Abstract
Immunotherapy based on the immune checkpoint blockade has emerged as the most promising approach for cancer therapy. However, the proportion of colorectal cancer patients who benefit from immunotherapy is small due to the immunosuppressive tumor microenvironment. Hence, combination immunotherapy is an ideal strategy to overcome this limitation. In this study, we developed a novel combination of CSF-1R (colony-stimulating factor 1 receptor) inhibitor (PLX3397), oncolytic viruses, and anti-PD-1 antibody. Our results demonstrated that the triple treatment synergistically conferred significant tumor control and prolonged the survival of mouse models of colon cancer. Approximately 43% and 82% of mice bearing the CT26 and MC38 tumor, respectively, survived long term following the triple treatment. This combination therapy reprogrammed the immunosuppressive tumor microenvironment toward a CD8+ T cell-biased anti-tumor immunity by increasing T cell infiltration in the tumor and augmenting anti-tumor CD8+ T cell function. Our results provide a robust strategy for clinical combination therapy.
Keywords: combination immunotherapy, tumor microenvironment, anti-PD-1 antibody, oncolytic virus, CSF-1R inhibitor
The tumor microenvironment develops multiple immune escape mechanisms, thus limiting the immunotherapy efficacy of monotherapy. Deng and colleagues design a novel combination of oncolytic viruses, CSF-1R inhibitor (PLX3397), and anti-PD-1. This combination strategy reprograms the immunosuppressive microenvironment to facilitate anti-tumor immunity, and it induces tumor regression and long-term protection in mouse colon cancer models.
Introduction
Immunotherapy has shown tremendous promise in cancer treatment.1, 2 Therapeutic antibodies targeting programmed cell death protein 1 (PD-1)/programmed cell death 1 ligand 1 (PD-L1) have resulted in major breakthroughs in cancer treatment.3, 4 Nevertheless, the durable responses induced by PD-1 or PD-L1 blockade alone are limited in patients with colorectal cancer,5, 6 and the poor therapeutic effects have been linked to tumor-intrinsic or -extrinsic mechanisms for escaping immune surveillance.7, 8, 9, 10 Recent preclinical studies have indicated that combination strategies would be an ideal approach to overcome immunosuppression, thus improving the therapeutic efficacy.
Tumor-associated macrophages (TAMs)—one of the limiting factors in anti-tumor immunity—are associated with the poor clinical outcome in most carcinomas, owing to their potential to promote angiogenesis and local invasion, increase the metastasis of tumor cells, and inhibit the anti-tumor immunity.11, 12 Macrophages are highly dependent on the CSF-1 (colony-stimulating factor 1)/CSF-1R (CSF-1 receptor) pathway for survival and differentiation in most tissues. Therapeutic disruption of the CSF-1/CSF-1R pathway is of significant interest, as CSF-1 is highly expressed in several tumors.13 Certain CSF-1R inhibitors (e.g., PLX3397) were shown to delay tumor growth in certain cancers.14, 15
Low T cell infiltration in most tumors is another limitation for poor outcomes of immunotherapy. Previous studies have indicated increased T cell infiltration to be an indispensable predictive biomarker for better prognosis.16, 17 Hence, therapies to increase T cell infiltration would theoretically exhibit a synergetic effect with anti-PD-1 immunotherapy. Oncolytic virotherapy is a potential approach to increase T cell infiltration of tumor due to its advantages to selectively infect and kill tumor cells.18, 19 Oncolytic viruses (OVs) can lyse tumor cells directly and release tumor-associated antigens, which are presented by antigen-presenting cells to activate host anti-tumor immunity.20 Thus, OVs provide an ideal means to reverse the immunosuppressive tumor microenvironment and render tumors sensitive to the immune checkpoint blockade.21
Although, a series of combination strategies has been tested in preclinical models and clinical settings, including combination with chemotherapy, radiotherapy, targeted therapy, and immunotherapy,22, 23, 24, 25 effective and rational combination approaches need to be explored to confer better therapeutic effects. Here we provide evidence that significant effects can be achieved by simultaneously combining a CSF-1R inhibitor (PLX3397) with OVs and anti-PD-1 antibody and this combination strategy could potentially overcome the limitations of low T cell infiltration and immunosuppression of TAMs and PD-1. This strategy might greatly improve the therapeutic efficacy of anti-PD-1 immunotherapy in colon cancer, the responses to which remain clinically limited.8 From the present investigation, we have proposed a novel combination strategy for improving the effects of immunotherapy in cancer patients.
Results
TAMs and PD-1/PD-L1 Are Highly Enriched in Tumors
To understand the immunosuppressive status within the tumor microenvironment, we determined the TAMs in CT26 and MC38 tumor-bearing mice. Results revealed that TAMs accounted for approximately 50% of the total tumor-infiltrating immune cells (Figures 1A and 1B). The M2 phenotype marker CD206 was expressed by 25%–45% of TAMs (Figure 1C). These M2 macrophages function to suppress the anti-tumor immunity.26 Our results further demonstrated that these tumor cells constitutively secreted CSF-1 in vitro (Figure 1D), suggesting an intrinsic mechanism for TAM recruitment and survival. These data indicated that immunosuppressive TAMs are enriched in the tumor microenvironment.
Figure 1.
Immunosuppressive Signals Are Enriched within the Tumor Microenvironment
(A and B) Flow cytometry analysis of TAMs (CD11b+F4/80+) within mouse colon cancer (CT26 and MC38) (A) and representative flow data (B) statistics, gated on the CD45+ cells (n = 3 mice per group). (C) The CD206 expression on TAMs (n = 3 mice per group). (D) CSF-1 expression by tumor cells in vitro (three technical replicates). (E–H) Flow cytometry analysis of co-inhibitory molecules on tumor-infiltrating T cells, gated on CD3+ cells. Representative histograms show the expressions of PD-1, TIGIT, and LAG-3 on CD4+ (E) and CD8+ (G) T cells within CT26 tumor. The expressions of PD-1, TIGIT, and LAG-3 on CD4+ and CD8+ T cells in mouse colon cancer (CT26 subcutaneous model, CT26 orthotopic models and MC38 model) are shown in (F) and (H), respectively (n = 3 mice per group). (I) PD-L1 expression on tumor cells in vitro. (J) IHC detection of macrophage (CD68) and PD-L1 expression in human colon cancer (scale bars, 100 μm).
