Abstract
The pathogenesis of Parkinson’s disease (PD) involves the accumulation of aggregated α-synuclein, which has been suggested to begin in the gastrointestinal tract. Here, we determined the capacity of the appendix to modify PD risk and influence pathogenesis. In two independent epidemiological datasets, involving more than 1.6 million individuals and over 91 million person-years, we observed that removal of the appendix decades before PD onset was associated with a lower risk for PD, particularly for individuals living in rural areas, and delayed the age of PD onset. We also found that the healthy human appendix contained intraneuronal α-synuclein aggregates and an abundance of PD pathology–associated α-synuclein truncation products that are known to accumulate in Lewy bodies, the pathological hallmark of PD. Lysates of human appendix tissue induced the rapid cleavage and oligomerization of full-length recombinant α-synuclein. Together, we propose that the normal human appendix contains pathogenic forms of α-synuclein that affect the risk of developing PD.
INTRODUCTION
Parkinson’s disease (PD) is clinically diagnosed on the basis of motor symptoms that result from the progressive loss of midbrain dopaminergic neurons of the substantia nigra (1, 2). However, it is now recognized that PD is a multisystem disorder involving both motor and nonmotor features, involving the central and peripheral nervous system. Gastrointestinal (GI) dysfunction is a common nonmotor symptom of PD (3), often preceding the onset of motor symptoms by as many as 20 years (4). PD pathology, consisting of aggregated α-synuclein, has been detected in enteric neurons of the GI tract in patients with PD (5–8). α-Synuclein aggregates in enteric neurons can occur early in the disease, before motor symptom onset, during the prodromal phase of PD (5, 9). Pathogenic accumulation of α-synuclein in the GI tract not only may contribute to the nonmotor symptoms of PD but also has been hypothesized to contribute to PD pathology in the brain (10). Experimental studies have shown that misfolded α-synuclein can spread in a prion-like fashion from cell-to-cell, triggering the formation of Lewy-like aggregates in neurons (11, 12). A truncal vagotomy, in which the vagal nerve connecting the GI tract to the brain is severed, has been associated with lower PD risk in some, but not all, epidemiological studies (13–15). Because of the early appearance of α-synuclein aggregates in the GI tract of patients with PD (5, 9) and their capacity to ascend the vagal nerve to the brain (16, 17), it has been suggested that the GI tract could be the origin of PD pathology (10, 18–20).
Aberrant accumulation of α-synuclein in the GI tract occurs in response to toxins and bacteria that activate the immune system (19, 21–23). This may signify that GI tract regions with regular interactions with environmental pathogens and enhanced immuno-surveillance have a greater risk of developing α-synuclein abnormalities involved in PD. In agreement with this hypothesis, the appendix was recently shown to contain an abundance of α-synuclein in prodromal and clinical PD cases, as well as in neurologically intact individuals (5, 24). Although the appendix is often considered to be a vestigial organ, its mucosa is rich in immune cells, and a primary function of the appendix is to assist the lymphatic system in the detection and removal of pathogens (25), as well as to regulate intestinal bacterial composition (26). Consequently, the appendix may be prone to accumulating α-synuclein pathology that affects PD risk, although this has yet to be investigated in detail.
Pathogenic aggregation of α-synuclein is thought to involve the formation of kinetically stable fibrils of β sheet structure that can seed further aggregation (27). The aggregation of α-synuclein is a nucleation-dependent process that requires amino acids 61 to 95, referred to as the nonamyloid β component (NAC) (28). Truncated α-synuclein at the C terminus proximal to the NAC domain enhances nucleation-dependent aggregation (29, 30). Truncated α-synuclein proteoforms are found in Lewy bodies (31) and are enriched in the PD brain (32). Immune tissues, like the appendix, contain proteases capable of cleaving the C terminus of α-synuclein (33–35). However, it is unknown whether GI tract lymphoid tissues, like the appendix, have an enhanced capacity to generate truncated α-synuclein amylogenic seeds relevant to PD.
In this study, we investigated the role of the vermiform appendix in PD. We analyzed two independent, yet complementary, epidemiological datasets: the nationwide Swedish National Patient Registry (SNPR) and the Parkinson’s Progression Markers Initiative (PPMI). The SNPR is unique in its magnitude, registering diagnoses in the entire population, covering over 91 million person-years. The PPMI data contain detailed information about PD diagnosis, age of onset, and other demographic factors, as well as genetic information. Analysis of the SNPR showed that an appendectomy was associated with a lower risk of developing PD. In addition, in both datasets, we found that an appendectomy occurring decades before PD was associated with a delayed age of PD onset/diagnosis. Next, we identified an abundance of aggregated α-synuclein in the mucosa and enteric plexuses of the healthy human appendix. These aggregates were present at all age groups, including young individuals (<20 years of age). Furthermore, the appendix contained soluble oligomeric α-synuclein and truncated forms of α-synuclein that are prone to rapid aggregation. Truncated α-synuclein was present in the healthy appendix but was more abundant in the appendix of patients with PD. Thus, our data suggest that the appendix may play a role in PD. The presence of pathogenic α-synuclein species in the appendix indicates a mechanism by which the appendix may contribute to, and possibly trigger, the development of PD.
RESULTS
Appendectomy is associated with a lower risk of PD
We sought to understand the contribution of the GI tract lymphoid tissue and enteric neurons in the vermiform appendix to initiation and progression of PD. We first investigated whether removal of the appendix reduces the risk for PD in a large population. Our study examined the health records of a total of 1,698,000 individuals followed up to 52 years (91,888,589 person-years) to determine PD risk among individuals with and without a previous appendectomy, using data obtained from the SNPR and Statistics Sweden (SCB; table S1). The patient registry contains medical diagnoses and surgical codes for the Swedish population starting in 1964, which allowed for this long observation period. We identified a total of 551,647 individuals who had an appendectomy. Each of these was matched to two control individuals without an appendectomy (matched on the basis of birth year, sex, and geographical location). In the follow-up, we identified patients with PD (2252 in total) from the first hospitalization discharge or outpatient summary, excluding individuals with secondary parkinsonism (i.e., related to neuroleptic medications for schizophrenia) and diseases that might cause secondary parkinsonism, such as neurosyphilis.
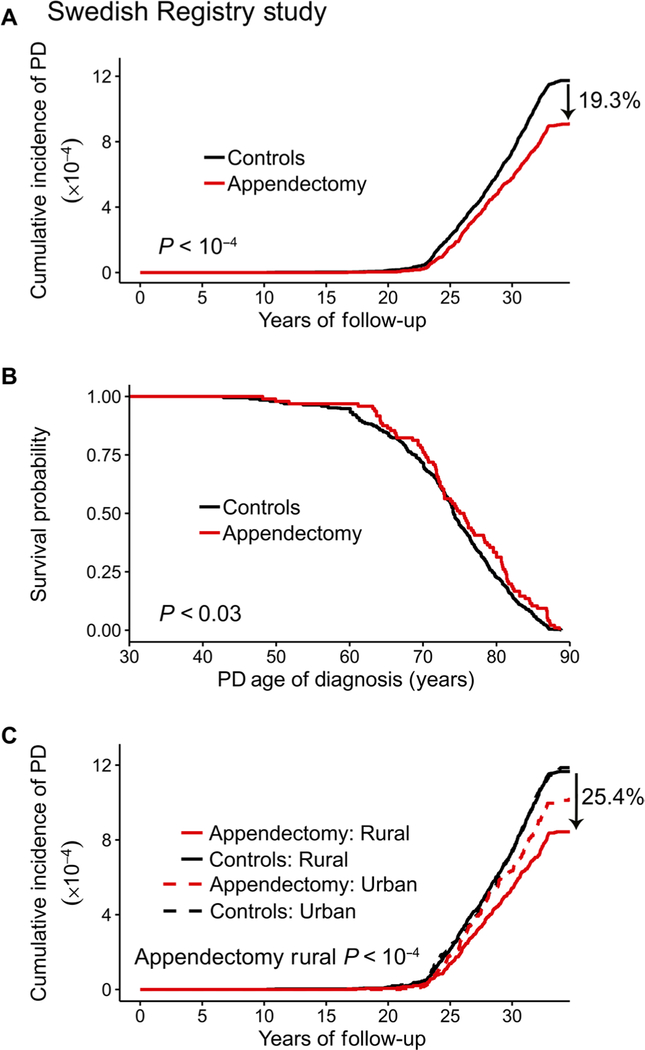
In individuals who had undergone an appendectomy, the overall risk for PD, as well as the age of PD diagnosis, was affected. First, the incidence of PD was lower in the appendectomy group compared to control individuals who did not have an appendectomy. PD incidence was 1.60 per 100,000 person-years among individuals who had an appendectomy [95% confidence interval (CI), 1.46 to 1.75] compared to 1.98 for controls (95% CI, 1.87 to 2.10; Fig. 1A). This signifies that the overall risk of developing PD was significantly decreased by 19.3% in appendectomized individuals compared to the general population (95% CI, 10.4 to 27.2%; P < 10−4; Fig. 1A). Second, the cumulative prevalence of PD (total proportion of cases) in people who had an appendectomy was also lower by 16.9% relative to the general population (95% CI, 9.3 to 24.4%; P < 10−4; Table 1). In total, PD was diagnosed in 1.17 out of every 1000 people who had an appendectomy (95% CI, 1.08 to 1.27) compared to 1.4 per 1000 in the general population (95% CI, 1.34 to 1.47). Third, we examined, among PD cases, whether a history of appendectomy was associated with a delayed age at first recorded diagnosis of PD. The age of PD diagnosis was, on average, 1.6 years later in individuals who had an appendectomy occurring 20 or more years prior than in cases without an appendectomy (95% CI, −0.09 to 3.34; P < 0.03; Fig. 1B, Table 1, and table S2). We also observed a significant delay in age of PD onset in individuals with an appendectomy 30 or more years prior (P < 0.006; table S2), but a limited size in this longer latency group precluded further analysis.
Fig. 1. An appendectomy reduces the risk of PD in a general population.
Data from the SNPR involving 1,698,000 individuals. (A) Cumulative incidence plot showing the rate of PD diagnosis in individuals who previously had an appendectomy and matched nonappendectomized controls. Incidence rate (PD cases per 100,000 person-years) in appendectomized individuals (n = 551,003 without PD, n = 644 with PD) was 1.60 (95% CI, 1.46 to 1.75); in the general population (n = 1,144,745 without PD, n = 1608 with PD), the PD incidence rate was 1.98 (95% CI, 1.87 to 2.10). (B) Age at PD diagnosis in individuals who had an appendectomy 20 or more years prior relative to nonappendectomized controls. n = 101 patients with PD with an appendectomy and n = 658 patients with PD without this surgery; hazard ratio, 0.793 (95% CI, 0.642 to 0.980). (C) Cumulative incidence plot showing the rate of PD diagnosis in appendectomized and nonappendectomized individuals living in rural or urban regions of Sweden. Incidence rate (PD cases per 100,000 person-years) in appendectomized individuals in the rural population: 1.49 (95% CI, 1.31 to 1.68); in appendectomized individuals in the urban population: 1.77 (95% CI, 1.55 to 2.02); in the general rural population: 2.00 (95% CI, 1.87 to 2.15); in the general urban population: 1.97 (95% CI, 1.79 to 2.16).
