) binds the extracellular domain of its cognate type I and type II receptors, activating an intracellular phosphorylation cascade that leads to transcription of SMAD-regulated target genes [6, 7]. But our complete understanding of TGF-β family action remains elusive, as cellular responses depend on specific growth factor-receptor pairings and on co-receptors that regulate the activities of some signaling complexes [8, 9]. Notably, many of these pairings, which combined form the TGF-β family interaction and signal transduction network, remain unknown.
Surface plasmon resonance (SPR) is a powerful and highly valuable tool for analysis of the BMP/TGF-β family interaction network [10]. It can identify specific growth factor–receptor–co-receptor complexes with great accuracy and precision [11–14]. It can also be used to characterize cooperative or inhibitory interactions [14–17]. In a standard SP-binding experiment, one binding partner (the “ligand” in SPR lingo) is immobilized or captured on a sensor chip, while the other binding partner (the “analyte” in SPR lingo) is injected over the sensor chip surface. Note that this protocol follows SPR convention and limits using the word “ligand” to the protein bound to the chip, in this case the BMP receptor. SPR is an optical method that measures the refractive index near the sensor chip surface. Binding is detected as a change in the refractive index. The refractive index increases as mass accumulates due to binding of the SPR “analyte” (the BMP) to the captured SPR “ligand” (the receptor). Changes in the refractive index reveal the association and dissociation of molecules, indicating the kinetic rates of an interaction [10]. Equilibrium binding constants can be derived from these kinetic rates. As SPR is extremely sensitive, it requires relatively small quantities of test material. Importantly, test proteins do not require molecular labels that can compromise their activity. Taken together, these characteristics make SPR especially useful and powerful for studying the TGF-β family and for elucidating the BMP interaction network.
The accurate SPR approach relies on highly reproducible capture methods using receptor-Fc fusion proteins (Fig. 1). The Fc moiety is used to capture the receptor (SPR “ligand”) on the sensor chip via ultrahigh-affinity protein A or anti-Fc antibody binding. Capture is simple; sensor chip regeneration is standardized, effective, and reproducible; and receptor activity is not compromised. Injection of BMP solution (SPR “analyte”) over the captured receptors provides highly reproducible and informative binding data that enables stratification of high-, medium-, and low-affinity BMP–receptor pairings (Fig. 2). The association and dissociation curves can be used to obtain binding rates and to derive equilibrium binding constants with good accuracy. In addition to mechanistic characterizations, this approach can be used for quality control. Binding curves for benchmarking are easily established, and the activity of a BMP or receptor over time or in different formulations can be monitored quickly and with great precision. The ability to capture any receptor-Fc-fusion protein without compromising receptor activity, combined with the ability to rapidly and reproducibly regenerate the sensor chip surface, permits a fast, high-throughput evaluation of any BMP–receptor complex.
Fig. 1.
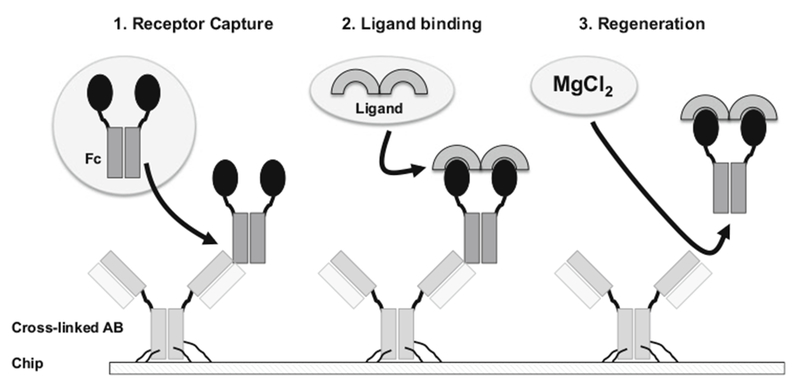
Schematic representation of the capture, binding, and regeneration cycle. The chip is prepared by cross-linking anti-Fc antibody (AB) on the sensor chip. In the first step of the reaction (1), receptor-Fc fusion protein is captured by the antibody (approximately 150–250 RUs of receptor-Fc fusion protein). In the second step of the reaction (2), 80 nM BMP is injected, and binding to the receptor moiety is detected. In the third step of the reaction (3), the BMP-receptor-Fc fusion complex is eluted with MgCl2 to regenerate the surface. To test binding of a different BMP, new receptor-Fc fusion protein has to be captured (step 1). A protein A chip can also be used to capture receptor-Fc proteins, but regeneration conditions need to be adapted
Fig. 2.