We next focused on the expression of co-inhibitory molecules on T cells. For CD4+ T cells, except for low expression of lymphocyte activation gene 3 (LAG-3), PD-1 and T cell immunoreceptor with immunoglobulin (Ig) and immunoreceptor tyrosine-based inhibition motif (ITIM) domain (TIGIT) expression ranged from 49.35% to 68.22% in the three colon cancer models (Figures 1E and 1F). However, PD-1 was highly expressed on almost all of the CD8+ T cells in the mouse tumor models (Figures 1G and 1H). TIGIT and LAG-3 expression levels ranged from 29.14% to 46.97% and from 27.41% to 42.4%, respectively (Figures 1G and 1H). We also detected PD-L1 expression on tumor cells in vitro (Figure 1I), and we observed low T cell infiltration at the tumor margin (Figures S1A–S1C). These data suggest that T cells infiltrating the tumor are low and severely exhausted.
To elucidate whether these observed results in mouse models are consistent in human colon cancer, we evaluated the status of enriched TAMs and co-inhibitory molecules in human colon cancer tissue samples. As expected, CD68 and PD-L1 were enriched in human colon cancers (Figure 1J). Taken together, these results indicate that the tumor microenvironment is armed with multiple immunosuppressive mechanisms.
Combination of PLX3397 and Anti-PD-1 Does Not Enhance T Cell Infiltration
Based on the above findings, we hypothesized that combining TAMs and PD-1 inhibition simultaneously could overcome the immunosuppressive tumor microenvironment and control the tumor growth in vivo. We established a subcutaneous CT26 colon cancer model, and we treated the mice with anti-PD-1 antibodies following the schedule (Figure 2A) upon achieving a tumor volume of 50–100 mm3. To simplify the description in the figures, the combinations of these components are referred to by their single initials, I (CSF-1R inhibitor PLX3397) and P (anti-PD-1), henceforth. We found that the therapeutic efficacy of a single agent was poor, whereas I + P exhibited synergistic effects and restrained the tumor progression (Figure 2A).
Figure 2.
The Anti-tumor Effects of PLX3397 and Anti-PD-1 Alone or Together
(A) Mice were treated as shown in the scheme; tumor size was monitored individually (n = 6 mice per group). (B) The percentage of TAMs in the tumor microenvironment after treatment, gated on CD45+ cells (n = 6 mice per group). (C) The gene expression of Arg1 and CD206 in tumor after PLX3397 treatment (n = 4–5 mice per group). (D) The CD206 expression on TAMs, gated on CD11b+F4/80+ (n = 6 mice per group). (E) The PD-1 expression on tumor-infiltrating T cells after anti-PD-1 treatment (n = 5 mice per group). (F) The percentage of total T cells in the tumor microenvironment after treatment, gated on total live cells (n = 6 mice per group). (G) The proportion of Tregs (CD4+CD25+FoxP3+) in tumor tissues after anti-PD-1 treatment (n = 5 mice per group). Mean ± SEM is shown. *p < 0.05, **p < 0.01, ***p < 0.001; ns, no statistical significance.
To explore the mechanisms behind this effect, flow cytometric analysis of tumor-infiltrating lymphocytes was performed, which demonstrated that the proportion of TAMs within the tumor was reduced upon PLX3397 treatment (Figure 2B). Further, we observed that the TAM-associated immunosuppression genes were also downregulated in tumor tissues (Figure 2C). A previous study reported that CSF-1R inhibition alters the TAM phenotype.14 However, we did not detect any significant alteration of CD206+ TAMs (Figure 2D); further gene expression analysis of TAMs isolated from the tumor tissues also confirmed this result (Figure S2A), suggesting that PLX3397 inhibits the immunosuppression by reducing the percentage of TAM population rather than polarization. Anti-PD-1 treatment downregulated the expression of PD-1 on CD8+ T cells rather than CD4+ T cells (Figure 2E), suggesting that anti-PD-1 reversed CD8+ T cell dysfunctions. The results demonstrate the ability of PLX3397 and anti-PD-1 to overcome immunosuppression in the tumor microenvironment and show significant tumor control in vivo. However, we did not observe any increase in T cell and the total immune cell population in the tumor after monotherapy or combination therapy (Figure 2F; Figure S2B). Further analysis demonstrated that anti-PD-1 did not alter the percentage of TAMs and regulatory T cells (Tregs) (Figures 2B and 2G). In addition, PLX3397 exhibited no effects on T cell function, which was evident from the PD-1 and inducible costimulatory molecule (ICOS) expression status (Figures S2C and S2D).
In brief, our results demonstrated that a combination of PLX3397 and anti-PD-1 repressed the non-redundant immune escape mechanisms and restrained tumor growth, but the therapeutic efficacy was undesirable. As no increased T cell infiltration was observed after combination treatment, we proposed that this might be one of the main reasons for the limited outcomes of the regimen.
OVs Lyse Tumor Cells and Promote T Cell Infiltration of Tumor
Previous research data have proven that an increased proportion of T cells is closely related to the effects of the PD-1 blockade.17 As indicated in the above results, a combination of PLX3397 and anti-PD-1 did not augment the number of tumor-infiltrating T cells. Theoretically, therapies that could induce T cell recruitment would be the ideal candidates to improve the therapeutic effects of such a regimen. Previous studies revealed that OVs possess the ability to modulate tumor microenvironment and attract T cells.21, 27, 28 Therefore, we chose oncolytic adenoviruses to overcome the low T cell infiltration and improve the outcome.