Table 1.
Summary of data from the SNPR and SCB for individuals with or without an appendectomy.
| Prevalence of PD in the general population | No PD diagnosis, n | PD diagnosis, n | Regression coefficient | Odds ratio (95% CI) | P | |
|---|---|---|---|---|---|---|
| All | Appendectomy | 551,003 | 644 | −0.18 ± 0.05 | 0.831 (0.756–0.907) | 8.1 × 10−5 |
| Controls | 1,144,745 | 1608 | ||||
| Rural | Appendectomy | 342,209 | 373 | −0.26 ± 0.06 | 0.769 (0.681–0.867) | 1.5 × 10−5 |
| Controls | 703,075 | 997 | ||||
| Urban | Appendectomy | 208,794 | 271 | −0.06 ± 0.07 | 0.939 (0.817–1.082) | 0.383 |
| Controls | 441,670 | 611 | ||||
| Age at PD diagnosis | Diagnosis age (years)* | Hazard ratio (95% CI) | ||||
| Appendectomy >0 years before PD | ||||||
| All | Appendectomy | 479 | 74.93 ± 0.51 | 0.990 (0.890–1.101) | 0.851 | |
| Controls | 1176 | 74.90 ± 0.31 | ||||
| Rural | Appendectomy | 277 | 75.09 ± 0.60 | 0.989 (0.860–1.138) | 0.877 | |
| Controls | 686 | 74.53 ± 0.41 | ||||
| Urban | Appendectomy | 202 | 74.71 ± 0.88 | 0.965 (0.818–1.137) | 0.666 | |
| Controls | 490 | 75.42 ± 0.47 | ||||
| Appendectomy ≥20 years before PD | ||||||
| All | Appendectomy | 101 | 74.85 ± 0.81 | 0.793 (0.642–0.980) | 0.027 | |
| Controls | 658 | 73.23 ± 0.33 | ||||
| Rural | Appendectomy | 63 | 74.09 ± 1.09 | 0.753 (0.575–0.988) | 0.034 | |
| Controls | 417 | 72.12 ± 0.44 | ||||
| Urban | Appendectomy | 38 | 76.12 ± 1.15 | 0.802 (0.567–1.135) | 0.202 | |
| Controls | 241 | 75.14 ± 0.45 | ||||
Means ± SEM.
Numerous epidemiological studies show that rural living is associated with a higher risk for PD, potentially because of the higher exposure to pesticides, which may contribute to the development of PD (36, 37). Therefore, we further examined whether the association between appendectomy and PD was different between individuals living in rural and urban areas of Sweden. We defined a rural area as a municipality containing less than 100,000 individuals and a population density that is below 200 people/km2. We found that an appendectomy had the greatest effect on rural residents. Individuals with an appendectomy living in rural areas had a significant 25.4% decrease in the risk of PD (95% CI, 14.1 to 35.6%; P < 10−4), but there was no benefit of an appendectomy for those dwelling in urban areas (P = 0.22; Fig. 1C, Table 1, and table S3). Among rural residents, an appendectomy 20 years prior was also associated with a significant delay in the age of PD diagnosis (P < 0.04), but again, no difference was found for urban residents (P = 0.20; Table 1). These findings suggest that appendectomy influences environmental risk factors for PD, specifically for individuals living in rural environments.
Appendectomy is associated with a delayed age of PD onset
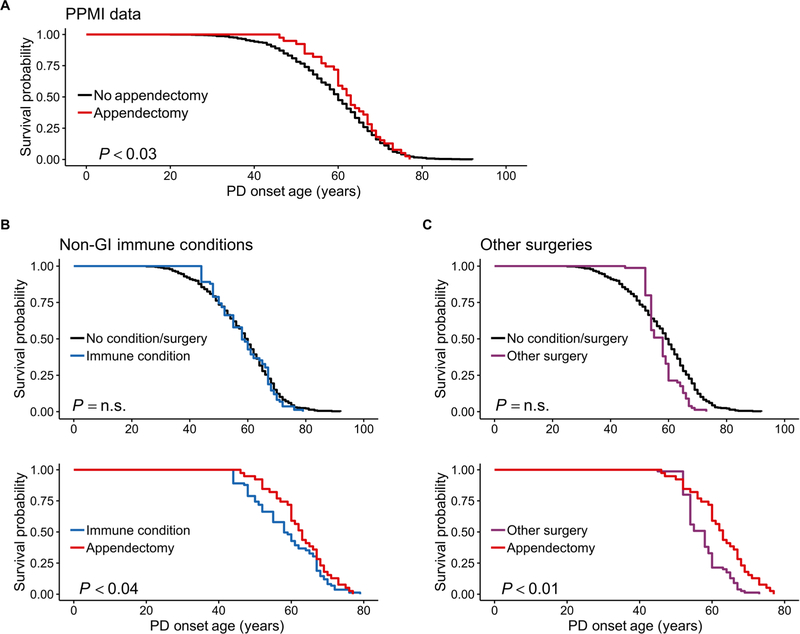
We further examined whether an appendectomy affected PD outcome using a large comprehensive clinical PD dataset, the PPMI study. In this dataset, there were 849 PD cases who met the rigorous diagnostic criteria for PD in the PPMI study, including DaTscan imaging evidence of dopamine deficiency (table S4). Previous medical history was ascertained as part of the study enrollment interview, including appendectomy and date of surgery. In the primary analysis, we compared the age of PD onset between individuals with (n = 54, 6.4% of patients with PD) and without a previous history of appendectomy. We then conducted two sensitivity analyses to examine whether our findings were specific to appendectomy, by examining the age of PD onset in individuals with a non–GI tract immune condition or surgery other than appendectomy. Given that the prodromal phase of PD can be decades long (4, 38), we considered the number of years that the appendectomy preceded PD onset and chose to focus on people who had undergone appendectomy ≥30 years before being diagnosed with PD.
Age of PD onset was significantly delayed, on average, by 3.6 years, in individuals who had an appendectomy at least 30 years before disease onset, relative to individuals without this surgery (95% CI, 1.03 to 6.13; P < 0.03; Fig. 2A and table S5). In contrast, the onset of PD was not associated with immune conditions that did not involve the GI tract (Fig. 2B and table S6), supporting the specific relevance of GI tract lymphoid tissue to PD. Furthermore, the delay in PD onset in patients with an appendectomy was not related to the surgical event itself, as confirmed by the analysis of cases with other age-matched surgeries (Fig. 2C and table S6). Last, once individuals developed PD, the severity of PD symptoms did not differ between those who had an appendectomy and those without this surgery, as determined by the unified PD rating scale (UPDRS) and the Hoehn and Yahr scale. This suggested that the appendix might primarily modify events before clinical onset of symptoms (table S7).
Fig. 2. Epidemiological analysis of PPMI data shows that an appendectomy delays the age of PD onset.
(A) Age of PD onset in patients who had an appendectomy 30 or more years prior relative to that of nonappendectomized patients. n = 39 patients with PD with an appendectomy (mean PD age of onset, 62.6; 95% CI, 60.1 to 65.0) and n = 780 PD controls without this procedure (mean age of PD onset, 59.0; 95% CI, 58.2 to 59.7). (B and C) PD age of onset in patients with PD with an immune condition that does not involve the GI tract (B) or a surgery that is not appendectomy (C). Patients with PD were matched on the basis of age of condition/surgery (i.e., age of appendectomy, non-GI immune condition diagnosis, and age of other surgery), sex, ethnicity, number of education years, family history, and mutation status. The effect of appendectomy, non-GI immune conditions, or other surgery occurring 30 or more years before PD was examined. Top panels show the age of PD onset in non-GI immune conditions or other surgeries compared to patients with PD without such conditions/other surgeries. Bottom panels show age of PD onset in patients who had an appendectomy relative to patients with non-GI immune conditions or other surgeries. Mean age of PD onset for the appendectomy group (n = 39), the non–GI tract immune condition group (n = 39), the other surgery group (n = 22), and the no-condition/surgery group (n = 366) is 62.6 (95% CI, 60.1 to 65.0), 58.6 (95% CI, 55.7 to 61.5), 57.7 (95% CI, 55.4 to 59.9), and 58.0 (95% CI, 56.8 to 59.2), respectively. n.s., not significant.
We further explored the interaction of appendix tissue with genetic and environmental PD risk factors. For our analysis, we assessed patients with PD with a moderate family history, defined as one to two affected family members (parents/siblings), because this group did not show an age of onset bias relative to individuals without family history (fig. S1). An appendectomy delayed age of PD onset in individuals with a family history of PD (P < 0.01) but had no effect in individuals without a family history of PD (fig. S2). Families can share both heritable and environmental risk factors for PD. We found that carriers of a common mutation relevant to familial PD (in α-synuclein, leucine-rich repeat kinase 2, or β-glucocerebrosidase) did not benefit from the removal of the appendix (fig. S2). This suggests that an appendectomy may be more protective against non-genetic, i.e., environmental, causes of PD.
The healthy human appendix contains pathology-associated α-synuclein
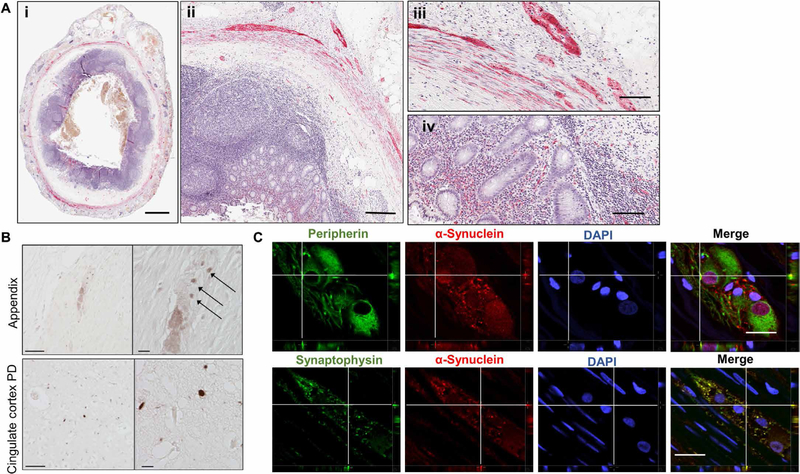
We next used immunohistochemistry to determine whether α-synuclein pathology is present in the appendix of the general population (n = 48 individuals without PD) (5, 39). We pretreated sections of appendix tissue with proteinase K, which digests free physiological α-synuclein, while leaving aggregated α-synuclein intact (5, 39). We found abundant proteinase K–resistant α-synuclein aggregates in the mucosa, submucosal plexuses, myenteric plexuses, and nerve fibers of the appendix in nearly all individuals (46 of 48 subjects examined; Fig. 3A, i to iv, and fig. S3A). We also found punctae immunoreactive for phosphorylated α-synuclein, similar to those observed in postmortem PD brain tissue (Fig. 3B). The α-synuclein aggregates were equally abundant in normal, acutely inflamed, and chronically inflamed appendiceal tissue (table S8) and, surprisingly, were apparent in both young (≤20 years old) and older individuals (aged 48 to 84; table S8). Large fibrillar α-synuclein aggregates are known to be thioflavin S positive, whereas small nonfibrillar aggregates (protofibrils) of α-synuclein are thioflavin S negative (40). In the appendix tissue samples, we found that the α-synuclein aggregates did not bind to thioflavin S (fig. S3B), which is characteristic of smaller, nonfibrillar aggregates (40).
