Abstract
A direct (3+2) cycloaddition between alkenes and vinyl diazo reagents using Cr or Ru photocatalysis is described. The intermediacy of a radical cation species enables a nucleophilic interception by vinyl diazo compounds, a departure from their traditional electrophilic behavior. A variety of cyclopentenes are synthesized using this method, and experimental insights implicate a direct cycloaddition instead of a cyclopropanation/rearrangement cascade process.
Keywords: chromium, cycloaddition, diazo compound, photocatalysis, ruthenium
Graphical Abstract
A radical departure: A direct (3+2) cycloaddition between alkenes and vinyl diazo reagents using Cr or Ru photocatalysis is described. The intermediacy of a radical cation species enables a nucleophilic interception by vinyl diazo compounds, a significant deviation from their traditional electrophilic behavior
Cycloadditions represent one of the most widely employed strategies for efficient molecule synthesis, emblematic of their strengths in convergency and regio- and stereocontrol. In comparison to the venerable Diels-Alder (4+2) cycloaddition for cyclohexene construction, the formation of cyclopentenes via (3+2) pathways is more limited in prominence.[1] Stereochemically and functionally diverse five-membered carbocycles are common cores in many bioactive and functional molecules (e.g., rocaglamide, chromodorolide B, merrilactone A),[2] and developments for their direct and convergent construction can be highly enabling. Vinyl and enol diazocarbonyl compounds are one such reagent that can serve as the 3-carbon component of a (3+2) cycloaddition.[3] These species have engaged in reactivity generally through metal carbene/carbenoid intermediacy. Here, diazo decomposition using Rh, Cu, or Au catalysis generates an electrophilic carbene species that can undergo the addition with alkenes.[4,5] Consequently, the electrophilic activation has generally necessitated the use of nucleophilic C=C partners, such as enol ethers, indoles, or vinyl azides (Figure 1a).[6,7] An alternative cyclopentene (3+2) approach would utilize a vinyl diazo reagent as a nucleophile. There have been isolated reports of vinyl diazo nucleophilicity with activated C=X and X=Y systems (e.g., oxocarbenium, iminium, LA-activated iodosyl).[8,9] Guerrero and coworkers demonstrated cycloaddition reactivity in principle in a cyclopentannulation pairing enol diazoacetates with conjugate acceptors (Figure 1b).[10] This net transformation involves a two-step sequence of TBSOTf-mediated conjugate addition, and cyclopropanation/ring opening to access the cyclopentene unit. A direct cyclopentene annulation using vinyl diazocarbonyl compounds as nucleophiles, however, has not been demonstrated. We hypothesized that generating a more reactive electrophilic 2-carbon partner (i.e., a radical cation) may engender direct and single-step reactivity with the less nucleophilic vinyl diazo species. Toward this goal of developing valuable (3+2) cycloaddition methods, herein we report a novel cyclopentene annulation process of vinyl diazo reagents mediated by Cr and Ru photoredox catalysis, with evidence implicating a direct cycloaddition instead of a cyclopropanation/rearrangement cascade.
Figure 1.
Cyclopentene formations from vinyl diazo (3+2) cycloadditions.
The emergence of photoredox catalysis has sparked broad interest in their diverse transformations and their intriguing mechanistic pathways.[11] Diazo reagents in these processes have seen limited use thus far.[12] As part of our program toward developing earth-abundant metals in photoredox catalysis, we recently described a Cr-catalyzed cyclopropanation between diazocarbonyl compounds and alkenes.[12c] This reaction proceeds via alkene oxidation to a radical cation intermediate by the excited state Cr complex.[13] We were curious whether vinyl diazo species would be able to undergo similar reactions to give cyclopentene compounds;[14] the process could be conceived to proceed via initial cyclopropanation, followed by vinylcyclopropane rearrangement.[15] Alternative, direct processes could also be possible, which would in turn raise further questions of regioselectivity in the cyclopentene formation (vide infra).