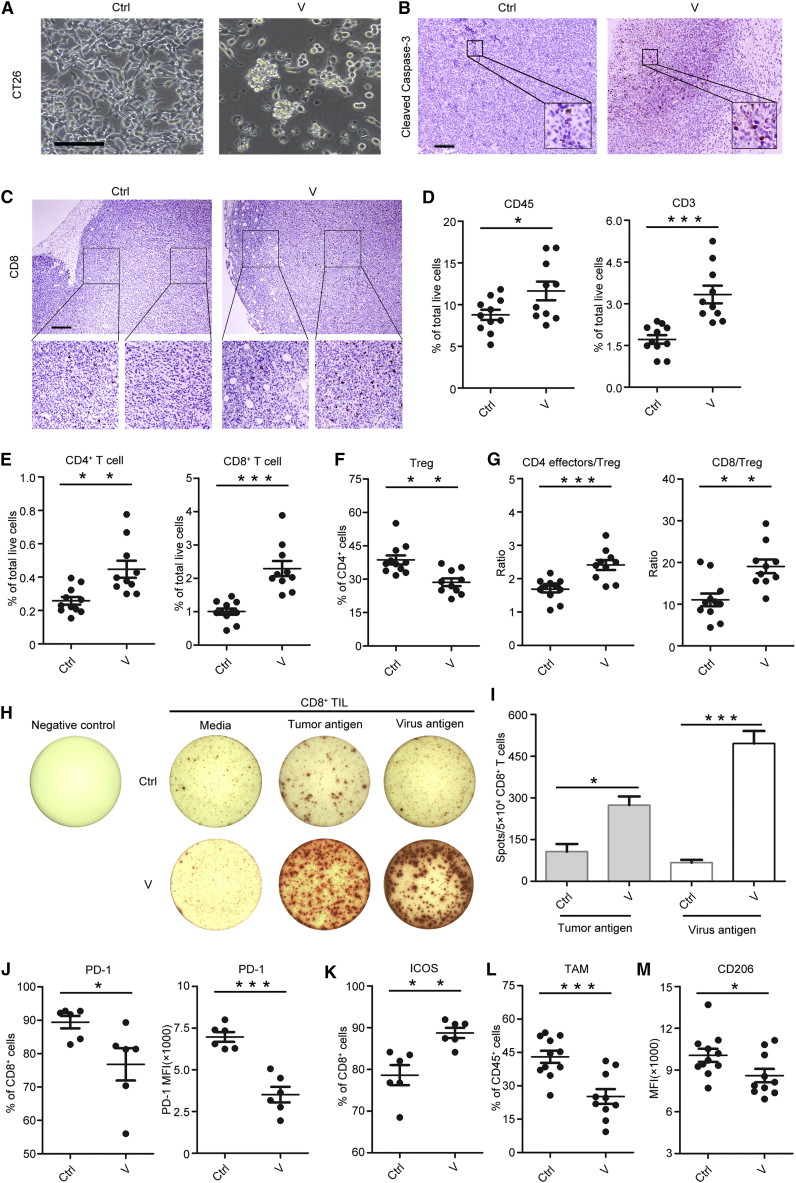
We initially evaluated the oncolytic abilities of the virus in vitro and in vivo. To simplify the description in the figures, the OVs are referred to as V henceforth. OV infection induced CT26 tumor cell lysis in vitro and promoted apoptosis in the tumor tissue (Figures 3A and 3B). Mice with intratumoral OV injection attracted more T cells from the tumor margin to the tumor center (Figure 3C; Figures S3A and S3B). Flow cytometry results also demonstrated the increase in the population of total immune cells (CD45+) and T cells (CD3+) in the tumor following OV injection (Figure 3D). Further analysis indicated that OVs increased the percentage of CD4+ and CD8+ T cells in the tumor (Figure 3E) and reduced the percentage of Tregs (Figure 3F), leading to an increase in the ratios of CD4+ effector T cells (CD44high CD62Llow) to Tregs and CD8+ T cells to Tregs (Figure 3G). This increased ratio of effector T cells to Tregs suggested that OVs alter the immune balance of tumor-infiltrating T cells and facilitate anti-tumor immunity.
Figure 3.
OVs Increase T Cell Infiltration
(A) CT26 tumor cells lyse after 72 hr by OV infection (MOI = 200) (scale bar, 100 μm). (B) Immunohistochemistry staining of cleaved caspase-3 within tumor after OV injection (scale bar, 100 μm). (C) Immunohistochemistry staining of CD8+ T cells within CT26 tumor after OV injection (scale bar, 250 μm). (D) The percentage of total immune cells (CD45+) and T cells (CD3+) in tumor after OV treatment (n = 10–11 mice per group). (E) The percentage of CD4+ T cells and CD8+ T cells within tumors (n = 10–11 mice per group). (F) The percentage of Tregs, gated on CD4+ T cells (n = 10–11 mice per group). (G) The ratios of CD4 effector cells (CD44high CD62Llow):Tregs (left) and CD8:Tregs (right) (n = 10–11 mice per group). (H) CD8+ tumor-infiltrating lymphocytes (TILs) induced by OVs respond to both tumor and virus antigen. (I) The amount of spot per 5 × 104 CD8+ TILs (n = 3 mice per group). (J) PD-1 expression on CD8+ T cells. The percentage and mean fluorescence intensity (MFI) are shown (n = 6 mice per group). (K) The expression of ICOS on CD8+ T cells (n = 6 mice per group). (L) The proportion of TAMs within tumor, gated on CD45+ cells (n = 10–11 mice per group). (M) The CD206 expression on TAMs (n = 10–11 mice per group). Mean ± SEM is shown. *p < 0.05, **p < 0.01, ***p < 0.001.
To test whether the increased T cells were tumor specific or virus specific, the tumor-infiltrating CD8+ T cells were isolated to detect interferon (IFN)-γ production using the enzyme-linked immunospot assay (ELISPOT) assay. Enhanced IFN-γ production was observed in response to stimulation with both tumor and viral antigens (Figures 3H and 3I). We also determined the expression of functional molecules on T cells. Results demonstrated that PD-1 expression was significantly downregulated on CD8+ T cells instead of CD4+ T cells upon OV treatment (Figure 3J; Figure S3C). Meanwhile, ICOS expression was upregulated on both CD4+ and CD8+ T cells (Figure 3K; Figure S3D), which indicated the activation of T cells. Moreover, OV treatment reduced the percentage of TAMs within the tumor microenvironment (Figure 3L). Importantly, OVs reduced CD206 expression (Figure 3M), suggesting a decrease in the M2 phenotype in the tumor.
These results demonstrated that OVs potentially reprogram the tumor microenvironment and facilitate T cell infiltration and activation.
Triple Combination Therapy Effectively Promotes Tumor Regression and Prolonged Survival
We next investigated the possibility of OVs to improve the therapeutic effects of PLX3397 and anti-PD-1. We established a subcutaneous CT26 colon cancer model and treated the mice following the schedule (Figure S4A). OVs and anti-PD-1 were administered when the tumor volume reached 50–100 mm3. We found that the single treatment failed to effectively restrain tumor growth; however, in combination, the therapeutic efficacy was enhanced, especially with the triple combination, which remarkably delayed the tumor growth, leading to complete tumor regression in 43% (3/7) of the mice (Figure 4A), and prolonged survival (Figure 4B).
Figure 4.
Triple Treatment Exhibits Significant and Specific Therapeutic Effects in Mouse Colon Cancer
(A and B) For subcutaneous CT26 model, (A) individual tumor growth and (B) overall survival. (C–F) For orthotopic CT26 model, (C and D), individual tumor growth is evaluated via bioluminescence on days 11 and 16 after inoculation (C, representative image of bioluminescence, n = 3 mice per group; D, average radiance, n = 8 mice per group). (E) Survival (n = 10 mice per group). (F) Tumor weight (n = 8 mice per group). (G–I) Mice surviving from triple treatment are re-challenged with a higher dose (1 × 106) of CT26 cells (G) and 4T1 cells (H) at 2 months after the cessation of OV and anti-PD-1 antibody injection; the incidence of tumor is evaluated on day 20 after rechallenge (n = 6–12 mice per group). (I) Tumor growth of mice that are re-challenged with 4T1 cells (n = 6 mice per group). Mean ± SEM is shown. *p < 0.05, **p < 0.01, ***p < 0.001; ns, no statistical significance.