Fig. 3. α-Synuclein pathology is prevalent in the healthy human appendix.
(A) Proteinase K–resistant α-synuclein (red) in the appendix of healthy individuals. Hematoxylin was used as a nuclear counterstain (purple). Tissue distribution of proteinase K–resistant α-synuclein aggregates in a representative cross section of human appendix (i and ii), in the muscularis externa (iii), and mucosa of the appendix (iv). Scale bars, 2 mm (i), 250 µm (ii), and 100 µm (iii and iv). (B) Phosphorylated α-synuclein in the human appendix. Sections were probed for α-synuclein phosphorylated at serine 129 (pSer129) using antibody AB51253 and detected with peroxidase-conjugated antibodies. Top panels depict plexus containing pSer129 puncta (arrows). Bottom panels depict pSer129 staining in the cingulate cortex in PD patient brain tissue. Scale bars, 50 µm (left) and 10 µm (right). (C) Cellular localization of human α-synuclein aggregates. Proteinase K–digested tissue sections were probed with antibodies against peripherin (top panels, green) or synaptophysin (bottom panels, green). Sections were also probed for proteinase K–resistant α-synuclein (red) and 4′,6-diamidino-2-phenylindole (DAPI) stain (blue). Sections were imaged by confocal microscopy. Depicted are orthogonal projections for each emission channel individually and with all channels merged in the last panel. Fluorescent signal in the z axis is depicted for the area of interest (crosshairs). All images are representative of staining done on n ≥ 9 individuals. Scale bars, 25 µm.
To define the precise localization of α-synuclein aggregates, we used double immunolabeling and confocal microscopy (Fig. 3C). In peripherin-immunoreactive neurons, aggregated α-synuclein was observed in both perinuclear and nuclear regions and occasionally in neurites (Pearson’s coefficient of colocalization for proteinase K– resistant α-synuclein and peripherin: 0.31 ± 0.02). In synaptophysin-immunostained sections through the appendix, α-synuclein aggregates were colocalized with synaptophysin-positive presynaptic terminals (Pearson’s coefficient of colocalization for proteinase K–resistant α-synuclein and synaptophysin: 0.64 ± 0.05; Fig. 3C). Therefore, the appendix from people not affected by PD was enriched for intraneuronal α-synuclein aggregates that are analogous to those seen in the PD brain (39).
The human appendix contains truncated α-synuclein proteoforms
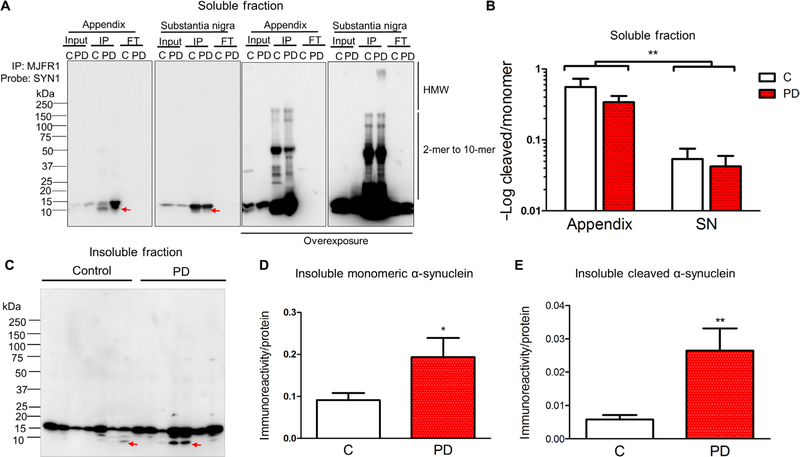
We sought to further characterize α-synuclein proteoforms in the appendix. We used surgical samples of healthy human appendix and compared these to PD appendix and substantia nigra tissue from healthy individuals and patients with PD. All assays were performed in the presence of protease inhibitors and at 4°C that effectively prevented α-synuclein proteolysis during tissue processing (fig. S4, A and B). We first performed a Triton X-100 solubility assay that found a similar abundance of soluble α-synuclein in healthy control appendix tissue (n = 5) and PD patient appendix tissue (n = 6; fig. S4, C and D). We then enriched for soluble α-synuclein from the appendix by immunoprecipitation and compared it to α-synuclein in the substantia nigra for both healthy individuals and patients with PD. There were several apparent high–molecular weight (25, 32, 37, 50, 145, and 160 kDa) SDS-resistant α-synuclein oligomers in the appendix and substantia nigra tissue samples of healthy individuals and patients with PD (n = 4 to 8 individuals per group; Fig. 4A). In the appendix, there was an enrichment of a truncated α-synuclein fragment (about 10 kDa) when compared to the sub-stantia nigra [12.9and 10.2-fold increase in the healthy and PD appendix, respectively; main effect of tissue: F(1,17) = 9.98, P < 0.006; Fig. 4B]. Truncated α-synuclein was observed in both the healthy and PD appendix. Therefore, the appendix contains an abundance of SDS-resistant oligomers and truncated α-synuclein, analogous to what is seen accumulating in Lewy bodies in PD (31, 41).
Fig. 4. The appendix contains an abundance of truncated α-synuclein proteoforms.
(A) Representative blot showing immunoprecipitation of α-synuclein from the Triton X-100–soluble fraction of substantia nigra (SN) and appendix of control individuals (C) and patients with PD (PD). α-Synuclein immunoprecipitation (IP), 20 µg of protein of the Triton X-100–soluble fraction (input), and 20 µg of protein of the remaining sample after immunoprecipitation (FT) were resolved on SDS–polyacrylamide gel electrophoresis (PAGE) and immunoblotted with the anti–α-synuclein antibody SYN1 (clone 42/α-synuclein). Low and high exposure included to show all immuno-reactive proteoforms. HMW, high molecular weight. (B) Densitometric analysis showing the ratio of α-synuclein cleavage product to monomer in the Triton X-100–soluble fraction. n = 8 healthy control appendix, n = 5 PD appendix, n = 4 healthy control substantia nigra, n = 4 PD substantia nigra. (C) Blot showing Triton X-100–insoluble fractions from appendix tissues. Triton X-100–insoluble fractions were extracted with 8 M urea and blotted using an antibody against α-synuclein (MJFR1). The relative abundance of (D) monomeric and (E) cleaved α-synuclein was determined by densitometric analysis. α-Synuclein immunoreactivity was normalized to in-gel protein abundance as measured by Coomassie blue staining. Data are representative of n = 8 healthy controls and n = 6 patients with PD. Red arrows highlight the position of cleavage product. *P < 0.05, **P < 0.01 by one-way or two-way analysis of variance (ANOVA).
In the PD brain, α-synuclein aggregates are commonly detected in the Triton X-100–insoluble fraction (42). Therefore, we measured the abundance of α-synuclein in the insoluble fraction from the appendix of healthy individuals (n = 8) and patients with PD (n = 6). We found increased monomeric α-synuclein in the PD appendix relative to the appendix of healthy controls [2.1-fold increase in PD; F(1,12) = 5.4, P < 0.04; Fig. 4, C and D, and fig S5]. Moreover, we detected higher levels of truncated α-synuclein (10 and 12 kDa) in the PD appendix than in the appendix of healthy individuals [4.5-fold increase in PD; F(1,12) = 12.0, P < 0.005; Fig. 4, C and E, and fig S5]. Therefore, the human appendix contains an abundance of insoluble α-synuclein similar to what has been found in the PD brain (42).
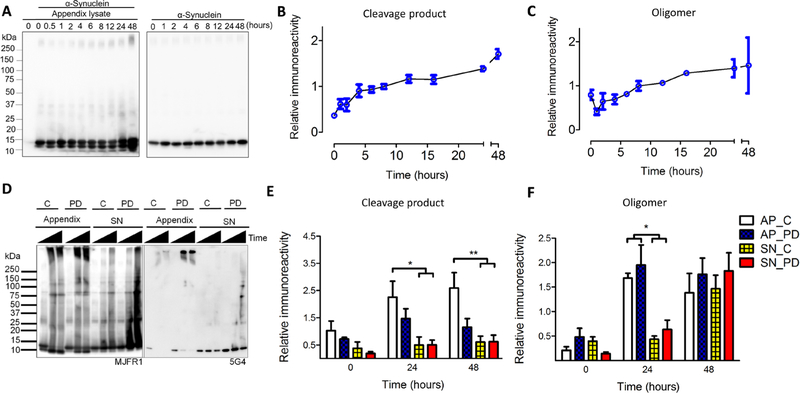
α-Synuclein is rapidly cleaved and aggregates in appendix lysates
α-Synuclein proteoforms in the GI tract have been suggested to act as seeds for pathogenic aggregation (18, 32). We defined whether α-synuclein from healthy human appendix could seed aggregation of recombinant α-synuclein using an in vitro assay. For this, we used a shaking assay where we “spiked” tissue lysates with purified full-length human α-synuclein and monitored cleavage and aggregation across time by SDS-PAGE. We found full-length α-synuclein to be both actively cleaved and subsequently aggregated as a function of time when appendix lysates were present (Fig. 5, A to C, and fig. S6). SDS-resistant oligomers were most abundant 24 and 48 hours after addition of appendix tissue lysate to the recombinant α-synuclein (1.8-fold increase in oligomers over 48 hours; P < 0.003). Proteolytic cleavage resulted in at least two apparent α-synuclein truncation products at ~12 and 10 kDa (4.7-fold increase in cleavage product over 48 hours; P < 0.001). α-Synuclein has been suggested to undergo proteolytic cleavage by matrix metalloproteinases, cysteine or serine proteases (such as calpain 1, neurosin, and caspase-1), the aspartate protease cathepsin D, and, most recently, asparagine endopeptidase (33–35). In the presence of a cocktail of inhibitors of all or some of these proteases, we found cleavage to be highly limited (fig. S7).
Fig. 5. Rapid cleavage and aggregation of α-synuclein in the human appendix.