Example of a multi-reaction cycle. (a) Three different receptor-Fc fusion proteins are captured sequentially on a four-channel sensor chip (R1-Fc, R2-Fc, and R3-Fc). Capture is highly reproducible as seen in the exact matching of ten capture cycles for R1-Fc (from approximately 200 to 300 s), R2-Fc (from approximately 550 to 650 s), and R3-Fc (from approximately 900 to 1000 s). (b) BMP at a concentration of 80 nM is injected over the sensor chip following receptor capture. In the example shown here, 15 different members of the TGF-β/BMP family were injected to test for binding to BMPRII-Fc. Each curve represents a different molecule. Each protein elicits a specific response as seen in the distinct association rates (from 0 to 300 s) and dissociation rates (from 300 to 1100 s). The strongest BMPRII-Fc binders were activin B (black response curve of approximately 110 RU) and nodal (dark gray response curve of approximately 100 RU). (c) Regeneration restores the baseline response ±5 RU (from 900 to 1000 s) by eluting the captured receptor-Fc fusion protein, including the receptor-bound protein. Regeneration conditions are highly reproducible with this approach as seen in this superposition of ten growth factor-binding/regeneration cycles. Regeneration steps are labeled as in the following panel. (d) In this close-up view of the sensor chip regeneration, the residual binding response (BMP, from 0 to 100 s) can be observed at the beginning of the regeneration cycle. Magnesium chloride (MgCl2, from 100 to about 450 s) elutes bound receptors and associated ligands. Re-equilibration with buffer (HBS, from about 450 to about 650 s) restores the baseline. A washing step with water (H2O, from about 650 to about 825 s) is necessary to avoid magnesium chloride accumulation and clogging of the microfluidics unit. A second equilibration with buffer (HBS, from about 825 to about 1000 s) completes the regeneration program, and the sensor chip returns to a constant RU baseline
2. Materials
2.1. Surface Plasmon Resonance (SPR) Instrument
Experiments presented here were carried out on a Biacore 2000 (GE Lifesciences, discontinued) (see Note 1).
2.2. Surface Preparation Reagents
0.4 M N-(3-Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC) dissolved in water. Solid EDC is hygroscopic and temperature sensitive and must be aliquoted as dry powder under N2 atmosphere. Sealed EDC aliquots can be stored at −20 °C. EDC solutions must be prepared fresh before use.
0.1 M N-Hydroxysuccinimide (NHS) dissolved in water. NHS solutions must be prepared fresh and immediately before use.
1.0 M Ethanolamine diluted in water. The pH is adjusted to 8.2 with concentrated HCl. A pH range between 8.0 and 8.5 is acceptable. Prepare ethanolamine fresh before use (see Note 2).
CM3 or CM5 chip (e.g., Biacore BR-1003-99) stored at 4 °C. Before use, equilibrate at room temperature for at least 30 min. This chemistry is used with the antibody in item 5. Sensor chip protein A (e.g., Biacore 29127557) is an alternative option that can be used out of the box.
Human Antibody Capture Kit (Biacore, BR-1008-39): stored at 4 °C. This kit contains 0.5 mg/mL antihuman IgG (Fc) antibody, 10 mM sodium acetate pH 5.0, and 3 M MgCl2 (see Notes 3 and 4).