We next determined whether the triple combination strategy would still be effective against a larger tumor by injecting OVs and anti-PD-1 simultaneously into the mice with the tumor volume of 200 mm3. As expected, the triple treatment arrested the tumor growth more effectively than single treatment or dual treatment (Figure S4B). Several clinical trials have shown that the inhibition of CSF-1R or combination of anti-PD-1 and anti-CTLA-4 are associated with severe toxicity.29, 30, 31 Strikingly, we did not observe any abnormal behavior in mice receiving PLX3397 alone or the triple combination during the course of the treatment. Further pathological staining of the heart, liver, spleen, lung, and kidney tissues also confirmed this observation (Figure S5). To mimic the actual disease progression, we implanted CT26-luc cells in situ and treated the mice with the triple combination. Our data demonstrated that all the mice receiving the triple treatment exhibited tumor regression (Figures 4C and 4D) and prolonged survival (Figure 4E). The therapeutic efficacy was further confirmed with respect to changes in the tumor weight and volume (Figure 4F; Figure S4C). These data provide strong evidence for the significant anti-tumor effects of the triple combination therapy.
Triple Combination Therapy Elicits a Durable and Specific Anti-tumor Immune Response
To investigate whether the combination strategy confers specific and lifelong therapeutic effects, we performed a re-challenge assay and adoptive treatment. All the mice surviving from the triple combination treatment completely rejected a re-challenge with a higher dose (1 × 106) of CT26 cells (Figure 4G). With respect to the long-term effects, none of the mice developed a tumor at the end of the experiment, which was 10 months after re-challenge (data not shown), indicating an effective immunological memory. However, when re-challenged with 1 × 106 4T1 breast tumor cells, long-term protection and tumor rejection were not observed (Figures 4H and 4I), suggesting a tumor-specific immune response. Similarly, T cells isolated from CT26 tumor-bearing mice treated with the triple combination successfully decreased the tumor burden after transfer to the mice bearing the same tumor (Figure S6A). However, the tumor control was abolished in mice bearing 4T1 breast tumors (Figure S6B), further verifying the specificity of anti-tumor immunity. In addition, we also observed that the mice receiving triple treatment generated tumor-specific endogenous antibodies, which recognized numerous tumor antigens (Figure S6C). These antibodies were not robust enough to induce tumor rejection, which was confirmed by serum adoptive assay (Figure S6D), suggesting that the antibody response was not essential for the therapeutic effect of this triple combination regimen. In summary, these data proved that the therapeutic effects generated by the triple combination are long term and highly specific.
Combination Therapy Increases T Cell Infiltration and Induces CD8+ T Cell-Dependent Anti-tumor Immune Response
Given the key roles of T cells in anti-tumor immunity,17 we attempted to determine the T cell infiltration in the tumor microenvironment via multiplex immunohistochemistry (mIHC). Combination therapy significantly increased the extent of CD4+ and CD8+ T cell infiltration in tumor tissues and reduced the Tregs population (Figure 5A; Figure S7A). To substantiate this result, we analyzed the T cell profiles in tumor tissues by flow cytometry. The triple combination therapy-receiving group exhibited more infiltration of T cells in the tumor microenvironment than the groups receiving dual combination treatments or the control group (Figures 5B–5D). We also observed that the combination therapy reduced Tregs (Figure 5E), leading to increasing ratios of CD4+ effector T cells (CD44high CD62Llow) to Tregs and CD8+ T cells to Tregs (Figures 5F and 5G). However, the combination of PLX3397 and anti-PD-1 did not induce any noticeable changes, which was in line with the previous data in Figure 2F. These results revealed that the triple combination therapy induced T cell infiltration in the tumor and reduced the percentage of Tregs. This, in turn, increased the ratios of CD4+ effector T cells to Tregs and effector CD8+ T cells to Tregs, and it promoted tumor cell killing by T cells. The increased T cell infiltration and reduced Tregs were also observed in the orthotopic colon cancer model (Figure S7B). Moreover, we also found increased CD8+ T cells responded to both tumor and virus antigen in triple treatment and dual treatment with OVs and anti-PD-1 (Figures S8A and S8B).
Figure 5.
Triple Treatment Increases T Cell Infiltration and Induces CD8+ T Cell-Dependent Anti-tumor Immunity
(A) Multiplex immunohistochemistry (mIHC) detection of T cell infiltration in CT26 tumor tissue (scale bars, 50 μm). (B–D) Infiltration of total T cells (B), CD4+ T cells (C), and CD8+ T cells (D) within CT26 tumor. (E) The percentage of Tregs (CD4+CD25+FoxP3+), gated on CD4+ T cells. (F) The ratios of CD4 effector T cells (CD44high CD62Llow) to Tregs. (G) The ratios of CD8+ T cells to Tregs (n = 10–11 mice per group for B–G). (H) Tumor growth of CD4+ T cells or CD8+ T cell depletion assay (n = 9–12 mice per group). Mean ± SEM is shown. *p < 0.05, **p < 0.01, ***p < 0.001; ns, no statistical significance.
To understand which T cell subsets are responsible for tumor cell killing, the CD4+ or CD8+ T cells were depleted. The CT26 tumor-bearing mice were treated as shown in Figure S4A, and the depletion efficacy was validated (Figure S7C). The mice with depleted CD8+ T cells showed complete abrogation of tumor rejection (Figure 5H). Hence, it is evident that the CD8+ T cells are required to achieve therapeutic efficacy.
In summary, these results indicated that the triple combination treatment attracted more T cells into the tumor microenvironment and elicited a CD8+ T cell-dependent anti-tumor immune response. Of note, the therapy reduced the Tregs in the tumor, leading to altered immune balance toward an anti-tumor bias at the cellular level.
Combination Therapy Reverses TAM-Mediated Immunosuppression
To study the effects of the triple combination treatment on TAMs within the tumor microenvironment, we analyzed their population and functional signaling molecules. The triple combination treatment inhibited the recruitment of TAMs into the tumor (Figures 6A and 6B) and reprogrammed TAMs toward the M1 phenotype, in which the percentage of CD206+ TAMs was reduced in mice receiving the triple treatment (Figure 6C). Further study of functional genes via qRT-PCR suggested that the triple combination downregulated the expression of immunosuppressive genes (Cd68, Cd206, Msr1, and Arg1) and upregulated the expression of the proinflammatory gene iNOS (Figure 6D). Taken together, the triple combination not only reduced the number of TAMs but also changed TAMs to the M1 phenotype.
Figure 6.