Triton X-100–soluble appendix lysates were combined with purified, full-length recombinant human α-synuclein and then vigorously shaken for up to 48 hours. α-Synuclein proteoforms were detected on SDS-PAGE by immunoblotting with MJFR1 antibody. (A to C) The formation of α-synuclein cleavage products and oligomers over a 48-hour time course. Representative blot (A) and densitometric analysis of cleavage products (B) and oligomers (C), n = 4 healthy appendix samples. (D to F) Time course using tissue lysate from appendix (AP) and substantia nigra (SN) from healthy individuals (C) and patients with PD. (D) Representative blots of shaking assay performed with purified α-synuclein combined with appendix or substantia nigra lysates. Left blot probed with anti-α-synuclein antibody (MJFR1); right blot probed with α-synuclein aggregate-preferring antibody (5G4). Time scale is 0, 24, and 48 hours. Densitometric analysis of cleavage products (E) and oligomers (F), n = 3 to 4 samples per group. Repeated-measures ANOVA for cleavage products showed a main effect of time [F(2,36) = 5.0, P < 0.05] and tissue [F(1,36) = 31.6, P < 10−5]; repeated-measures ANOVA for oligomers showed a main effect of time [F(2,30) = 30.0, P < 10−7] and tissue [F(1,30) = 9.3, P < 0.005]. *P < 0.05, **P < 0.01 by post hoc Tukey test.
Next, we performed the aggregation assay with tissue lysates from appendix or substantia nigra of healthy individuals and patients with PD to define whether α-synuclein cleavage or aggregation is influenced by tissue type or disease (Fig. 5, D to F). The healthy appendix was found to rapidly accumulate a higher amount of α-synuclein cleavage product than did the healthy and PD substantia nigra after 24 and 48 hours (4.2-to 4.5-fold more in the healthy appendix at 24 and 48 hours; P < 0.05). The healthy and PD appendix showed an accelerated formation of SDS-resistant α-synuclein oligomers relative to the healthy and PD substantia nigra (3.1-and 3.9-fold more in the appendix at 24 hours; P < 0.05); levels of oligomers in the substantia nigra reached those of the appendix only after 48 hours (Fig. 5, D to F). Immunoblotting with an antibody (5G4) that preferentially detected pathological aggregates of α-synuclein showed that several α-synuclein species formed (>250 kDa and 37 to 150 kDa) were conformationally similar to pathological aggregates (Fig. 5D).
The healthy human appendix contains N-and C-terminal truncated α-synuclein
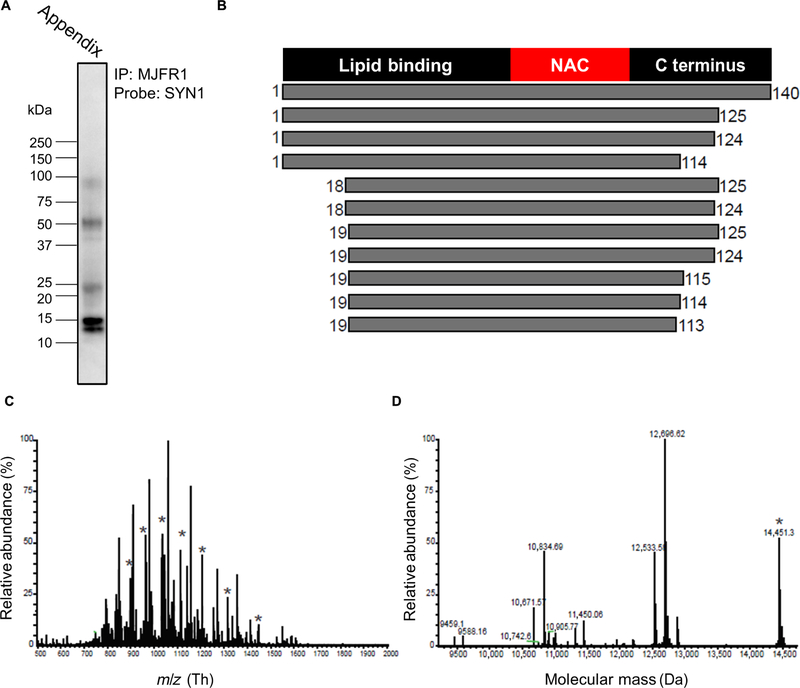
We used a top-down mass spectrometry (TD-MS) approach to identify the α-synuclein proteoforms in the healthy human appendix. α-Synuclein immunoprecipitations included a cocktail of protease inhibitors (as above) to prevent artifactual proteolysis. The characteristic bands of α-synuclein proteoforms in the appendix were confirmed by immunoblotting (Fig. 6A). Subsequent TD-MS analysis identified 11 α-synuclein proteoforms (Fig. 6, B to D, and fig. S8). These included full-length α-synuclein and 10 forms that were truncated at the C terminus at amino acids 113/114/115 or 124/125. Several proteoforms were also truncated at the N terminus at amino acids 18/19. In all identified proteoforms, the NAC domain was intact. Therefore, α-synuclein cleavage products with the aggregation-prone NAC domain were prominent in the healthy human appendix.
Fig. 6. Identification of α-synuclein truncation products in the human appendix using TD-MS.
(A) Blot showing α-synuclein immunoprecipitated from the healthy human appendix. (B) Graphical representation of the α-synuclein proteoforms identified by TD-MS. Analysis of the TD-MS fragmentation data by TDPortal identified 11 α-synuclein proteoforms present in the human appendix (n = 1 individual), corresponding to full-length α-synuclein and 10 α-synuclein truncation products. α-Synuclein proteoforms in the appendix are depicted with the amino acid start and end position marked. (C) MS spectrum shows full-length α-synuclein (marked with “*”) in addition to truncation products. m/z, mass/charge ratio. (D) Deconvoluted spectrum showing relative abundance of the α-synuclein proteoforms in the human appendix and their respective protein masses.
DISCUSSION
Recent studies have implicated α-synuclein misfolding and the GI tract in the etiopathogenesis of idiopathic PD. Here, we established, in two independent epidemiological datasets with large sample sizes (a general population of 1.6 million in the SNPR study and 849 patients with PD in PPMI), that an appendectomy occurring decades before disease onset was protective against PD. In the SNPR study, we found that an appendectomy was associated with a substantial decrease in the risk for PD and postponed the age of diagnosis. In the PPMI study, the age of PD symptom onset was delayed by 3.6 years in individuals with an appendectomy. Together, this evidence supports the hypothesis that the appendix plays a role in the development or triggering of PD.
We then analyzed appendix tissue obtained from people not diagnosed with PD who underwent routine appendectomies and found that they contained high levels of intraneuronal α-synuclein aggregates. The tissue was also rich in truncated forms of α-synuclein, analogous to those seen in Lewy body pathology in PD. Appendix tissue from healthy individuals induced a rapid aggregation of recombinant monomeric α-synuclein in vitro. Collectively, our results suggest a model where pathological α-synuclein species, which are capable of seeding α-synuclein aggregates, are formed within the appendix. Our results signify that the appendix is a reservoir for pathogenic forms of α-synuclein, which may contribute to PD initiation and development.
In 2014, Gray et al. (24) reported that α-synuclein is present in the appendiceal mucosa of neurologically intact people and suggested that the impact of appendectomy on PD should be tested. Subsequent epidemiological studies have yielded conflicting results on the effect of an appendectomy on PD risk and age of onset (43–46). Specifically, an initial case-control study suggested that appendectomy was associated with a delayed onset of late-onset PD (43). However, three subsequent studies largely found a null association with either PD risk or age of onset (44–46). Two of these used a similar study design to our SNPR study, i.e., a comparison of PD risk between individuals with and without appendectomy using large medical record databases. In the Canadian study, Marras et al. (44) reported a higher PD incidence within 5 years of appendectomy but not there-after. The authors attributed this increase to ascertainment bias and further acknowledged that the study had a relatively short follow-up time (up to 17 years) that was inadequate for determining the effects of an appendectomy in early life on PD (44). In a Danish registry study, which also largely showed a null association, Svensson et al. (46) highlighted the many challenges in examining the role of the appendix or appendectomy in PD development. For example, because the accumulation of aggregation-prone α-synuclein in the appendix is hypothesized to trigger a decades-long PD pathogenic process, epidemiological studies need to carefully account for temporal relationships between appendectomy and PD. An appendectomy typically occurs in early adulthood, and as a result, a complete ascertainment of appendectomy history and adequate length of follow-up are important but challenging in many study settings. Furthermore, epidemiological registry studies need to rely on the age at first recorded PD diagnosis, which is often delayed relative to the age at PD motor symptom onset. Thus, the time lag between onset of motor symptoms and clinical diagnosis of PD may lead to an underestimation of the effects of appendectomy on PD. The current study faced the same challenges; however, we approached the research question with two complementary studies. Our analysis using the SNPR was considerably larger and had a longer follow-up period than did previously published studies (43–46). In addition, the results were complemented by the PPMI study, which contained carefully collected clinical data from well-characterized patients with PD. Overall, the two studies consistently indicated that appendectomy in early life may be protective against PD development. Further, well-designed epidemiological studies with large sample sizes, long follow-up periods, and accurate exposure and outcome assessments should be performed to confirm the effect of appendectomy on PD risk and age of onset.
In the SNPR, we had access to data on over 1.6 million individuals who had been followed up for up to 52 years (resulting in a total of 91 million person-years). This large population-based study (which included 551,647 appendectomized individuals) revealed that there is a decreased absolute risk of PD by 19.3% in individuals who have previously had an appendectomy. In addition, we found that the age at first hospital diagnosis of PD was delayed (by 1.6 years) in people with PD who had undergone appendectomy at least 20 years prior. Upon further analysis, an appendectomy was protective against PD for individuals living in rural areas but not for those in urban communities. In rural areas, PD is linked to environmental factors, particularly to the use of pesticides (36, 37, 47). Hence, our findings suggest that the involvement of the appendix in the development of PD may be modified by environmental risk factors. Together, the evidence from our large population-based study shows that the appendix affects both the absolute risk of PD and age at PD diagnosis. The effect was most pronounced when appendectomy was performed early in people living in rural areas, which implies that the appendix could function in the mechanisms triggering PD pathology and that the actual trigger could be environmental in origin.
In the PPMI study, patients with PD who had an appendectomy decades earlier (≥30 years) had an onset of motor symptoms that was, on average, 3.6 years later than patients with PD who had not undergone an appendectomy. This association was specific to appendectomy, as it was not observed in patients with an age-matched surgery or non-GI immune condition. An appendectomy did not delay PD onset in individuals who carried PD mutations in α-synuclein, leucine-rich repeat kinase 2, or β-glucocerebrosidase. By contrast, patients with PD who had family members with PD (a parent or sibling) had a delay in symptom onset after an appendectomy. In these individuals, genetic factors may have a role, but given the absence of an effect of appendectomy in carriers of PD mutations, a possible explanation is that the beneficial effects of an appendectomy may only be apparent in individuals with environmental causes of PD. Hence, both the PPMI and SNPR findings tentatively suggest that the effect of an appendectomy is most protective in cases where there is an underlying environmental trigger of PD.