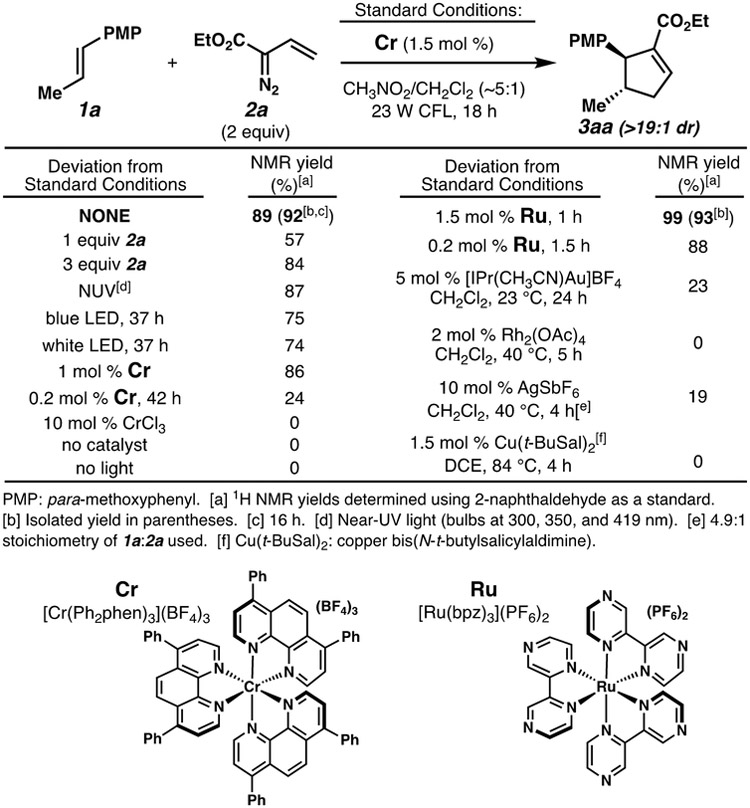
Our initial study of this reaction is outlined in Scheme 1. Using vinyl diazoacetate 2a with trans-anethole (1a) as the alkene substrate, we found that our catalytic chromium conditions (Cr: [Cr(Ph2phen)3](BF4)3, CH3NO2, 23 W CFL) indeed provided cyclopentene 3aa in excellent yield. Notably, this product was observed as a single diastereomer and with exclusive regioselectivity. Some deviations from these conditions were relevant, while others had minimal effects. Fewer than 2 equiv of the vinyl diazo reagent was detrimental to yield. Near UV irradiation was acceptable, but both blue and white LEDs afforded diminished yields. Both light and the photoredox catalyst were essential to reactivity; the simple CrCl3 salt did not catalyze the cyclopentene annulation. Interestingly, the highly oxidizing ruthenium photocatalyst Ru ([Ru(bpz)3](PF6)2) was also effective, with considerably shorter reaction times. Substantially lower catalyst loadings (0.2 mol %) were detrimental for Cr, but less so for Ru. In comparison to the photocatalytic systems, reactions that proceed through carbene/carbenoid manifolds[3-5] were inferior (0-23% yield). Given the previously observed differential behavior of these two oxidizing photocatalysts,[16] we decided to pursue both in the subsequent reaction scope studies.
Scheme 1.
Reaction optimization.