Triple Treatment Reverses Immunosuppressive Microenvironment to Favor Anti-tumor Immunity
(A) Immunofluoresence staining of TAMs (F4/80+) within CT26 tumor tissue (scale bar, 50 μm). (B) The percentage of TAMs in CT26 tumor, gated on CD45+ cells (n = 10–11 mice per group). (C) The CD206 expression on TAMs, gated on CD11b+F4/80+ cells (n = 10–11 mice per group). (D) qRT-PCR analysis of macrophage function-associated gene expression (n = 7 mice per group). (E) ICOS expression level on CD8+ T cells (n = 5–6 mice per group). (F) Expression of co-inhibitory molecules (PD-1, LAG-3, TIGIT, and Tim-3) on CD8+ T cells (n = 5–9 mice per group). (G) The percentage of PD-1+LAG-3+, PD-1+TIGIT+, and PD-1+Tim-3+ double-positive CD8+ T cells (n = 5–9 mice per group). (H) Co-expression of PD-1, TIGIT, and LAG-3 on CD4+ and CD8+ T cells, respectively (n = 8–9 mice per group). (I) Enrichment of genes related to T cell recruitment within CT26 tumor tissue after therapy (n = 7 mice per group). (J) Upregulation of genes associated with T cell activation and response in CT26 tumor after treatment (n = 7 mice per group). Mean ± SEM is shown. *p < 0.05, **p < 0.01, ***p < 0.001; ns, no statistical significance.
Combination Therapy Decreases the Levels of Co-inhibitory Molecules and Enhances T Cell Activation
To further explore the detailed mechanism of the observed anti-tumor effects, we investigated the expression levels of co-stimulatory and co-inhibitory molecules on T cells within the tumor microenvironment. The triple combination treatment significantly increased the expression levels of ICOS on CD8+ T cells, but not on CD4+ T cells (Figure 6E; Figure S9C). In addition, it also induced the expansion of IFN-γ-producing CD8+ T cells, and not CD4+ T cells, in the spleen and draining lymph nodes (Figures S9A and S9B).
We next focused on the co-inhibitory molecules expressed on CD8+ T cells, which are tightly related with T cell dysfunction. Mice receiving the triple combination treatment showed reduced expression levels of PD-1, LAG-3, TIGIT, and Tim-3 (Figure 6F). In addition, the numbers of PD-1+Tim-3+, PD-1+LAG-3+, and PD-1+TIGIT+ double-positive CD8+ T cells, which indicate a severely exhausted status,32 were significantly decreased (Figure 6G). Furthermore, the percentage of PD-1+TIGIT+LAG-3+ T cells was also reduced in both CD4+ and CD8+ T cells (Figure 6H). We also analyzed the expression of these functional molecules in CD4+ T cells, in which the triple combination treatment downregulated the expression of LAG-3 and TIGIT, but not PD-1 and Tim-3 expression (Figure S9E). The percentage of PD-1+LAG-3+ and PD-1+TIGIT+ cells were reduced, but not PD-1+Tim-3+ cells, after the treatment (Figure S9F). We also observed that the genes associated with T cell recruitment (e.g., CCL5 and CXCL10) and with the T cell activation or response (e.g., Gram B, perforin, and IFN-γ) were enriched within the tumor following the triple treatment (Figures 6I and 6J).
These results indicated that the triple combination not only increased the T cell infiltration and the ratio of effector T cells to Tregs (Figures 5A–5G) but also activated the T cells and restrained multiple co-inhibitory signals besides PD-1. Taken together, we conclude that the triple combination treatment removed the harness mediated by co-inhibitory molecules on T cells and activated effective T cells, resulting in enhanced anti-tumor immunity and tumor killing.
Triple Combination Treatment Abrogates Tumor Development in MC38 Colon Cancer Model
Finally, to extend the application of the triple combination treatment to another colon cancer model, we tested the combination strategies in mouse MC38 colon cancer. The triple treatment elicited a notable anti-tumor ability, induced complete tumor clearance in 82% (9/11) of the mice (Figure 7A), and prolonged the overall survival (Figure 7B). Analysis of immune cell profiles demonstrated an increase in T cell population in the tumor tissues after therapy (Figure 7C). Tregs were reduced in mice receiving treatment (Figure 7D), resulting in increased ratios of CD4+ effector T cells to Tregs and CD8+ T cells to Tregs (Figure 7E). Then, we evaluated the T cell function by analyzing the expression of co-stimulatory and co-inhibitory molecules. The expression of ICOS on both CD4+ and CD8+ T cells was significantly upregulated in mice after triple treatment (Figure 7F), suggesting a higher activation level and strong tumor-killing effects. With regard to co-inhibitory molecules, the expressions of PD-1, LAG-3, TIGIT, and Tim-3 on both CD4+ and CD8+ T cells were significantly reduced in the mice after therapy (Figure 7G; Figure S10A), indicating a weak immunosuppression to the T cells. Further analysis revealed a substantial reduction in the percentage of PD-1+Tim-3+, PD-1+LAG-3+, and PD-1+TIGIT+ double-positive CD8+ T cells (Figure 7H), as well as PD-1+TIGIT+LAG-3+ T cells (Figure 7I). Similar results were also observed in CD4+ T cells (Figures S10B and S10C). Reduced inhibitory signals, in turn, improved T cell activation and the immune response, in line with the previous results. Finally, we assessed TAM infiltration in the tumor. The triple treatment reduced the percentage of TAMs without altering the M2 phenotype (Figure S10D), indicating that the main impact of triple treatment on TAMs was to decrease the TAM population without changing the M2 phenotype.
Figure 7.
Triple Treatment Is Also Effective in Mouse MC38 Colon Cancer
(A) Tumor growth (n = 10–11 mice per group). (B) Overall survival (n = 10–11 mice per group). (C) The percentage of total T cells (CD3+), CD4+ T cells, and CD8+ T cells in tumor (n = 5 mice per group). (D) The percentage of Tregs (CD4+CD25+FoxP3+), gated on CD4+ T cells (n = 5 mice per group). (E) The ratios of CD4 effector T cells (CD44high CD62Llow) to Tregs (left) and CD8+ T cells to Tregs (right) (n = 5 mice per group). (F) The expression of ICOS on CD4+ T and CD8+ T cells (n = 5 mice per group). (G) The expression of co-inhibitory molecules (PD-1, LAG-3, TIGIT, and Tim-3) on CD8+ T cells (n = 5 mice per group). (H) The percentages of PD-1+Tim-3+, PD-1+LAG-3+, and PD-1+TIGIT+ double-positive CD8+ T cells (n = 5 mice per group). (I) Co-expression of PD-1, TIGIT, and LAG-3 on CD8+ T cells (n = 5 mice per group). Mean ± SEM is shown. *p < 0.05, **p < 0.01, ***p < 0.001.