We detected α-synuclein aggregates in the appendix of young and old people (infants to 84 years of age), both those diagnosed with PD and those without neurological disease, and in individuals with a normal appendix and in individuals with an inflamed appendix (i.e., incidental appendectomy and appendectomy due to clinical signs of appendicitis). These α-synuclein deposits were proteinase K resistant and, in a separate analysis, were immunoreactive for α-synuclein phosphorylated at serine 129. We call these α-synuclein accumulations aggregates based on their resistance to proteinase K degradation and found that they were enriched in nerve terminals of the enteric plexuses of the appendix. Although the majority of pathology was associated with neuronal structures, it is possible that proteinase K–resistant α-synuclein is also present in other non-neuronal cell types. Enteric plexuses in the appendix are innervated by the vagal nerve, which has previously been suggested to act as a conduit for the spread of aggregated α-synuclein from the gut to the brainstem (10, 48, 49). The initial impetus for this concept came from observations of α-synuclein aggregates in other parts of the gut of patients with PD (7, 50–52). The concept was supported by a recent report that the vermiform appendix contains high levels of total α-synuclein (24) and a study that discovered phosphorylated and aggregated forms of α-synuclein in the healthy and PD appendix (5). When we characterized the α-synuclein present in the appendix of healthy people and patients with PD, we invariably found an unexpected abundance of truncated α-synuclein forms, exceeding the quantities we observed in the substantia nigra of both patients with PD and normal subjects. Thus, a high degree of proteolytic turnover of α-synuclein is a likely feature of the appendix. Aberrant proteolytic turnover of α-synuclein has been hypothesized to play a role in PD pathogenesis, and several proteases that cleave α-synuclein have been identified (33–35). α-Synuclein truncation products are enriched in Lewy bodies (53–55) and can serve as potent seeds for further α-synuclein aggregation in experimental model systems (56). Thus, the appendix is rich in α-synuclein proteoforms relevant to PD.
When we added lysates of appendix tissue to recombinant α-synuclein monomers in vitro, we found that small oligomers (<250 kDa; i.e., not full-length fibrils) and cleavage products formed rapidly. We also determined that the cleavage products in the human appendix were a combination of N-and C-terminal truncations. 03B1-Synuclein truncation products identified in the appendix contained an intact NAC domain, which is necessary and sufficient for α-synuclein aggregation (27, 28). Because truncation at the C and N termini of α-synuclein enhances aggregation kinetics of α-synuclein in vitro and in vivo (30, 32, 56), their abundance in the appendix would likely make this tissue prone to PD pathology. Further-more, proteoforms lacking the membrane-binding and -stabilizing N terminus may have greater propensity to travel within and potentially across neurons (57, 58). α-Synuclein cleavage fragments in the appendix may also exacerbate the formation of other pathogenic conformers (32, 56, 59). We found that in appendix tissue lysates, the formation of α-synuclein cleavage products closely paralleled the production of oligomers. Accumulating evidence supports that oligomeric α-synuclein spreads more efficiently across neurons than do higher-order molecular fibril assemblies (60, 61). Furthermore, retrograde transport of oligomers from gut to brain via the vagal nerve has been demonstrated in rodents in vivo (16). Thus, the proteolytic turnover of α-synuclein in the appendix might be important in generating protein conformers that are readily able to undergo axonal transport to the brainstem.
There are some limitations to our study. Although we show that the vermiform appendix is abundant in pathological forms of α-synuclein, we do not present data from other peripheral tissues, so it is unclear whether other GI tissues rich in immune cells, besides the appendix, also contain pathological α-synuclein. Regardless, appendectomy alone affected PD risk and age of onset, and therefore, this tissue contributes to PD, even if the effect is not wholly unique to the vermiform appendix. Another limitation is that the genetic screening of patients in the PPMI dataset did not include all known PD risk alleles, and therefore, it is plausible that undetermined genetic risk factors play a role in modulating the risk associated with appendectomy.
In addition, the two epidemiological datasets used in this study have different, but complementary, strengths and limitations. The SNPR study is nationwide and has the strengths of accurate appendectomy hospital admission data and a large number of PD cases and controls to examine association of appendectomy and the risk for PD. Although a large epidemiological registry study, the SNPR study has limitations in PD diagnosis accuracy. An earlier study comparing diagnoses identified from the SNPR with clinical evaluations demonstrated a 70.8% accuracy in PD diagnosis (62). Further, the date of PD identification depended on hospitalization and clinical visits, which may have occurred later than the clinical symptom onset of PD (62). By contrast, in the PPMI, PD diagnosis is robust with detailed clinical examinations and DaTscan evidence, and its age at onset information is likely reliable (63). However, the PPMI participants represent a select group of patients with PD who are committed to multiple comprehensive clinical examinations, neuro-imaging, and longitudinal follow-ups, and the diagnostic accuracy is likely higher than among patients with late-onset sporadic PD. Also, in the PPMI, appendectomy surgery is based on patient recollection. Although this can be viewed as a limitation, emergency room visits or hospital inpatient admissions (as would be required for an appendectomy) have been shown to be highly reliable in self-reporting versus administrative data comparisons (>90%) (64), probably in part due to the characteristic surgical scar left by the operation. As early disease stage (<2 years) is an inclusion criterion for patients with de novo PD in the PPMI, we expect that most patients with PD would have sufficient cognitive integrity to reliably recall this information. In support of this, the average age of appendectomy (22.07 ± 0.28) and appendectomy prevalence (6.4%) in the PPMI cohort are in agreement with the reported average age for an appendectomy (21 to 30 years old) and lifetime risk of appendicitis (6 to 9%) in the general population (65–71). Overall, consistent findings from two independent populations lend support for a potential protective role of appendectomy in PD.
GI symptoms are a salient feature of prodromal PD, which may coincide with the development of α-synuclein pathology in the gut. However, reports concerning the distribution and prevalence of α-synuclein pathology throughout gut tissues have been conflicting (72). A neuropathological study of 92 autopsy cases failed to find α-synuclein pathology in the gut in the absence of central nervous system (CNS) pathology (50, 72), although such prior studies did not examine the appendix. Here, we show an abundance of α-synuclein aggregates in the vermiform appendix of healthy individuals across a wide range of chronological ages. Our findings demonstrate that α-synuclein aggregates are present in the appendix of healthy individuals (including young people under 20 years old) who presumably lack CNS pathology. This signifies that α-synuclein aggregation in the appendix is a common phenomenon (much more common than PD) and therefore is not likely to be implicitly disease causing. Rather, it suggests that other biological processes that suppress the spread of α-synuclein aggregates or are responsible for the clearance of α-synuclein aggregates in the appendix may be crucial determinants in PD development. Hence, the abundance of α-synuclein aggregates in the healthy appendix signifies that, in certain individuals, there might be a “secondary hit” that aids in the accumulation and uninhibited spread of α-synuclein from the gut to the brain, eventually causing PD. In addition, removal of the appendix may reduce GI tract inflammation and thereby reduce systemic inflammatory cytokines, which contribute to PD as recent studies show (73–75).
Therapeutic strategies preventing the pathological accumulation of α-synuclein have been a major focus of researchers (76, 77). Unfortunately, the molecular details that precede pathological aggregation in vivo have remained elusive, which subsequently impedes the development of effective therapeutics. Data here suggest that the proteolytic cleavage of α-synuclein in the gut may be a viable target for therapeutics. Selective protease inhibitors, specifically for metalloproteases, have been developed and have undergone U.S. Food and Drug Administration testing for the treatment of cancer (78). Repurposing of these drugs to target the proteolytic cleavage of α-synuclein may be an effective strategy for the treatment or even prevention of PD. Furthermore, a key therapeutic solution may be to slow or prevent the spread of pathological forms of α-synuclein from the appendix through retrograde neuronal transportation to the CNS. Activation of the immune system, by noninfectious or infectious factors, can modulate PD pathology through the rate of accumulation and transportation of the α-synuclein aggregates (23, 79, 80). Thus, factors affecting the gut inflammatory and microbial environment, such as altering the gut microbiome (81), might serve to slow the pathological process, although this has yet to be tested.
How can one envision the contribution of the appendix to the pathology of PD? The simplest explanation would be as a source of seeding-competent aggregates of full-length or truncated α-synuclein, and that these α-synuclein conformers can migrate into the CNS via the vagal nerve (fig. S9). Previous studies have shown that a truncal vagotomy is associated with a reduced subsequent risk of developing PD (13), and in animal models, the vagal nerve has been demonstrated to transport misfolded α-synuclein to the brainstem (16). By analogy, if the appendix constitutes a prominent source of misfolded α-synuclein that is transmitted along the vagal nerve, then the propagation of α-synuclein aggregates would be inhibited after appendectomy. We consistently found α-synuclein aggregates in the appendix from people not affected by PD, even at young ages (≤20 years old), and earlier work has shown that α-synuclein upregulation can occur in the intestinal wall of children (23). On the basis of these observations, one could predict that the effect of appendectomy on PD pathogenesis would be maximally protective the earlier the appendectomy occurred, and this is what we observed in our epidemiological data. It is conceivable that transport of α-synuclein could still occur through vagal afferents from other parts of the GI tract, but in lower quantities due to the abolished source of input from the appendix. One could further speculate that the appendix may be a site of initiation in PD cases with early GI tract abnormalities, whereas PD without prevalent prodromal GI tract issues may originate in other tissue regions, such as the olfactory bulb (82, 83). Future studies should examine whether the PD subgroup with prodromal GI tract dysfunction is protected against PD by an appendectomy.
Given recent evidence linking gut inflammation to PD (23, 73, 74) and the role of the appendix in immunosurveillance and microbiome regulation (25, 26), it is possible that the appendix contributes to PD via inflammation and microbiome alterations. Given that the appendix is responsible for monitoring and repopulating the microbiome in the small and large intestine (26), any subclinical inflammation in the appendix may signify the presence of proinflammatory bacteria, which would then be dispersed throughout the intestine. Studies in germ-free mice have also shown that the PD microbiome can release metabolites (short-chain fatty acids) that promote microglial activation in the brain and PD-like symptoms (19). If the appendix were to house bacteria that release metabolites promoting inflammation in the brain, this may also increase the risk of developing PD.
In sum, our study shows that the appendix is a rich, lifelong source of misfolded α-synuclein, and early removal of the appendix is associated with a reduced risk of developing PD. Compounds that limit aberrant α-synuclein cleavage and accumulation in the appendix and other GI tract lymphoid tissue may be a potential therapeutic strategy for PD.
MATERIALS AND METHODS
Study design
This study was designed to investigate whether the appendix contributes to PD risk. We first conducted two complementary epidemiological studies. The first epidemiological study involved a large administrative database, the SNPR, and contained 1.6 million individuals with up to a 52-year follow-up period. The second epidemiological study used PPMI data that involved patients with PD who were clinically well characterized. Next, we conducted immunohistochemical investigations of appendix tissue from the general population. We examined the pathology associated with PD, aggregated α-synuclein, and its cellular localization. Last, we performed biochemical assays, along with TD-MS, to characterize the α-synuclein proteoforms in the appendix. Biochemical assays were also used to determine the propensity for α-synuclein aggregation in the appendix relative to that of substantia nigra, examining tissues from both healthy individuals and patients with PD.