2.3. SPR Disposables and Running Buffers
Capped glass and plastic vials for storing the “analyte,” “ligand,” and regeneration solutions that are compatible with instrument control, injection needle, and sample racks. For the Biacore 2000 sample racks, use 9 mm glass vials, 7 mm plastic vials, and 8 mm polyethylene dust cover caps.
HBS-EPS/BSA running buffer: 0.01 M HEPES, 0.5 M NaCl, 3 mM EDTA, 0.005% (v/v) Tween 20, and 0.1% BSA, pH 7.4 (see Notes 5 and 6).
2.4. BMPs and Fc-Fusion Receptors
100 μg/mL BMPs dissolved in 4 mM HCl, 0.1 M acetic acid, or 1× PBS. Buffer selection is BMP dependent (follow manufacturer’s instructions). 10 μL aliquots are stored at −20 °C for 3 months or at −80 °C for 1 year (see Note 7).
Expression-ready plasmids are not publicly available. The pFUSE-hIgG1-Fc1 vector system (InvivoGen) can be used to create Fc-fusion receptor expression plasmids for transiently and stably transfected mammalian cell cultures. Alternatively, Fc-fusion receptors can be obtained from a number of vendors, including R&D Systems (e.g., BMPRII-Fc, 811-BR) and Prosci (see Note 8).
3. Methods
3.1. Receptor Preparation and Storage
Transfect plasmids encoding the Fc-receptor-fusion gene into mammalian cells. For transient suspension cultures, use the ThermoFisher Scientific FreeStyle 293 or ThermoFisher Expi-CHO Expression systems. For stable suspension cultures, use the ThermoFisher Scientific pcDNA3.3 TOPO, ThermoFisher Scientific Freedom CHO-S, or Lonza’s GS Gene Expression systems (see Note 9).
Purify secreted Fc-fusion receptors from conditioned medium using protein A capture chromatography (e.g., HiTrap MabSelect SuRe column, GE11-0034-94). An additional step of size exclusion chromatography (e.g., Superdex 200 16/60, GE 28989335) is recommended to remove aggregated Fc-fusion receptors and degraded components.
Combine fractions containing monodisperse, dimeric receptor-Fc fusions, and dialyze in 1× PBS overnight at 4 °C.
Some receptor-Fc fusions might require addition of stabilizers such as trehalose after dialysis to maintain activity over time. The need for stabilizers can be determined by monitoring binding affinities over time. Alternatively, the addition of stabilizers is often mentioned in vendor instructions. These instructions could be followed for guidance.
Filter sterilize dialyzed Fc-fusion receptors and store aliquots at −20 ° C.
Purchased Fc-fusion receptors are reconstituted according to the manufacturer’s instructions and stored in aliquots at −20 °C.
3.2. Anti-Fc Chip Preparation and Storage
The following example is based on the application wizard protocol in the Biacore 2000 control software.
The CM5 chip is incubated at room temperature at least for 30 min before it is placed into SPR (Biacore) instrument.
For anti-Fc chip preparation, water is used as running buffer, and the surface is primed at least one time before starting the surface activation program.
Separately aliquot 120 μL of 0.4 M EDC, 120 μL of 0.1 M NHS, and 100 μL of 1.0 M ethanolamine pH 8.2 into 7 mm plastic vials. Use a separate vial for each solution and each channel that will be activated, i.e., use one vial for EDC, one vial for NHS, one vial for ethanolamine, and one empty vial for EDC/NHS mixing per channel that will be activated.
Mix 0.4 M EDC and 0.1 M NHS at a 1:1 ratio.
Inject the EDC/NHS mixture separately into each flow channel at 5 μL/min for 400 s.
To ensure adequate antibody immobilization, at least a 100 response unit (RU) increase should be achieved following activation with EDC/NHS. Using this protocol, the response increase is between 400 and 600 RUs, which is optimal for this method (see Note 10).
Dilute 13.5 μL 0.5 mg/mL antihuman IgG (Fc) antibody in 876 μL of 10 mM sodium acetate pH 5.0 to a final concentration of 7.7 μg/mL. Immobilize the antibody to both blank and experimental flow channels (i.e., FC 1, 2, 3, and 4) by injecting the antibody containing solution at 10 μL/min for 16 min. 210 μL of diluted antibody is needed for each channel (see Note 11).