Taken together, these data provided evidence that the triple combination decreased inhibitory molecules and triggered a successful anti-tumor immune response in the MC38 colon cancer model.
Discussion
Recent years have seen enormous breakthroughs in the field of cancer immunotherapy based on the successful development of immune checkpoint blockade.1, 23 However, not all the cancer types effectively respond to immune checkpoint blockade, including colon cancer.6, 8, 33 In our study, the effect of single treatment of anti-PD-1 antibody, PLX3397, or OVs was poor in mouse colon cancer. Accumulating evidence has now clarified that many factors operating within the tumor microenvironment contribute to the unsatisfactory outcomes of immunotherapy, which includes the recruitment of TAMs, MDSC, and Tregs and low T cell infiltration into the tumor.9 Similar findings were reported in our study. These factors work together to suppress the effects of anti-PD-1.33 The therapeutic outcomes were greatly improved by a triple combination of CSF-1R inhibitor, OVs, and anti-PD-1.
It is clear that TAMs play a pivotal role in tumor progression and resistance to the PD-1 blockade,11, 34, 35 and they are tightly associated with poor prognosis.36, 37, 38 In our study, TAMs were highly enriched within the tumor in both mouse colon cancers and human colon cancer tissues, expressing the M2 phenotype marker CD206, which indicates the immunosuppressive functions of TAMs. Our findings suggest that targeting TAMs with PLX3397 (CSF-1R inhibitor) could effectively reduce TAMs and their mediated immunosuppression. These immune modulations induced by PLX3397 remarkably boosted the anti-PD-1 therapy, and the combination of PLX3397 and anti-PD-1 exhibited more effective tumor control. The therapeutic effects of PLX3397 alone were poor, similar to previous reports, which demonstrated that the therapeutic effects achieved by targeting the CSF-1/CSF-1R pathway alone with inhibitors or antibodies are limited in animal models and human cancers.39, 40 To date, objective responses for single-agent treatment have been achieved in patients with diffuse-type giant cell tumors.15 We propose that this limited efficacy is partly due to the alternative mechanisms that support tumor progression. Previous reports also demonstrated that the therapeutic effects were significantly augmented upon combining CSF-1R inhibitor with chemotherapy or immune checkpoint blockade.14, 41, 42
T cell infiltration is an important predictive biomarker for the PD-1 blockade, and it is closely associated with a good prognosis.17 In our study, we found that T cells were low and exhausted within the tumor microenvironment and were distributed in the tumor margin. Anti-PD-1 treatment reduced PD-1 expression on CD8+ T cells, thereby activating them. On the other hand, the treatment did not increase T cell infiltration in the tumor and reduced the number of Tregs. Therefore, it may be one of the possible explanations for the poor effects of anti-PD-1 monotherapy in our results.
OVs can induce extensive effects on tumor microenvironment, including direct tumor killing, recruitment of the T cells, activation of anti-tumor immunity, and disruption of the tumor neo-vasculature.18, 20, 43 Local OV injection could induce tumor regression in both injected tumor and distant tumors, suggesting a systemic immune response elicited by local injection.44 Thus, OVs would be an ideal approach to overcome the situation of low T cell infiltration and improve the effects of anti-PD-1. Our results also revealed that OVs lyse tumor cells directly and induce tumor cell apoptosis in vivo. Furthermore, OVs were responsible for the increased T cell infiltration and activation as well as the change in the ratio of effector T cells to Tregs, because none of the remaining components could induce an increase in T cell population. OV treatment also decreased the percentage of TAMs and altered their phenotypes. As expected, triple treatment remarkably enhanced the therapeutic effects. Previous studies also showed that significant tumor inhibition could be achieved by combining OVs with other therapies, such as chimeric antigen receptor (CAR)-redirected T cell or PD-1/PD-L1 blockade.21, 45, 46, 47
Despite so many advantages of oncolytic virotherapy, limited efficacy of OVs alone was observed in our study. We speculated that it might be attributed to other immunosuppressive factors operating within the tumor microenvironment, such as TAMs, as we observed that the therapeutic effects were improved in mice treated with PLX3397 and OVs, and the triple combination resulted in better effects than the dual combination. Moreover, the antiviral immunity induced by OVs themselves might be yet another limitation, because the tumor grew rapidly following the last OV injection, suggesting the short-term effects of OVs. We also detected the presence of virus-specific CD8+ T cells in the tumor, but it was unexpected that the antivirus immunity could actually potentiate the immunotherapeutic efficacy.48 Furthermore, increased CD8+ T cells after OV injection seemed to be more virus specific in our study. However, it was time dependent and variable. The immune responses were predominantly to the virus at an early stage after OV injection, but they shifted to anti-tumor immunity at the later stage when the viruses were eliminated.
The choice of agent and timing would be important considerations for the combination therapy. For instance, local chemotherapy, but not systemic chemotherapy, greatly improved the efficacy of anti-PD-1 treatment to confer anti-tumor immunity,49 because systemic chemotherapy produced indiscriminate cytotoxicity on both the tumor cells and T cells, dampening the immunotherapy and diminishing the synergistic effect. In this combination strategy, each of the three components could overcome one or more immunosuppressive factors, and thus work synergistically to combat the tumor effectively. Continuous PLX3397 treatment restrained TAMs within the tumor microenvironment, sensitizing the tumor to the subsequent OVs and anti-PD-1 treatment. OV treatment was the key factor in this regimen, as it potentially kills the tumor cells directly and releases the tumor-associated antigens to activate T cells and boost T cell infiltration of the tumor microenvironment. Furthermore, OVs reversed the immune balance within the T cells and led to an increased ratio of effector T cells to Tregs. However, this increased immune response might be a double-edged sword, playing a role in both tumor killing and OV clearance to resume rapid tumor growth at a later stage, leading to unsatisfactory therapeutic outcomes. In addition, OV treatment could activate the PD-1/PD-L1 axis in tumor cells to attenuate the inflammatory responses,47 which is likely related to the natural stimulation of checkpoint molecules during chronic viral infections to reduce tissue damage.50, 51 Hence, to promote and expand anti-tumor immunity, it was extremely important to combine anti-PD-1 to further evoke the exhausted effector T cells and maintain proinflammatory conditions within the tumor microenvironment.
As expected, our data from the animal models clearly reflect these synergistic effects, demonstrating the effectiveness of the triple combination treatment compared to the possible dual combinations, which are themselves better than the single-agent treatment. These powerful anti-tumor immune responses were specific and long term, as all survivors completely rejected the second tumor when re-challenged and lived for 10 months without any tumor development. Triple combination therapy boosted T cell infiltration and activation; interestingly, the decreased expression of co-inhibitory molecules on T cells was not confined to PD-1 that is theoretically caused by anti-PD-1 treatment. The expressions of other co-inhibitory molecules, such as TIGIT, LAG-3, and Tim-3, were also apparently reduced.