For all experiments, no statistical methods were used to predetermine sample size. The study parameters for the nationwide SNPR study and the PPMI dataset are detailed below. For immunohistochemistry studies, experimenters were blinded during sample processing and scoring. The blinding process involved a third party sectioning, mounting, and assigning randomized tissues a unique serial number. For all biochemical assays, quantitative measures were done using software and normalized to loading controls by experimenters blind to assay outcome until after statistical analysis performed by two independent investigators. The number of experimental replicates is specified within each figure legend and elaborated for specific experiments in Results.
Epidemiological study in a general population
The SNPR has been collecting information on the diseases and surgical treatments of individuals living in Sweden since 1964 (84). The SNPR has records on hospital discharge diagnoses coded according to the Swedish Revisions of the International Classification of Diseases (ICD) from 1964, and outpatient diagnoses were added to the registry from 2001 (84). There are more than 50 million inpatient and outpatient discharges in the SNPR. Using this dataset, we identified all individuals who had had an appendectomy from 1964 to 2015 (ICD codes: JEA00, JEA01, JEA10, 4510, 4511, 0058, and 0059). For each individual, we obtained the following information: surgical procedure, year of birth, year of surgical procedure, sex, geographic location (municipality), and, when applicable, date and cause of death. In addition, each individual who had an appendectomy was matched to two controls who did not have this procedure. Control individuals were identified by SCB and matched to appendectomized individuals on the basis of year of birth, sex, and geographic area (municipality). Control individuals were confirmed to have no history of appendectomy by the SNPR. When applicable, date and cause of death were provided for controls.
For both appendectomy individuals and matched controls, SNPR gathered information on PD diagnosis (codes for ICD-7: 350.99; ICD-8: 342; ICD-9: 332A; ICD-10: G20). We defined PD cases as individuals with a primary or secondary diagnosis of PD in the SNPR. Age of PD hospital diagnosis was defined as the date of first-ever hospital admission or outpatient record. Control individuals and individuals who underwent appendectomy were followed up until their first diagnosis of PD disease, date of death, or 31 December 2015, whichever came first. We also identified and excluded individuals with the following confounding conditions: secondary PD, schizophrenia (in which medications can cause secondary parkinsonism), and degenerative brain diseases that can involve secondary parkinsonism, such as olivopontocerebellar atrophy and cerebral syphilis [codes for ICD-7: 25.99, 26, 300.99, and 355; ICD-8: 66, 94.1, 94.9, 94.91, 94.98, 295 to 295.99, 342.08, and 342.09; ICD-9: 094X, 295(A to X), 331X, 332B, 333, and 333A; ICD-10: G21; G22, G23, A52.1, and F20 to F20.9]. Furthermore, we identified individuals with ulcerative colitis (codes for ICD-7: 57220 and 57221; ICD-8: 56310 and 56902; ICD-9: 556X; ICD-10: K51.0) or Alzheimer’s disease (codes for ICD-7: 305; ICD-8: 290.1; ICD-9: 290B; ICD-10: G30-G30.9) to verify that these conditions would not affect our results. Validation analysis of SNPR hospital discharge diagnoses of PD against detailed clinical diagnoses has shown a positive predictive value of 70.8% and a sensitivity of 72.7% (62). In addition, the SNPR has a high sensitivity for surgical procedures (>90%) (85). All data obtained from the SNPR and SCB were de-identified, and the data request and analysis were approved by the Lund University ethical review board (reference no. 2016/8).
To determine whether prevalence of PD differed in individuals who had an appendectomy versus those who did not, logistic regression adjusted for sex and urban/rural municipality was used. To determine whether the incidence of PD differed in Swedish persons who had an appendectomy versus those who did not, Poisson regression with person-years as an offset, adjusted for sex and urban/ rural municipality, was used. Person-years were calculated as the number of years between birth and PD diagnosis, death, or end of observational period (31 December 2015), whichever came first. To determine whether the age of PD diagnosis differed between Swedish persons who had an appendectomy versus those who did not, the two samples were matched on sex, urban/rural dweller, and birth year using the genetic matching algorithm in the R package “Matchit,” which calculates weights that optimally balance the covariates (86). On the basis of the matching, a weighted Cox proportional hazards regression, adjusted for urban/rural dweller and sex, was fit. The above survival analysis was performed restricting the number of years between the appendectomy and PD diagnosis to >0 and ≥20 years. An individual was identified as a rural dweller if he or she resided in a municipality with a population of less than 100,000 and a density of less than 200 people/km2 (87). We also excluded immigrants and emigrants from our analysis (n = 37,913), and a sensitivity analysis was performed confirming that the inclusion of immigrants and emigrants did not change the study results. In the study, there were 3002 individuals with ulcerative colitis (of whom 16 had PD) and 551 individuals with Alzheimer’s disease (of whom 12 had PD). A sensitivity analysis verified that exclusion of individuals with ulcerative colitis or Alzheimer’s disease did not change the results. Last, a parametric survival regression with an assumed extreme value distribution (distribution was chosen on the basis of minimization of the Akaike information criterion) and adjusted for sex and urban/rural dweller showed results consistent with the Cox model, confirming that the results were not sensitive to the proportional hazards assumption (88). All analyses were performed in R v. 3.3.0 (www.r-project.org/), and survival analyses were performed using the “survival” package (89). All P values were calculated via a two-sided likelihood ratio test. Reported survival times are the restricted means (90).
Epidemiological analysis of the PPMI dataset
PPMI is a large, international, multicenter study designed to identify biomarkers of PD progression (www.ppmi-info.org/study-design/). Detailed descriptions of the study have been published elsewhere (63). All patients were recruited via clinics specialized in neurology and movement disorders. PPMI data are publicly available and we downloaded data for this analysis on 24 March 2017 from its website, including demographic information, clinical characteristics, medical history, family history, genetic testing results, and outcomes of clinical tests. The data included 849 patients with PD. Of these patients, 478 were originally recruited for a de novo PD participant group who met the following inclusion criteria: aged 30 years or older; diagnosis of PD based on the presence of (i) asymmetrical resting tremor, (ii) asymmetrical bradykinesia, or (iii) at least two of the following: resting tremor, bradykinesia, and rigidity; disease duration of ≤24 months; Hoehn and Yahr stage of 1 to 2; and presence of striatal dopamine transporter deficit on 123I-ioflupane SPECT imaging (DaTscan) (63). In addition, we included patients with PD in the PPMI genetic cohort (n = 190) and genetic registry (n = 181), both of which have patients with a mutation in LRRK2, GBA, or SNCA. Patients with PD from the de novo and genetic groups were equivalent in age of appendectomy, age of PD onset, sex, and ethnicity (table S9). A detailed medical history (including appendectomy and age of surgery) was provided by patients as part of the enrollment interview for PPMI. PD age of onset refers to the age of first motor symptom onset.
In the PPMI dataset, we identified individuals who had an appendectomy, non-GI immune disorder (defined as an individual who had allergies, eczema, asthma, anemia, hyperthyroidism, fibromyalgia, Graves’ disease, or Hashimoto’s disease), or other surgery (individuals who had a surgery or resection other than LASIK and never had an appendectomy or tonsillectomy). Individuals who had multiple surgeries or immune conditions, or both, were classified on the basis of the first diagnosis. A sensitivity analysis excluding individuals who fit into both groups confirmed that results were not changed by this classification. The appendectomy, non-GI immune conditions, other surgery, and no immune conditions/surgery were non-overlapping sample groups. Family history was defined as having one to two parents, siblings, or half-siblings with PD. Individuals with more than two relatives were excluded, as only the no-appendectomy group had individuals with more than two relatives and there was evidence that these individuals biased effects away from the null (fig. S1). We also excluded 14 patients with PD who had an unspecified ethnicity and three appendectomy cases who had appendectomy after PD onset (a total of 19 excluded samples from n = 849).
Lognormal parametric survival regression, stratified by having an appendectomy more than 0, 10, 20, and 30 years before PD onset and adjusted for age, sex, ethnicity (white and nonwhite), number of education years, family history, and PD mutation status (known mutation/no known mutation), was used to determine whether having an appendectomy was significantly associated with delayed PD onset at α = 0.05 significance level. Individuals with a non-GI immune condition or surgery not involving the appendix served as negative controls for these analyses. To infer whether PD onset was delayed by an appendectomy, and not by an imbalance in covariates or by the age at which procedures/diagnoses occurred, individuals who had an appendectomy were matched with individuals with another surgery or immune condition separately, based on procedure age, sex, ethnicity, number of years of education, family history, and mutation status using the R package Matchit as described above. A weighted Cox proportional hazards model, adjusted for the same covariates as above, was then fit to assess whether the hazard ratio differed significantly from 1. A lognormal survival regression, adjusted for the same covariates, was also fit to assess sensitivity of the results to the proportional hazards assumption. Matched individuals with a non–GI tract immune condition or other surgery were then compared to the no-condition/surgery group to verify the effect that only occurs in individuals who had an appendectomy and does not occur in an unrelated disorder or surgery. To test whether there were any reported differences in the UPDRS or Hoehn and Yahr scores for individuals who had had an appendectomy versus those who did not, a proportional odds logistic regression using the R package “MASS” (91), adjusted for sex, ethnicity, number of education years, family history, and mutation status, was fit to each outcome. The P values for the estimated effect an appendectomy had on each outcome were then false discovery rate corrected to adjust for multiple testing. Furthermore, a sensitivity analysis that included only patients with de novo PD showed that the results did not change (table S10). We confirmed that clinic site did not bias the results using a frailty model with clinic site as a random effect. All analyses were performed in R.
Statistical analyses
For the immunohistochemistry assays, statistical analyses were performed using R v. 3.3.0. A logit mixed-effects regression was used for the analysis of aggregated α-synuclein staining in young versus old and normal versus inflamed appendix. Pearson’s correlations were determined to assess the cellular colocalization of α-synuclein aggregates. Biochemical assays were analyzed using Statistica v. 13.3. We used a one-way, two-way, or repeated-measures ANOVA with the appropriate between-subjects (diagnosis and tissue) or within-subjects (time) factors. Significant main effects were followed by Tukey’s honest significant difference post hoc comparisons. Statistical significance was P < 0.05 and denoted by *P < 0.05, **P < 0.01, or ***P < 0.001.