To deactivate the surface, inject 1.0 M ethanolamine pH 8.2 to blank and experimental flow channels (i.e., FC 1, 2, 3, and 4) at 5 μL/min for 360 s.
Each flow channel (FC) is prepared individually when using the application wizard in the Biacore control software. All steps, from surface activation, antibody cross-linking, to surface deactivation, are carried out for one channel before continuing with the next channel. Solutions must be kept in a separate vial for each tube and channel.
If the chip surface is prepared manually, all channels can be prepared simultaneously. EDC/NHS solutions can be premixed and are loaded into all four channels at once.
3.3. Running Conditions
Run the SPR instrument at 50 μL/min to minimize mass transport artifacts.
3.4. Fc-Fusion Receptor Capture
150–250 RU Fc-fused receptors are captured separately on each experimental channel. (For the Biacore 2000 used here as example, flow channels 1 and 3 can be used as blanks.) In the following example, channel 1 is used as control, and Fc-fusion receptors are immobilized in experimental flow channels 2–4 (see Note 12).
To get desired receptor levels on the surface, approximately 3.0 μg/mL Fc-fusion receptor in HBS-EPS/BSA running buffer is injected for 20 μL at 20 μL/min per channel.
Low surface loading is critical to minimize mass transport artifacts and receptor dissociation from the antibody (see Note 13).
During the receptor capture step, channels are loaded individually. After the receptor capture step, all channels are opened simultaneously. BMP binding and surface regeneration steps are carried out in all four channels at once (see Note 14).
3.5. BMP Binding
To prepare the BMP solution, dilute the BMP aliquot to a final concentration of 80 nM BMP directly from storage conditions into HBS-EPS/BSA running buffer.
It is important to prepare at least 320 μL of BMP dilution when using 7 mm plastic tubes and 350 μL of BMP dilution when using 9 mm glass vials in order to prevent injection of air bubbles.
For the BMP–receptor association step, inject 250 μL BMP solution at a rate of 50 μL/min into the four channels at once (see Note 15).
For the BMP–receptor dissociation step, flow running buffer for at least 750 s after sample injection is complete. This dissociation time works well when considering the affinity of a typical BMP–receptor complex (see Note 16).
3.6. Surface Regeneration
Regenerate the anti-Fc chip surface by injecting 60 μL of 3.0 M MgCl2 at 10 μL/min (see Note 17).
To prevent MgCl2 accumulation on the surface, inject 30 μL water at 10 μL/min between cycles.
To get the same level of Fc-fusion receptor capture in multi-injection experiments, which is critical for high-throughput binding comparisons and for determination of kinetic parameters, the baseline of the anti-Fc cross-linked sensor chip should reach exactly the same level before Fc-fusion receptor immobilization (±10 RU).
3.7. Evaluating a Binding Experiment
Evaluate binding data using software such as BIAevaluation, Scrubber, or Clamp. In the following example, the y-axis represents RU (response units), and the x-axis represents time (seconds).
The following steps are carried out with BMP sample injections to obtain binding information and also with running buffer alone to remove spikes and shifts, which disturb data fitting (see Note 18).
Biacore instruments automatically subtract the blank control channel (i.e., FC 1) from experimental channels (i.e., FC 2, 3, and 4) to obtain a difference between experimental and control channel responses (i.e., 2-1, 3-1, and 4-1).
Blank channel subtracted injection curves (i.e., 2-1, 3-1, and 4-1 if FC 1 is the blank channel) are transformed to a zero baseline on the y-axis using BIaevaluation software. This step is called “y-transformation.”
Blank channel subtracted binding curves (i.e., 2-1, 3-1, and 4-1) are aligned on the x-axis so that the BMP injection time point equals 0 s. This step is called “x-transformation.”
Running buffer injections are subtracted from BMP injections. This process of data analysis (steps 3–5) is called “double referencing.”