Treatment-related adverse events have been a serious issue in clinical application. Severe toxicity has been reported in clinical trials using antibody blockade of CSF-1R (20 mg/kg), PD-1, and CTLA-4.29, 30, 31 However, apparent side effects were not observed in our study with PLX3397 treatment alone or in combination. This might be due to the lower dose (20 mg/kg) used in our study compared to the previous reports that used 40–275 mg/kg in mice.52, 53, 54 Furthermore, a clinical trial has reported that an oral dose of 1,000 mg/day is well tolerated.55 The different drug properties between antibodies and the small-molecule inhibitors might be another reason for this diversity. Meanwhile, the dose of anti-PD-1 used in this study was also lower than previous studies, with 200 μg per mouse.14, 24, 49 As a consequence, our triple combination strategy demonstrated significant anti-tumor ability with fewer side effects.
In summary, our study demonstrates a novel and effective combination strategy for cancer immunotherapy, making it a potent candidate for cancer therapy in near future.
Materials and Methods
Mice and Cell Lines
The 6- to 8-week-old C57BL/6 and BALB/c female mice were purchased from Beijing Huafukang Bioscience (Beijing, China). The mouse colon carcinoma cell line CT26 was purchased from American Type Culture Collection (ATCC); CT26-luc was generated using lentiviral vectors. Mouse MC38 colon cancer cell line was provided by Innovent Biologics (Suzhou, Jiangsu, China).
Viruses and Antibodies
Recombinant oncolytic adenoviruses were obtained from Biowit Technologies (Shenzhen, China). These are type 5 adenovirus with depleted E1b and E3 genes while retaining the E1a gene, and human telomerase reverse transcriptase promoter (hTERTp) was inserted upstream of the E1A gene to control virus replication. Therapeutic mouse anti-PD-1 antibodies were provided by Innovent Biologics. Anti-CD4 (GK1.5) and anti-CD8 (2.43) for cell depletion were purchased from Bio X Cell. Antibodies to CD3 (17A2), CD4 (RM4-5), CD25 (3C7), CD45 (30-F11), CD11b (M1/70), F4/80 (BM8), Ly-6G (1A8), CD206 (C068C2), TIGIT (1G9), LAG-3 (C9B7W), Tim-3 (RMT3-23), PD-1 (RMP1-30), ICOS (7E.17G9), CD44 (IM7), CD62L (MEL-14), MHCII (M5/114.1 5.2), and Ki67 (16A8) were purchased from BioLegend. Antibodies to CD8 (53-6.7), FoxP3 (MF23), and IFN-γ (XMG1.2) were purchased from BD Biosciences.
Tumor Model and Treatment
To establish the subcutaneous tumor models, 5 × 105 CT26 cells or 1 × 106 MC38 cells in 100 μL serum-free medium were injected subcutaneously (s.c.) into the right flank of the mice. Tumor size was measured with a digital caliper every other day and terminated upon exceeding a tumor volume of 2,000 mm3. Mice that succumbed to tumors were recorded for overall survival statistics. An orthotopic colon cancer model was established as described in the previous report.56 Briefly, 6- to 8-week-old female BALB/c mice were anesthetized by inhalation of 3% isoflurane in oxygen. The mice were placed in a supine position and an incision was made to expose the cecum. 2 × 105 CT26-luc cells in 40 μL serum-free medium were injected into the cecum subserosa, and the abdominal wall and skin were closed and sterilized with iodophor. Tumor development was monitored using IVIS Lumina III (PerkinElmer) after intraperitoneal injection of D-luciferin (Gold Biotechnology).
For treatment in subcutaneous CT26 and MC38 tumor models, PLX3397 (20 mg/kg) was given daily for 30 days by gavage from the day after tumor cell inoculation, OVs (intratumoral [i.t.], 5 × 108 pfu per mouse) and anti-PD-1 antibody (intravenous [i.v.], 100 μg per mouse) were injected simultaneously every 2 days for 3 times when the tumor volume reached 50–100 mm3. In CT26 orthotopic colon cancer model, PLX3397 was administered as above; OVs (i.t., 1 × 109 pfu per mouse) were injected once with microsyringe on day 6 after tumor cell inoculation via surgery to expose the tumor. Anti-PD-1 antibody (i.v., 100 μg per mouse) was injected on days 6, 9, and 12 after tumor cell inoculation. All experiments were performed in accordance with the Animal Care and Use Committee of West China Hospital, Sichuan University, China.
Flow Cytometry
Animals were euthanized on day 5 after the last injection of OVs and anti-PD-1; tumors were harvested, minced, and digested in RPMI-1640 medium containing collagenase IV (0.1%; Gibco), nuclease, and 1% fetal bovine serum (FBS) at 37°C for 40–60 min; and the cell suspensions were filtered. Fixable Viability Stain 620 (BD Biosciences) was used to discriminate live and dead cells. The cells were then blocked with Fc-block (BD Biosciences) and stained using antibodies. For detecting nuclear factors, cell surface markers were stained and permeabilized using FoxP3 fixation and permeabilization kit (eBioscience). Intracellular cytokine staining was performed using the Fixation/Permeabilization Kit (BD Biosciences). Data were acquired on a NovoCyte flow cytometer.
T Cell Depletion
For CD4+ and CD8+ T cell depletion, anti-CD4 (clone GK1.5, Bio X Cell) and anti-CD8 (clone 53-6.7, Bio X Cell) antibodies were used, and 500 μg antibodies per mouse were intraperitoneally injected on day 4 after tumor cell inoculation to deplete CD4+ and CD8+ T cells. Then, follow-up injections of 250 μg per mouse on days 8, 12, and 16 were administered. The depletion efficacy was validated by flow cytometry detection of CD4+ or CD8+ T cells in peripheral blood mononuclear cells (PBMCs).
Adoptive Assay
Spleen and lymph nodes were harvested from mice untreated or treated with triple combination therapy, and they were mechanically ground and filtered using a 70-μm cell strainer to obtain a single-cell suspension. T cells were enriched using Percoll (GE Healthcare) and further purified with CD3 Microbead kit (Miltenyi Biotec). Isolated T cells (5 × 106 per mouse) were i.v. transferred into mice on days 4, 7, 10, 13, and 16 following tumor cell inoculation. For serum adoption, serum (100 μL per mouse) collected from untreated or treated mice was i.v. transferred into the mice on days 4, 7, 10, 13, and 16 following tumor cell inoculation. Tumor size was measured using a digital caliper every other day.