Supplementary Material
Acknowledgments:
We thank J. Ma for providing purified human α-synuclein. We thank J. Saunders for technical assistance and C. DeHart for advice on mass spectrometry work. We thank Van Andel Research Institute core services including pathology and biorepository, confocal and quantitative imaging, and bioinformatics and biostatistics. Some data used in the preparation of this article were obtained from the PPMI database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
Funding: V.L. was supported by grants from the Alzheimer’s Society of Canada (1615), the Scottish Rite Charitable Foundation of Canada (15110), the Department of Defense (PD170089), and a Gibby & Friends vs. Parky award. L.B. was supported by a grant from the Michael J. Fox Foundation (14939). P.B. reports relevant grants from NIH (R01DC016519-01 and 5R21NS093993-02), Department of Defense (W81XWH-17-1-0534), The Michael J. Fox Foundation for Parkinson’s Research, and Cure Parkinson’s Trust. Mass spectrometry work was supported by the National Resource for Translational and Developmental Proteomics (P41 GM108569). PPMI, a public-private partnership, is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including Abbvie, Allergan, Avid Radiopharmaceuticals, Biogen, BioLegend, Bristol-Myers Squibb, Denali, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal, Roche, Sanofi Genzyme, Servier, Takeda, Teva, UCB, and Golub Capital.
Competing interests: P.B. has received commercial support as a consultant from Renovo Neural, Roche, Teva, Lundbeck A/S, AbbVie, NeuroDerm, Fujifilm Cellular Dynamics, Living Cell Technologies, ClearView Healthcare, FCB Health, IOS Press Partners, Capital Technologies, and Axial Biotherapeutics. In addition, P.B. has received commercial support for grants/research from Renovo, Roche, Teva, and Lundbeck. P.B. has ownership interests in AcouSort AB. The other authors declare that they have no competing interests.
Footnotes
Data and materials availability: Data used in the SNPR study can be obtained with ethical approval from the SNPR and SCB (www.socialstyrelsen.se/register/halsodataregister/patientregistret/inenglish). Data for the PPMI study can be obtained from www.ppmi-info.org. All other data associated with this study are in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G, MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord 30, 1591–1601 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Fearnley JM, Lees AJ, Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 114 (Pt. 5), 2283–2301 (1991). [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Zhao EJ, Zhang W, Lu Y, Liu R, Huang X, Ciesielski-Jones AJ, Justice MA, Cousins DS, Peddada S, Meta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosis. Transl. Neurodegener 4, 1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savica R, Carlin JM, Grossardt BR, Bower JH, Ahlskog JE, Maraganore DM, Bharucha AE, Rocca WA, Medical records documentation of constipation preceding Parkinson disease: A case-control study. Neurology 73, 1752–1758 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P, Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann. Neurol 79, 940–949 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Barrenschee M, Zorenkov D, Böttner M, Lange C, Cossais F, Scharf AB, Deuschl G, Schneider SA, Ellrichmann M, Fritscher-Ravens A, Wedel T, Distinct pattern of enteric phospho-alpha-synuclein aggregates and gene expression profiles in patients with Parkinson’s disease. Acta Neuropathol. Commun 5, 1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beach TG, Corbille AG, Letournel F, Kordower JH, Kremer T, Munoz DG, Intorcia A, Hentz J, Adler CH, Sue LI, Walker J, Serrano G, Derkinderen P, Multicenter assessment of immunohistochemical methods for pathological alpha-synuclein in sigmoid colon of autopsied Parkinson’s disease and control subjects. J. Parkinsons Dis 6, 761–770 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbillé A-G, Letournel F, Kordower JH, Lee J, Shanes E, Neunlist M, Munoz DG, Derkinderen P, Beach TG, Evaluation of alpha-synuclein immunohistochemical methods for the detection of Lewy-type synucleinopathy in gastrointestinal biopsies. Acta Neuropathol. Commun 4, 35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, Broughton E, Hagan H, Carroll C, Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol 127, 235–241 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Hawkes CH, Del Tredici K, Braak H, Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol 33, 599–614 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM-Y, Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao X, Ou MT, Karuppagounder SS, Kam T-I, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin J-H, Kang HC, Zhang J, Xu J, Chen R, Park H, Andrabi SA, Kang SU, Gonçalves RA, Liang Y, Zhang S, Qi C, Lam S, Keiler JA, Tyson J, Kim D, Panicker N, Yun SP, Workman CJ, Vignali DA, Dawson VL, Ko HS, Dawson TM, Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353, aah3374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, Svenningsson P, Chen H, Wirdefeldt K, Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology 88, 1996–2002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sorensen HT, Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol 78, 522–529 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Tysnes O-B, Kenborg L, Herlofson K, Steding-Jessen M, Horn A, Olsen JH, Reichmann H, Does vagotomy reduce the risk of Parkinson’s disease? Ann. Neurol 78, 1011–1012 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, Wang Z-Y, Roybon L, Melki R, Li J-Y, Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128, 805–820 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Ulusoy A, Rusconi R, Pérez-Revuelta BI, Musgrove RE, Helwig M, Winzen-Reichert B, Di Monte DA, Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Mol. Med 5, 1119–1127 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visanji NP, Brooks PL, Hazrati L-N, Lang AE, The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol. Commun 1, 2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet M-F, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK, Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480.e12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knudsen K, Fedorova TD, Hansen AK, Sommerauer M, Otto M, Svendsen KB, Nahimi A, Stokholm MG, Pavese N, Beier CP, Brooks DJ, Borghammer P, In-vivo staging of pathology in REM sleep behaviour disorder: A multimodality imaging case-control study. Lancet Neurol 17, 618–628 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Pan-Montojo F, Schwarz M, Winkler C, Arnhold M, O’Sullivan GA, Pal A, Said J, Marsico G, Verbavatz J-M, Rodrigo-Angulo M, Gille G, Funk RHW, Reichmann H, Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci. Rep 2, 898 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM, Colonic bacterial composition in Parkinson’s disease. Mov. Disord 30, 1351–1360 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Stolzenberg E, Berry D, Yang D, Lee EY, Kroemer A, Kaufman S, Wong GCL, Oppenheim JJ, Sen S, Fishbein T, Bax A, Harris B, Barbut D, Zasloff MA, A role for neuronal alpha-synuclein in gastrointestinal immunity. J. Innate Immun 9, 456–463 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray MT, Munoz DG, Gray DA, Schlossmacher MG, Woulfe JM, Alpha-synuclein in the appendiceal mucosa of neurologically intact subjects. Mov. Disord 29, 991–998 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Zahid A, The vermiform appendix: Not a useless organ. J. Coll. Physicians Surg. Pak 14, 256–258 (2004). [PubMed] [Google Scholar]
- 26.Masahata K, Umemoto E, Kayama H, Kotani M, Nakamura S, Kurakawa T, Kikuta J, Gotoh J, Motooka D, Sato S, Higuchi T, Baba Y, Kurosaki T, Kinoshita M, Shimada Y, Kimura T, Okumura R, Takeda A, Tajima M, Yoshie O, Fukuzawa M, Kiyono H, Fagarasan S, Iida T, Ishii M, Takeda K, Generation of colonic IgA-secreting cells in the caecal patch. Nat. Commun 5, 3704 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM-Y, Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. U.S.A 106, 20051–20056 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giasson BI, Murray IV, Trojanowski JQ, Lee VM-Y, A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J. Biol. Chem 276, 2380–2386 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Crowther RA, Jakes R, Spillantini MG, Goedert M, Synthetic filaments assembled from C-terminally truncated α-synuclein. FEBS Lett 436, 309–312 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Murray IVY, Giasson BI, Quinn SM, Koppaka V, Axelsen PH, Ischiropoulos H, Trojanowski JQ, Lee VM-Y, Role of α-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry 42, 8530–8540 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T, Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol 152, 879–884 (1998). [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, West N, Colla E, Pletnikova O, Troncoso JC, Marsh L, Dawson TM, Jäkälä P, Hartmann T, Price DL, Lee MK, Aggregation promoting C-terminal truncation of α-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc. Natl. Acad. Sci. U.S.A 102, 2162–2167 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi D-H, Kim Y-J, Kim Y-G, Joh TH, Beal MF, Kim Y-S, Role of matrix metalloproteinase 3-mediated α-synuclein cleavage in dopaminergic cell death. J. Biol. Chem 286, 14168–14177 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Nguyen LTT, Burlak C, Chegini F, Guo F, Chataway T, Ju S, Fisher OS, Miller DW, Datta D, Wu F, Wu C-X, Landeru A, Wells JA, Cookson MR, Boxer MB, Thomas CJ, Gai WP, Ringe D, Petsko GA, Hoang QQ, Caspase-1 causes truncation and aggregation of the Parkinson’s disease-associated protein α-synuclein. Proc. Natl. Acad. Sci. U.S.A 113, 9587–9592 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Kang SS, Liu X, Ahn EH, Zhang Z, He L, Iuvone PM, Duong DM, Seyfried NT, Benskey MJ, Manfredsson FP, Jin L, Sun YE, Wang J-Z, Ye K, Asparagine endopeptidase cleaves α-synuclein and mediates pathologic activities in Parkinson’s disease. Nat. Struct. Mol. Biol 24, 632–642 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ, The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology 50, 1346–1350 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT, Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci 3, 1301–1306 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Savica R, Rocca WA, Ahlskog JE, When does Parkinson disease start? Arch. Neurol 67, 798–801 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Neumann M, Müller V, Kretzschmar HA, Haass C, Kahle PJ, Regional distribution of proteinase K-resistant α-synuclein correlates with Lewy body disease stage. J. Neuropathol. Exp. Neurol 63, 1225–1235 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Lee H-J, Lee S-J, Characterization of cytoplasmic α-synuclein aggregates. Fibril formation is tightly linked to the inclusion-forming process in cells. J. Biol. Chem 277, 48976–48983 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Tsigelny IF, Crews L, Desplats P, Shaked GM, Sharikov Y, Mizuno H, Spencer B, Rockenstein E, Trejo M, Platoshyn O, Yuan JX-J, Masliah E, Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer’s and Parkinson’s diseases. PLOS ONE 3, e3135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Zhou J, Broe M, Huang Y, Anderson JP, Gai W-P, Milward EA, Porritt M, Howells D, Hughes AJ, Wang X, Halliday GM, Changes in the solubility and phosphorylation of α-synuclein over the course of Parkinson’s disease. Acta Neuropathol 121, 695–704 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Mendes A, Gonçalves A, Vila-Chã N, Moreira I, Fernandes J, Dámasio J, Teixeira-Pinto A, Taipa R, Lima AB, Cavaco S, Appendectomy may delay Parkinson’s disease onset. Mov. Disord 30, 1404–1407 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Marras C, Lang AE, Austin PC, Lau C, Urbach DR, Appendectomy in mid and later life and risk of Parkinson’s disease: A population-based study. Mov. Disord 31, 1243–1247 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Yilmaz R, Bayram E, Ulukan C, Altinok MK, Akbostanci MC, Appendectomy history is not related to Parkinson’s disease. J. Parkinsons Dis 7, 347–352 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Svensson E, Horváth-Puhó E, Stokholm MG, Sørensen HT, Henderson VW, Borghammer P, Appendectomy and risk of Parkinson’s disease: A nationwide cohort study with more than 10 years of follow-up. Mov. Disord 31, 1918–1922 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Barbeau A, Roy M, Bernier G, Campanella G, Paris S, Ecogenetics of Parkinson’s disease: Prevalence and environmental aspects in rural areas. Can. J. Neurol. Sci 14, 36–41 (1987). [DOI] [PubMed] [Google Scholar]