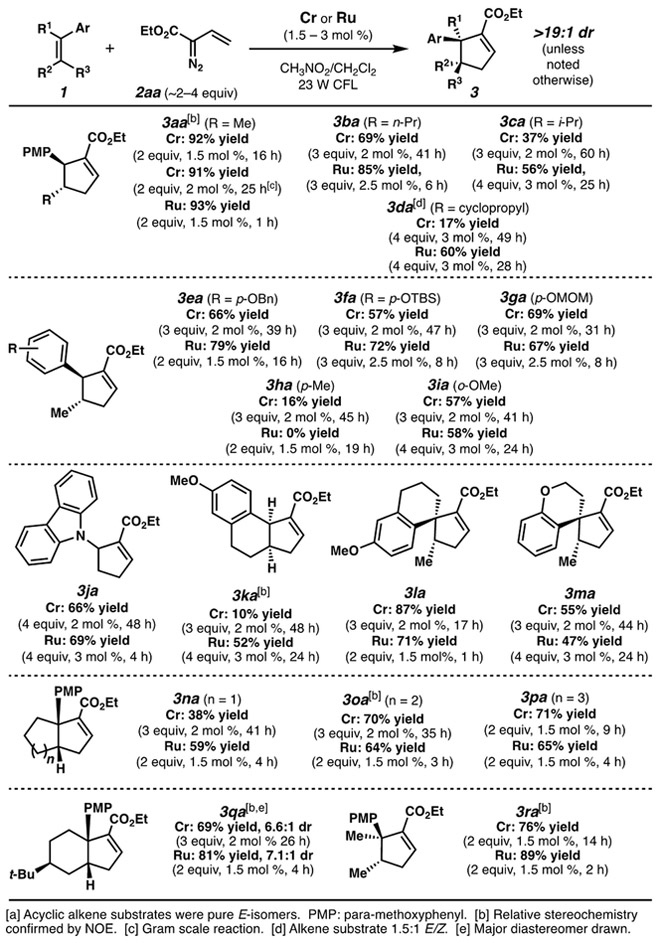
A variety of cyclopentenes could be synthesized via this (3+2) cycloaddition. We found that 1.5-3 mol % catalyst loading, and ~2-4 equiv of the diazo reagent were sufficient for effective reactivity across several substrates evaluated (Schemes 2 and 3). Alkene variation is presented in Scheme 2. The main requirement is an electron rich substituent to facilitate single electron oxidation of the alkene species. Acyclic disubstituted alkenes afforded the trans-substituted cyclopentene compounds. Oxygenated arenes were optimal for this requirement, as the aromatic group could be substituted with alkyl ethers, silyl ethers, and acetals. An alkyl arene was only modestly reactive with the Cr complex and unreactive with the Ru catalyst (3ha). A carbazole-based enamine could engage in the cycloaddition to afford nitrogenated cyclopentene 3ja. Both spirocyclic and fused ring systems can also be synthesized via this cycloaddition (3ka-3qa). In both families of ring systems, very high diastereoselectivities can be achieved.[17] Acyclic trisubstituted alkenes are also competent reactants (3ra).
Scheme 2.
Photocatalyzed (3+2) cycloaddition - Alkene scope.
Scheme 3.
Photocatalyzed (3+2) cycloaddition - Diazo scope.
Diazo variation was also examined (Scheme 3). Other diazoesters were largely effective to afford cyclopentenoates, including activated phenyl ester 3ad. Ketones were tolerated with the Ru system (3ae). β-Alkyl and β-aryl substitutions on the diazo reagent were also tolerated.
A few additional constraints were noted in our evaluation (Scheme 4). Tetrasubstituted alkene 1s and stilbene (1t) were unreactive, likely due to steric hindrance. Enol diazoacetate 2j was not productive, presumably because of competitive enol oxidation by the catalyst. Finally, γ-substitution on the diazo reagent was not tolerated. This limitation could also be due to steric considerations, although we also found background pyrazole formation mediated by heat/light (eq 1)[18] was particularly fast in these cases.[19] Adding the diazo reagent in up to three portions over the course of the reaction was generally beneficial to minimize background processes.[20]
Scheme 4.
Observed reaction constraints.
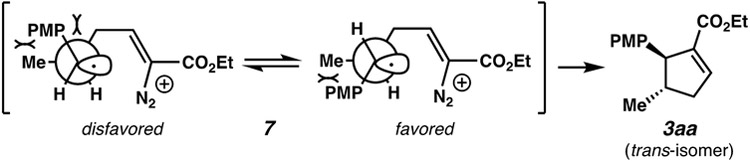
Mechanistic possibilities considered for this transformation are depicted in Figure 2, using trans-anethole and ethyl vinyl diazoacetate as model reagents. The excited state catalyst oxidizes the alkene to form radical cation 4, consistent with measured reduction potentials.[21,22] At this stage, one scenario would involve cyclopropanation on C(α) or C(γ) of the diazo reagent, followed by vinylcyclopropane rearrangement and isomerization to the enoate. We have ruled out this possibility (vide infra). Alternatively, the vinyl diazo reagent can attack electron deficient intermediate 4 as a nucleophile.[8,23] Benzylic radical stabilization of the resulting intermediate (7) would offer a rationale for the observed high regioselectivity.[24,25] A 5-endo radical cyclization generates intermediate 8. Reduction of this species and loss of N2 produces the observed cyclopentene. Another possible pathway from intermediate 7 would involve a 4-exo cyclization to species 9. Ring expansion with concomitant loss of N2, and reduction would afford the observed cyclopentene. If expansion is not immediate, an off-cycle reduction of intermediate 9 would yield cyclobutane 11.