3.8. Calculating Equilibrium Binding Constants
Association (ka) and dissociation rate constants (kd) are obtained by fitting binding data to the X and Υ transformed curves obtained by following the steps described in Subheading 3.5.
To obtain kd and ka, from a single curve, the separate fitting approach can be used with the high-throughput, single-injection format described in this manuscript (see Note 19).
1:1 binding models are typically used for data analysis (see Note 20).
BIAevaluation, Scrubber, Clamp, or similar software can be used for data fitting. In this example, we use BIAevaluation.
In the separate fitting of a single injection curve, begin by selecting the start and end of the dissociation phase.
Fit the selected area to the 1:1 Langmuir model. This step yields the kd.
In the next step, select the start and end of the association phase.
By using the kd obtained in step 6 and the concentration of the injected BMP, fit the selected association phase to the 1:1 Langmuir model. This step yields the ka.
The equilibrium dissociation constant (Kd) is calculated from the ratio of kd/ka. (see Note 21).
4 Notes
The following example can be used with all Biacore instruments that have four flow cells (Biacore 2000, 3000, T100, T200, and S200). Experiments presented here were carried out on a Biacore 2000. Biacore instruments with a different number of flow cells can also be used. Likewise, these methods can be adapted to other SPR instruments.
Filtered and vacuum degassed type 1 ultrapure or distilled water should be used for all steps of the anti-Fc chip preparation, especially when preparing the EDC and NHS solutions. EDC, NHS, and ethanolamine should be made freshly before chip preparation. These chemicals are temperature sensitive. In addition, EDC is hygroscopic and labile.
Although alternate anti-Fc antibodies could be used, their stability, binding specificity, capture, and regeneration conditions would have to be determined. Using the Antibody Capture Kit is therefore advised.
The anti-Fc chips are kept at room temperature in the SPR machine. The lifetime of an anti-Fc chip averages 3 months when HBS-EPS/BSA running buffer is also used as standby buffer. The use of water as standby solution and dry storage outside of the SPR instrument significantly reduces the antibody/chip half-life.
The running buffer, reconstitution solutions, and in-house purified protein samples should be sterile filtered (0.2 μm).
HBS buffer is preferred over PBS to reduce salt crystal formation after regeneration in the microfluidics unit.
Purchased BMPs are reconstituted in sterilized buffers and need not be filtered after reconstitution.
We previously found that the linker that connects the ecto-domain with the Fc moiety can affect the binding affinity [13], possibly by limiting the range of motion of the receptor domain. A generously sized tag is therefore preferred. We use 20–30 amino acid linkers. Shorter linkers have been used, but this can limit the flexibility and accessibility of the receptor domain and lead to an underestimation of the equilibrium dissociation constant.
Adherent and suspension cultures can be used for Fc-fusion receptor expression; however, suspension cultures are recommended for larger-scale production.
SPR instruments measure a refractive index change (detected as a RU). The change in refractive index is correlated with mass accumulation on the surface of the chip. A minimum of 100 RU from EDC/NHS activation is required to capture sufficient antibody.
This method results in the cross-linking of ~8000 RU of antihuman IgG (Fc) antibody per channel, which is optimal. Excessive antibody cross-linking (>12,000 RU) can lead to Fc-fusion receptor leakage and baseline drift, compromising baseline integrity, exacerbating mass-transport artifacts. Insufficient antibody (<5000 RU) may limit receptor capture and reduce the shelf life of the chip.
This approach relies on a simple, standardized capture method of an Fc-tagged protein. Significant advantages are the ability to stably capture the Fc tag with a well-characterized monoclonal antibody or using protein A chemistry and the ability to thoroughly regenerate the surface without compromising its activity. Direct cross-linking of the BMP or receptor domain to the sensor chip via EDC/NHS chemistry may not be desirable, as side chains with primary amines (mainly lysine but also arginine) in these domains can be found at the BMP–receptor interface [11, 18]. Modification of these side chains could therefore restrict BMP–receptor complexation. In addition, regeneration conditions can be harsh and lead to very limited lifetime of the cross-linked receptor or BMP. A particular advantage of this approach is that all captured receptors have the same orientation.