- 48.Angot E, Steiner JA, Hansen C, Li JY, Brundin P, Are synucleinopathies prion-like disorders? Lancet Neurol 9, 1128–1138 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Braak H, Braak E, Pathoanatomy of Parkinson’s disease. J. Neurol 247 (suppl. 2), II3–II10 (2000). [DOI] [PubMed] [Google Scholar]
- 50.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White III CL, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG; Arizona Parkinson’s Disease Consortium, Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119, 689–702 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lebouvier T, Chaumette T, Damier P, Coron E, Touchefeu Y, Vrignaud S, Naveilhan P, Galmiche JP, Bruley des Varannes S, Derkinderen P, Neunlist M, Pathological lesions in colonic biopsies during Parkinson’s disease. Gut 57, 1741–1743 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F, Parkinson’s disease: The presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76, 217–221 (1988). [DOI] [PubMed] [Google Scholar]
- 53.Games D, Valera E, Spencer B, Rockenstein E, Mante M, Adame A, Patrick C, Ubhi K, Nuber S, Sacayon P, Zago W, Seubert P, Barbour R, Schenk D, Masliah E, Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates, neurodegeneration and propagation in Parkinson’s disease-like models. J. Neurosci 34, 9441–9454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kellie JF, Higgs RE, Ryder JW, Major A, Beach TG, Adler CH, Merchant K, Knierman MD, Quantitative measurement of intact alpha-synuclein proteoforms from post-mortem control and Parkinson’s disease brain tissue by intact protein mass spectrometry. Sci. Rep 4, 5797 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grassi D, Howard S, Zhou M, Diaz-Perez N, Urban NT, Guerrero-Given D, Kamasawa N, Volpicelli-Daley LA, LoGrasso P, Lasmézas CI, Identification of a highly neurotoxic α-synuclein species inducing mitochondrial damage and mitophagy in Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A 115, E2634–E2643 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulusoy A, Febbraro F, Jensen PH, Kirik D, Romero-Ramos M, Co-expression of C-terminal truncated alpha-synuclein enhances full-length alpha-synuclein-induced pathology. Eur. J. Neurosci 32, 409–422 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Bartels T, Ahlstrom LS, Leftin A, Kamp F, Haass C, Brown MF, Beyer K, The N-terminus of the intrinsically disordered protein α-synuclein triggers membrane binding and helix folding. Biophys. J 99, 2116–2124 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terada M, Suzuki G, Nonaka T, Kametani F, Tamaoka A, Hasegawa M, The effect of truncation on prion-like properties of α-synuclein. J. Biol. Chem 293, 13910–13920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dufty BM, Warner LR, Hou ST, Jiang SX, Gomez-Isla T, Leenhouts KM, Oxford JT, Feany MB, Masliah E, Rohn TT, Calpain-cleavage of α-synuclein: Connecting proteolytic processing to disease-linked aggregation. Am. J. Pathol 170, 1725–1738 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V, α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522, 340–344 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Borghammer P, How does Parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov. Disord 33, 48–57 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Feldman AL, Johansson ALV, Gatz M, Flensburg M, Petzinger GM, Widner H, Lew MF, Pedersen NL, Wirdefeldt K, Accuracy and sensitivity of Parkinsonian disorder diagnoses in two Swedish national health registers. Neuroepidemiology 38, 186–193 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parkinson Progression Marker Initiative, The Parkinson Progression Marker Initiative (PPMI). Prog. Neurobiol 95, 629–635 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Short ME, Goetzel RZ, Pei X, Tabrizi MJ, Ozminkowski RJ, Gibson TB, Dejoy DM, Wilson MG, How accurate are self-reports? Analysis of self-reported health care utilization and absence when compared with administrative data. J. Occup. Environ. Med 51, 786–796 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richards W, Watson D, Lynch G, Reed GW, Olsen D, Spaw A, Holcomb W, Frexes-Steed M, Goldstein R, Sharp K, A review of the results of laparoscopic versus open appendectomy. Surg. Gynecol. Obstet 177, 473–480 (1993). [PubMed] [Google Scholar]
- 66.Ferris M, Quan S, Kaplan BS, Molodecky N, Ball CG, Chernoff GW, Bhala N, Ghosh S, Dixon E, Ng S, Kaplan GG, The global incidence of appendicitis: A systematic review of population-based studies. Ann. Surg 266, 237–241 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Hardin DM Jr., Acute appendicitis: Review and update. Am. Fam. Physician 60, 2027–2034 (1999). [PubMed] [Google Scholar]
- 68.Stewart B, Khanduri P, McCord C, Ohene-Yeboah M, Uranues S, Vega Rivera F, Mock C, Global disease burden of conditions requiring emergency surgery. Br. J. Surg 101, e9–e22 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Khan MN, Fayyad T, Cecil TD, Moran BJ, Laparoscopic versus open appendectomy: The risk of postoperative infectious complications. JSLS 11, 363–367 (2007). [PMC free article] [PubMed] [Google Scholar]
- 70.Guller U, Hervey S, Purves H, Muhlbaier LH, Peterson ED, Eubanks S, Pietrobon R, Laparoscopic versus open appendectomy: Outcomes comparison based on a large administrative database. Ann. Surg 239, 43–52 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mentes O, Eryilmaz M, Harlak A, Ozer T, Balkan M, Kozak O, Tufan T, Can d-dimer become a new diagnostic parameter for acute appendicitis? Am. J. Emerg. Med 27, 765–769 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Lionnet A, Leclair-Visonneau L, Neunlist M, Murayama S, Takao M, Adler CH, Derkinderen P, Beach TG, Does Parkinson’s disease start in the gut? Acta Neuropathol 135, 1–12 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Hui KY, Fernandez-Hernandez H, Hu J, Schaffner A, Pankratz N, Hsu N-Y, Chuang L-S, Carmi S, Villaverde N, Li X, Rivas M, Levine AP, Bao X, Labrias PR, Haritunians T, Ruane D, Gettler K, Chen E, Li D, Schiff ER, Pontikos N, Barzilai N, Brant SR, Bressman S, Cheifetz AS, Clark LN, Daly MJ, Desnick RJ, Duerr RH, Katz S, Lencz T, Myers RH, Ostrer H, Ozelius L, Payami H, Peter Y, Rioux JD, Segal AW, Scott WK, Silverberg MS, Vance JM, Ubarretxena-Belandia I, Foroud T, Atzmon G, Pe’er I, Ioannou Y, McGovern DPB, Yue Z, Schadt EE, Cho JH, Peter I, Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci. Transl. Med 10, eaai7795 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peter I, Dubinsky M, Bressman S, Park A, Lu C, Chen N, Wang A, Anti-tumor necrosis factor therapy and incidence of Parkinson disease among patients with inflammatory bowel disease. JAMA Neurol 75, 939–946 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andersson RE, Olaison G, Tysk C, Ekbom A, Appendectomy and protection against ulcerative colitis. N. Engl. J. Med 344, 808–814 (2001). [DOI] [PubMed] [Google Scholar]
- 76.Brundin P, Dave KD, Kordower JH, Therapeutic approaches to target alpha-synuclein pathology. Exp. Neurol 298 (Pt. B), 225–235 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Agnaf OM, Paleologou KE, Greer B, Abogrein AM, King JE, Salem SA, Fullwood NJ, Benson FE, Hewitt R, Ford KJ, Martin FL, Harriott P, Cookson MR, Allsop D, A strategy for designing inhibitors of α-synuclein aggregation and toxicity as a novel treatment for Parkinson’s disease and related disorders. FASEB J 18, 1315–1317 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Zucker S, Cao J, Chen W-T, Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene 19, 6642–6650 (2000). [DOI] [PubMed] [Google Scholar]
- 79.Beatman EL, Massey A, Shives KD, Burrack KS, Chamanian M, Morrison TE, Beckham JD, Alpha-synuclein expression restricts RNA viral infections in the brain. J. Virol 90, 2767–2782 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen SG, Stribinskis V, Rane MJ, Demuth DR, Gozal E, Roberts AM, Jagadapillai R, Liu R, Choe K, Shivakumar B, Son F, Jin S, Kerber R, Adame A, Masliah E, Friedland RP, Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci. Rep 6, 34477 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P, Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord 30, 350–358 (2015). [DOI] [PubMed] [Google Scholar]
- 82.Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, Lee VM-Y, Brundin P, Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J. Exp. Med 213, 1759–1778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White III CL, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG; Arizona Parkinson’s Disease Consortium, Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117, 613–634 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Socialstyrelsen, The National Patient Register; www.socialstyrelsen.se/register/halsodataregister/patientregistret/inenglish (2017).
- 85.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim J-L, Reuterwall C, Heurgren M, Olausson PO, External review and validation of the Swedish national inpatient register. BMC Public Health 11, 450 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ho D, Imai K, King G, Stuart EA, MatchIt: Nonparametric preprocessing for parametric causal inference. J. Stat Softw 42, 1–28 (2011). [Google Scholar]
- 87.Organisation for Economic Co-operation and Development (OECD), Functional urban areas in OECD countries: Sweden; www.oecd.org/cfe/regional-policy/functional-urban-areas-all-sweden.pdf (2016).
- 88.George B, Seals S, Aban I, Survival analysis and regression models. J. Nucl. Cardiol 21, 686–694 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Therneau T, A Package for Survival Analysis in S. Version 2.38; https://CRAN.R-project.org/package=survival (2015).
- 90.Miller RG Jr., Gong G, Munoz A, Survival Analysis (John Wiley and Sons, 1981). [Google Scholar]
- 91.Venables WN, Ripley BD, Modern Applied Statistics with S (Springer-Verlag, ed. 4, 2002). [Google Scholar]
- 92.Friedman DB, Quantitative proteomics for two-dimensional gels using difference gel electrophoresis. Methods Mol. Biol 367, 219–239 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Butt RH, Coorssen JR, Coomassie blue as a near-infrared fluorescent stain: A systematic comparison with Sypro Ruby for in-gel protein detection. Mol. Cell. Proteomics 12, 3834–3850 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, Matsubara T, Nakagaki T, Yamanaka H, Shirabe S, Yamada M, Mizusawa H, Kitamoto T, Klug G, McGlade A, Collins SJ, Nishida N, Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat. Med 17, 175–178 (2011). [DOI] [PubMed] [Google Scholar]
- 95.Wessel D, Flügge UI, A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem 138, 141–143 (1984). [DOI] [PubMed] [Google Scholar]
- 96.Ntai I, Toby TK, LeDuc RD, Kelleher NL, A method for label-free, differential top-down proteomics. Methods Mol. Biol 1410, 121–133 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.