Figure 2.
Proposed mechanism for cyclopentene annulation.
Information toward mechanistic elucidation was accrued experimentally. We subjected two vinylcyclopropanes, from potential direct (5) or vinylogous (6) addition, to the reaction conditions (eq 2). From either compound, there was no formation of the cyclopentene product (3aa), thereby eliminating this potential pathway. When cis-anethole (1acis) is subjected to the standard catalytic conditions, the trans product (3aa) is the only diastereomer observed (eq 3). Trans-anethole was not recovered when this transformation was run partway, suggestive that a bond rotation occurs along the reaction pathway that ultimately leads to the single diastereomer. This observed stereoconvergency validates the likely intermediacy of species 7, where the second bond forming event then establishes the high stereoselectivity. Figure 3 depicts the favorability of the trans-substituted product via intermediate 7; in the favored conformer the PMP group has only one gauche interaction instead of two. Finally, when we subjected independently-synthesized diazo cyclobutane 11 to the photocatalytic conditions, cyclopentene 3aa was formed in essentially quantitative yield (eq 4).[26] We have not observed this cyclobutane compound in reaction mixtures, but given the comparatively rapid formation of 3aa from 11 it could exist as an off-cycle product. We presently favor the 5-endo pathway due to the formation of a less strained intermediate;[27] further experimentation will elucidate whether either (or both) of these pathways are indeed viable.[28]
Figure 3.
Conformational analysis of intermediate 7.
In general, the cycloaddition reaction times for the ruthenium catalyst were shorter than those for the chromium catalyst. Reduction potentials for the two metal catalyst excited states suggest similar reactivity would be expected.[21] In the previously studied radical cation (4+2) cycloadditions, there have been observed mechanistic differences that appear to reflect radical chain (Ru) vs. photocatalyst turnover (Cr) processes,[16,29] that may extrapolate to observed differences here. We have observed reactivity distinctions between the Cr-catalyzed (4+2) and these (3+2) reactions;[30] further studies will seek to establish photocatalyst roles in both the Ru and Cr systems.
The cycloaddition products can also be readily diversified; representative transformations of compound 3aa are shown in Scheme 5. Enoate cyclopropanation and epoxidation both occur with excellent diastereoselectivity. Both allylic halogenation/azidation and oxidation[31] functionalize the γ-methylene. Michael additions and deconjugative alkylations were also successful, both in high diastereoselectivity. Diol 18 can be attained in excellent dr, and it can be further transformed into piperidine 19 via oxidative cleavage and reductive amination. Cycloaddition with TMS-diazomethane[32] works well, and a further desilylative elimination yields aminonitrile 21. Reduction to alcohol 22 followed by Johnson orthoester Claisen rearrangement affords alkene 23. Diastereoselective hydrogenation affords ester 24;[33] importantly, the electron-rich arene can be oxidatively converted to carboxylic acid 25, illustrating substrate requirements we observe in radical cation formation are ultimately not restrictive in accessing product diversity.
Scheme 5.
Cycloaddition product diversification. Reagents: a) m-CPBA; b) Me3S(O)I, NaH; c) NBS, AIBN, then NaN3; d) Pd(OH)2/C, TBHP, K2CO3; e) CH3NO2, DBU; f) KHMDS, BnBr, HMPA; g) OsO4, NMO; h) Pb(OAc)4, then NaBH3CN, BnNH2, AcOH; i) Me3SiCHN2, n-BuLi; j) TsOH; k) DIBAL; l) MeC(OEt)3, PivOH; m) Pd/C, H2; n) RuCl3·3H2O, NaIO4.