A low amount of captured receptor is essential to minimize mass transport limitation artifacts, which frequently leads to overestimation of kinetic rate constants. Limiting receptor levels below 300 RU is therefore advised.
As three experimental channels are typically available (Biacore 2000, 3000, T100, T200, S00), three different receptor-Fc fusions or variants can be tested simultaneously.
Before starting real binding injections, 2–3 blank injections should be done to equilibrate the Biacore instrument. At least three additional blank injections should be done between binding injections to obtain blanking curves.
Importantly, at least a 5% decrease in the SPR response should be observed to obtain an accurate dissociation rate constant.
This removes the captured receptors as well as the BMPs that are bound by the receptors. For each new cycle, new receptor-Fc fusions have to be captured.
Fc-fusion receptors, BMPs, and blank solutions are prepared and dialyzed in the same running buffer to minimize bulk shift effects, which cause significant problems during the fitting process. In addition, the needle should be dipped in running buffer before injecting BMP to minimize spikes on the sensograms. Small spikes might be observed when the running buffer injection pump is filled, but these are easy to eliminate in the analysis process. Also, use the “Extraclean” command after each injection to minimize contamination from different samples.
High-throughput single injection vs. low-throughput titration. Although multiple curves from an “analyte” titration generally give more accurate kinetic and equilibrium constants, we found that, in a well-executed SPR experiment, a single injection can also yield accurate binding rate constants [12]. In contrast to a low-throughput titration, such an approach can be applied in a high-throughput format, allowing for rapid interrogation of multiple BMP–receptor complexes. In order to get a good agreement with Kd values obtained from titration, the concentration in the single injection should be significantly above Kd. 80 nM is a good starting point for members of the BMP family. Thus, a single-injection approach using the suggested BMP concentrations and captured receptor totals can help rapidly produce a BMP–receptor interaction map, as well as kinetic rates and equilibrium binding constants with good approximation. Notably, any approach that relies on multiple injections is not high-throughput.
Many different kinetic models can be used for fitting. In the separate fitting, only the 1:1 Langmuir binding model is available. When simultaneously fitting multiple titration curves, the most commonly used models are “1:1 Langmuir binding,” “1:1 binding with mass transfer,” “1:1 binding with drifting baseline,” “bivalent analyte,” “conformational change,” “heterogeneous analyte,” and “heterogeneous ligand.” “Ligand” and “analyte” are used here to reflect the SPR nomenclature. Fitting parameters help identify the appropriate fitting model, i.e., the goodness of the fit, as indicated by the Chi2 (RU2) value, shows if a particular model is acceptable. A small Chi2 value indicates a good fit. For the Biacore 2000, this value should not be above 1.0. Relative residuals should be equally distributed around zero. If the fit is good, the standard error (SE) of the fit becomes negligible. More complex binding models and stoichiometries not described here can also be investigated. For binding analysis of a single injection, separate fitting is used. By contrast, global fitting is preferred when at least five concentrations of one BMP have been sampled.
High-throughput BMP binding to Fc-fusion receptors can be studied at a fixed concentration of “analyte”/BMP as suggested in this manuscript. This leads to good Kd estimates. A titration experiment can then be designed based on the estimated Kd value. At least five protein concentrations of “analyte”/BMP between 0.5 and 20 times the Kd concentration for that binding pair should be used to obtain accurate kinetic parameters. Ideally, binding saturation should be reached at the highest selected analyte concentration.
To obtain the SE for the kinetic rates and equilibrium binding constants, the experiment should be repeated at least three times.
In order to get steady-state binding kinetics, the flow rate could be reduced, or injection time could be increased. Both approaches give BMPs more time to bind the captured receptors.
While BMPs are extremely sensitive to storage conditions, Fc-fusion receptors are stable at 4 °C for at least 2 weeks.