In summary, we have developed a photocatalyzed (3+2) cycloaddition between alkenes and vinyl diazo compounds. Both Ru and Cr complexes catalyze this reactivity, and the transformation appears to proceed via vinyl diazo nucleophilic interception of a radical cation. High diastereoselectivities are obtained in the transformation, and the cycloadducts are readily diversified. To our knowledge, this is the first report of a vinyl diazo species reacting with a radical cation electrophile. We anticipate this reaction can serve as an excellent platform for accessing an array of cyclopentane-based compounds; further mechanistic studies, expansions toward enantioselective variants, and applications in chemical synthesis are currently underway.
Supplementary Material
Acknowledgements
This work was supported by the NSF and EPA through the Catalysis Collaboratory for Light-Activated Earth Abundant Reagents (C-CLEAR) (CHE-1339674). F.J.S. is supported by the NSF Graduate Research Fellowship Program (038550-02). Mass spectrometry data was acquired on an instrument supported by the NIH (S10RR028859). We thank Prof. Todd Harrop and Phan Truong for assistance with electrochemical measurements.
References
- [1].Iwasawa N, Thermal and Metal-Induced [3+2] Cycloadditions In Comprehensive Organic Synthesis II (Eds.: Knochel P, Molander GA), Elsevier, Amsterdam, 2014; vol. 5, pp 273–350. [Google Scholar]
- [2].For a recent review of cyclopentane natural product synthesis approaches, see: Ferreira AJ; Beaudry CM Tetrahedron 2017, 73, 965. [Google Scholar]
- [3].a) For recent reviews on the use of vinyl and enol diazocarbonyl compounds, see: Cheng Q-Q, Deng Y, Lankelma M, Doyle MP, Chem. Soc. Rev. 2017, 46, 5425; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) López E, González-Pelayo S, López LA, Chem. Rec. 2017, 17, 312; [DOI] [PubMed] [Google Scholar]; c) Cheng Q-Q, Yu Y, Yedoyan J, Doyle MP, ChemCatChem 2018, 10, 488. [Google Scholar]
- [4].a) For seminal examples using vinyl diazocarbonyls, see: Davies HML, Hu B, Tetrahedron Lett. 1992, 33, 455; [Google Scholar]; b) Davies HML, Hu B, Saikali E, Bruzinski PR, J. Org. Chem. 1994, 59, 4535; [Google Scholar]; c) Davies HML, Xiang B, Kong N, Stafford DG, J. Am. Chem. Soc. 2001, 123, 7461; [DOI] [PubMed] [Google Scholar]; d) Lian Y, Davies HML, J. Am. Chem. Soc. 2010, 132, 440; [DOI] [PubMed] [Google Scholar]; e) Briones JF, Davies HML, J. Am. Chem. Soc. 2013, 135, 13314; [DOI] [PubMed] [Google Scholar]; f) López E, Lonzi G, González J, López LA, Chem. Commun. 2016, 52, 9398; [DOI] [PubMed] [Google Scholar]; g) López E, González J, López LA, Adv. Synth. Catal. 2016, 358, 1428; [Google Scholar]; h) López E, López LA, Angew. Chem. Int. Ed. 2017, 56, 5121; Angew. Chem 2017, 129, 5203; [Google Scholar]; i) López E, Lonzi G, López LA, Synthesis 2017, 49, 4461. [Google Scholar]
- [5].a) For seminal examples using enol diazocarbonyls, see:Smith AG, Davies HML, J. Am. Chem. Soc. 2012, 134, 18241; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xu X, Leszczynski JS, Mason SM, Zavalij PY, Doyle MP, Chem. Commun. 2014, 50, 2462; [DOI] [PubMed] [Google Scholar]; c) Deng Y, Yglesias MV, Arman H, Doyle MP, Angew. Chem. Int. Ed. 2016, 55, 10108; Angew. Chem 2016, 128, 10262; [DOI] [PubMed] [Google Scholar]; d) Jing C, Cheng Q-Q, Deng Y, Arman H, Doyle MP, Org. Lett. 2016, 18, 4550; [DOI] [PubMed] [Google Scholar]; e) Deng Y, Massey LA, Rodriguez Núñez YA, Arman H, Doyle MP, Angew. Chem. Int. Ed. 2017, 56, 12292; Angew. Chem 2017, 129, 12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) C=O and C=N bonds have also been utilized as the 2-atom component to form heterocycles. For examples, see: Doyle MP, Hu W, Timmons DJ, Org. Lett. 2001, 3, 3741; [DOI] [PubMed] [Google Scholar]; b) Doyle MP, Yan M, Hu W, Gronenberg LS, J. Am. Chem. Soc. 2003, 125, 4692; [DOI] [PubMed] [Google Scholar]; c) Barluenga J, Lonzi G, Riesgo L, López LA, Tomás M, J. Am. Chem. Soc. 2010, 132, 13200. [DOI] [PubMed] [Google Scholar]
- [7].For a related (3+2) cycloaddition between aryl diazoacetates and alkynes, see: Park EJ, Kim SH, Chang S, J. Am. Chem. Soc. 2008, 130, 17268. [DOI] [PubMed] [Google Scholar]
- [8].a) For isolated cases of vinyl diazo nucleophilicity, see:ref 6b; [Google Scholar]; b) Barluenga J, Lonzi G, Riesgo L, Tomás M, López LA, J. Am. Chem. Soc. 2011, 133, 18138; [DOI] [PubMed] [Google Scholar]; c) Jadhav AM, Pagar VV, Liu R-S, Angew. Chem. Int. Ed. 2012, 51, 11809; Angew. Chem 2012, 124, 11979; [DOI] [PubMed] [Google Scholar]; d) Pagar VV, Jadhav AM, Liu R-S, J. Org. Chem. 2013, 78, 5711; [DOI] [PubMed] [Google Scholar]; e) Pagar VV, Liu R-S, Org. Biomol. Chem. 2015, 13, 6166; [DOI] [PubMed] [Google Scholar]; f) Xu G, Zhu C, Gu W, Li J, Sun J, Angew. Chem. Int. Ed. 2015, 54, 883; Angew. Chem. 2015, 127, 897. [DOI] [PubMed] [Google Scholar]
- [9].The enol subunit of an enol diazocarbonyl has been invoked as a nucleophile (e.g., Mukaiyama aldol-type, etc.). See ref 3a for discussion and references.
- [10].a) Del Bel M, Rovira A, Guerrero CA, J. Am. Chem. Soc. 2013, 135, 12188; [DOI] [PubMed] [Google Scholar]; b) For a related example in synthesis, see, Hou S-H, Tu Y-Q, Liu L, Zhang F-M, Wang S-H, Zhang X-M, Angew. Chem. Int. Ed. 2013, 52, 11373; Angew. Chem 2013, 125, 11583. [Google Scholar]
- [11].a) For select reviews, see:Prier CK, Rankic DA, MacMillan DWC, Chem. Rev 2013, 113, 5322; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xuan J, Xiao W-J, Angew. Chem. Int. Ed 2012, 51, 6828; Angew. Chem 2012, 124, 6934; [DOI] [PubMed] [Google Scholar]; c) Narayanam JMR, Stephenson CRJ, Chem. Soc. Rev 2011, 40, 102; [DOI] [PubMed] [Google Scholar]; d) Angnes RA, Li Z, Correia CRD, Hammond GB, Org. Biomol. Chem 2015, 13, 9152; [DOI] [PubMed] [Google Scholar]; e) Yoon TP, ACS Catal. 2013, 3, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12] a).Huang X, Webster RD, Harms K, Meggers E, J. Am. Chem. Soc. 2016, 138, 12636; [DOI] [PubMed] [Google Scholar]; b) Rybicka-Jasinska K, Ciszewski LW, Gryko D, Adv. Synth. Catal. 2016, 358, 1671; [Google Scholar]; c) Sarabia FJ, Ferreira EM, Org. Lett. 2017, 19, 2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) For reports of this process using triarylaminium catalysts, see: Stufflebeme G, Lorenz KT, Bauld NL, J. Am. Chem. Soc. 1986, 108, 4234; [Google Scholar]; b) Bauld NL, Stufflebeme GW, Lorenz KT, J. Phys. Org. Chem. 1989, 2, 585; [Google Scholar]; c) Yueh W, Bauld NL, J. Am. Chem. Soc. 1995, 117, 5671. [Google Scholar]
- [14].For a photoredox-catalyzed (3+2) cycloaddition between acylcyclopropanes and alkenes, see: Lu Z, Shen M, Yoon TP, J. Am. Chem. Soc. 2011, 133, 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15] a).For a review, see: Baldwin JE, Chem. Rev. 2003, 103, 1197; [DOI] [PubMed] [Google Scholar]; b) For an example of a radical-cation based VCP rearrangement, see, Dinnocenzo JP, Conlon DA, J. Am. Chem. Soc. 1988, 110, 2324. [Google Scholar]
- [16].Higgins RF, Fatur SM, Shepard SG, Stevenson SM, Boston DJ, Ferreira EM, Damrauer NH, Rappé AK, Shores MP, J. Am. Chem. Soc. 2016, 138, 5451. [DOI] [PubMed] [Google Scholar]
- [17].The diastereoselectivity observed in spirocycles 3la and 3ma likely arises due to similar principles as those in the 1,2-trans substituted alkenes. The diastereoselectivity of 3qa presumably arises from preferential facial attack opposite the large t-Bu group.