For weekly cleaning of Biacore instruments, the anti-Fc chips can be taken out for a few hours. Changes in temperature should be minimized to prevent formation of bubbles on the surface.
Water should be avoided as running or standby buffer, as prolonged exposure to water decreases the lifetime of the anti-Fc chips.
References
- 1.Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A (2011) Bone morphogenetic proteins: a critical review. Cell Signal 23 (4):609–620. 10.1016/j.cellsig.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Goumans MJ, Mummery C (2000) Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 44(3):253–265 [PubMed] [Google Scholar]
- 3.Werner S, Alzheimer C (2006) Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine Growth Factor Rev 17 (3):157–171. 10.1016/j.cytogfr.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 4.Wakefield LM,Hill CS(2013) Beyond TGFbeta: roles of other TGFbeta superfamily members in cancer. Nat Rev Cancer 13(5):328–341. 10.1038/nrc3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massague J (2008) TGFbeta in cancer. Cell 134(2):215–230. 10.1016/j.cell.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113(6):685–700 [DOI] [PubMed] [Google Scholar]
- 7.Weiss A, Attisano L (2013) The TGFbeta superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol 2(1):47–63. 10.1002/wdev.86 [DOI] [PubMed] [Google Scholar]
- 8.Massague J (2012) TGFbeta signalling in context. Nat Rev Mol Cell Biol 13(10):616–630. 10.1038/nrm3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moustakas A, Heldin CH (2009) The regulation of TGFbeta signal transduction. Development 136(22):3699–3714. 10.1242/dev.030338 [DOI] [PubMed] [Google Scholar]
- 10.Olaru A, Bala C, Jaffrezic-Renault N, Aboul-Enein HY (2015) Surface plasmon resonance (SPR) biosensors in pharmaceutical analysis. Crit Rev Anal Chem 45(2):97–105. 10.1080/10408347.2014.881250 [DOI] [PubMed] [Google Scholar]
- 11.Townson SA, Martinez-Hackert E, Greppi C, Lowden P, Sako D, Liu J, Ucran JA, Liharska K, Underwood KW, Seehra J, Kumar R, Grinberg AV (2012) Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex. J Biol Chem 287(33):27313–27325. 10.1074/jbc.M112.377960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aykul S, Martinez-Hackert E (2016) Transforming growth factor-beta family ligands can function as antagonists by competing for type II receptor binding. J Biol Chem 291 (20):10792–10804. 10.1074/jbc.M115.713487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sako D, Grinberg AV, Liu J, Davies MV, Castonguay R, Maniatis S, Andreucci AJ, Pobre EG, Tomkinson KN, Monnell TE, Ucran JA, Martinez-Hackert E, Pearsall RS, Underwood KW, Seehra J, Kumar R (2010) Characterization of the ligand binding functionality of the extracellular domain of activin receptor type IIb. J Biol Chem 285 (27):21037–21048. 10.1074/jbc.M110.114959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aykul S, Ni W, Mutatu W, Martinez-Hackert E (2015) Human cerberus prevents nodal-receptor binding, inhibits nodal signaling, and suppresses nodal-mediated phenotypes. PLoS One 10(1):e0114954 10.1371/journal.pone.0114954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aykul S, Martinez-Hackert E (2016) New ligand binding function of human Cerberus and role of proteolytic processing in regulating ligand-receptor interactions and antagonist activity. J Mol Biol 428(3):590–602. 10.1016/j.jmb.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aykul S, Martinez-Hackert E (2016) Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis. Anal Biochem 508:97–103. 10.1016/j.ab.2016.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castonguay R, Werner ED, Matthews RG, Presman E, Mulivor AW, Solban N, Sako D, Pearsall RS, Underwood KW, Seehra J, Kumar R, Grinberg AV (2011) Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J Biol Chem 286 (34):30034–30046. 10.1074/jbc.M111.260133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber D, Kotzsch A, Nickel J, Harth S, Seher A, Mueller U, Sebald W, Mueller TD (2007) A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor. BMC Struct Biol 7:6 10.1186/1472-6807-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]