- [18].a) Taylor EC, Turchi IJ, Chem. Rev. 1979, 79, 181; [Google Scholar]; b) Huisgen R, Angew. Chem. Int. Ed. Engl. 1980, 19, 947; Angew. Chem 1980, 92, 979. [Google Scholar]
- [19].Pyrazole formation was also observed in minute amounts in reactions using non-γ-substituted vinyl diazo compounds.
- [20].See the Supporting Information for details.
- [21].Measurements: [Cr(Ph2phen)3]3+ in CH3NO2 (ref 17); [Ru(bpz)3]2+ in CH3CN (ref 11a); 1a in CH3NO2 (ref 17); 2a in CH3NO2 (Supporting Information).
- [22].Interestingly, the (3+2) could also be mediated using Bauld’s oxidizing triarylaminium catalyst, albeit in moderate yield (47%). See the Supporting Information.
- [23].The scheme depicts the nucleophile attack on the alkene radical cation by 2a as a 2-electron process, consistent with analogous proposed 2-electron attacks on similar species. For example, see, Wu F, Wang L, Chen J, Nicewicz DA, Huang Y, Angew. Chem. Int. Ed. 2018, 57, 2174; Angew. Chem 2018, 130, 2196. [Google Scholar]
- [24].a) Vinyldiazo 2a could also be conceived as a 1-electron reactant, which has been invoked for non-vinyl diazo esters and other diazo compounds. This radical mode of nucleophilic attack essentially yields resonance forms of intermediate 7/8, which may or may not be more significant contributors to the hybrid depending on specific substitution patterns. For isolated examples invoking non-vinyl diazo esters and other diazo compounds as 1-electron reactants, see:ref 12b; [Google Scholar]; b) Dang H-S, Roberts BP, J. Chem. Soc., Perkin Trans. 1 1996, 769; [Google Scholar]; c) Li Y, Chen C, Li F, Liao L, Liu L, Polym Chem. 2017, 8, 2881; [Google Scholar]; d) Wang Y, Wen X, Cui X, Wojtas L, Zhang XP, J. Am. Chem. Soc 2017, 139, 1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].This regioselectivity could not be forced in the other direction via intramolecular tethering. See the Supporting Information for details.
- [26].Both catalyst and light were necessary for this rearrangement.
- [27].5-endo radical cyclizations are rare but have been reported.
- [28].For additional mechanistic discussion, see the Supporting Information.
- [29].Cismesia MA, Yoon TP, Chem. Sci. 2015, 6, 5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].In contrast to the Cr-catalyzed (4+2) cycloadditions, O2 was not beneficial for this reaction.
- [31].Yu J-Q, Corey EJ, J. Am. Chem. Soc. 2003, 125, 3232. [DOI] [PubMed] [Google Scholar]
- [32].O’Connor M, Sun C, Lee D, Angew. Chem. Int. Ed. 2015, 54, 9963; Angew. Chem 2015, 127, 10101. [DOI] [PubMed] [Google Scholar]
- [33].Mg/MeOH reduction afforded a ~1:1 mixture of diastereomers, and the other diastereomer could then be obtained pure after ester cleavage. See the Supporting Information for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.