IFN-γ ELISPOT Assay
Single-cell suspension of the tumor was prepared as described above. Tumor-infiltrating CD8+ T cells were enriched using 40%–70% Percoll (GE Healthcare), and they were further purified with a CD8+ Microbead kit (Miltenyi Biotec). Isolated CD8+ T cells (5 × 104) were stimulated with 1 × 104 irradiated C26 cells or 4T1 cells infected with OVs (MOI = 500). IFN-γ-producing CD8+ cells were determined using a mouse IFN-γ ELISPOT Kit (BD Biosciences), according to the manufacturer’s instructions.
Immunohistochemistry
Tumor tissues and organs were fixed in 4% paraformaldehyde (for staining CD68, PD-L1, and cleaved Caspase-3) or IHC Zinc Fixative (BD Biosciences; for staining CD3, CD4, CD8, and Foxp3) at room temperature for 48 hr, and they were dehydrated in alcohol gradient and embedded with a Leica tissue embedder. 4-μm-thick sections were cut and transferred to a tissue flotation bath. Slides were then dried at room temperature overnight and stored at 4°C. Slides were incubated at 60°C for 10 min to melt the paraffin and subjected to deparaffinization (xylene, 10 min, 2 times; 100% ethanol, 3 min; 95% ethanol, 3 min; 85% ethanol, 3 min; 75% ethanol, 3 min; and water, 3 min). The slides were then incubated with H2O2 to quench endogenous peroxidase. Heat-mediated antigen unmasking was performed in citrate buffer for CD68, PD-L1, cleaved Caspase 3, and FoxP3. After blocking with goat serum, slides were stained using primary antibody and horseradish peroxidase (HRP)-labeled secondary antibody, signals were detected using 3,3'-diaminobenzidine (DAB), and the cell nucleus was stained with hematoxylin.
mIHC detection of T cell populations within the tumor was performed as described in the previous study, with slight modifications.57 Briefly, slides were deparaffinized and blocked with H2O2. After washes in TBST, slides were incubated with ready-to-use normal goat blocking serum (Vector Laboratories) for 20 min. Rat anti-CD8 (1:50, BD Biosciences) was added to the slide and incubated for 45 min at room temperature. After washes in TBST, anti-rat HRP secondary antibody (Vector Laboratories) was added to the slides, incubated for 10 min at room temperature, and subsequently washed with TBST. Slides were incubated with tyramide-fluorophore reagent (PerkinElmer, 1:100 in amplification buffer) for 10 min at room temperature and washed with TBST. Slides were treated with antibody-stripping buffer (0.1 M glycine [pH 3] and 0.5% Tween) for 20 min at room temperature. The same cycles were performed for rat anti-CD4 (BioLegend, 1:50), rat anti-CD3 (eBioscience, 1:100), and rabbit anti-FoxP3 (Cell Signaling Technology, 1:100). Heat-mediated antigen unmasking was performed in citrate buffer before FoxP3 staining. After TBST washes, DAPI (PerkinElmer) was added and incubated for 5 min at room temperature. Slides were then rinsed with TBST and water, and they were examined using a Vectra multispectral imaging system (PerkinElmer).
Real-Time PCR
Total RNA was extracted using Trizol (Life Technologies). After measuring the concentration, RNA was reverse transcribed, and mRNA expression analysis was performed using PrimeScript RT reagent Kit (TaKaRa) on a LightCycler 96 System (Roche). The gene expression was normalized to the housekeeping gene β-actin.
Statistical Analysis
The data were analyzed using Prism (GraphPad Prism version 5). Statistical significance was analyzed using the unpaired t test. Animal survival was presented using Kaplan-Meier survival curves and analyzed via the log-rank test. The value of p < 0.05 was considered to be statistically significant. In the figures, the symbols used to denote significance are as follows: *p < 0.05, **p < 0.01, ***p < 0.001, and ns (no statistical significance).
Author Contributions
G.S., L.C., and Q.J. were involved in the acquisition and analysis of the data. H.D. designed the research, performed the experiments, revised the manuscript, and obtained funding. Y. Liu, Y.Li, Y.Z., and X.S. performed animal study. Qin Wang and H.Z. were involved in qPCR assay. S.Y., Q.Y., and Y. Lin conducted flow cytometry and mIHC assay. L.D., L.L., Y.Y., and S.Z. provided advice. J.L. and Z.L. provided anti-PD-1 antibodies. H.W. helped with macrophage and T cell sorting. Qingan Wang and X.Z. were involved in colon cancer tissue collection and IHC. D.Y. and Y.W. were involved in obtaining funding and study supervision.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This study was supported by a National Key R&D Program of China grant (2017YFA0105702) and National Natural Science Foundation of China Program grants (81772939, 81472195, and 81372445). We are grateful to Xiaogang Wang (PerkinElmer) for helping offering access to the multispectral scanner Vectra and InForm software.
Footnotes
Supplemental Information includes ten figures and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.11.010.
Supplemental Information
References
- 1.Baumeister S.H., Freeman G.J., Dranoff G., Sharpe A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu. Rev. Immunol. 2016;34:539–573. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 2.Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov. 2016;15:235–247. doi: 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- 3.Carbone D.P., Reck M., Paz-Ares L., Creelan B., Horn L., Steins M., Felip E., van den Heuvel M.M., Ciuleanu T.E., Badin F., CheckMate 026 Investigators First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou W., Wolchok J.D., Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C., Long G.V., Brady B., Dutriaux C., Maio M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., Kalinka-Warzocha E. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 6.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitt J.M., Vétizou M., Daillère R., Roberti M.P., Yamazaki T., Routy B., Lepage P., Boneca I.G., Chamaillard M., Kroemer G., Zitvogel L. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 2016;44:1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Bindea G., Mlecnik B., Tosolini M., Kirilovsky A., Waldner M., Obenauf A.C., Angell H., Fredriksen T., Lafontaine L., Berger A. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A., Locati M. Macrophage Metabolism Shapes Angiogenesis in Tumors. Cell Metab. 2016;24:653–654. doi: 10.1016/j.cmet.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 12.De Palma M., Lewis C.E. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Pollard J.W. Trophic macrophages in development and disease. Nat. Rev. Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y., Knolhoff B.L., Meyer M.A., Nywening T.M., West B.L., Luo J., Wang-Gillam A., Goedegebuure S.P., Linehan D.C., DeNardo D.G. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassier P.A., Italiano A., Gomez-Roca C.A., Le Tourneau C., Toulmonde M., Cannarile M.A., Ries C., Brillouet A., Müller C., Jegg A.M. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16:949–956. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 16.Tumeh P.C., Harview C.L., Yearley J.H., Shintaku I.P., Taylor E.J., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang A.C., Postow M.A., Orlowski R.J., Mick R., Bengsch B., Manne S., Xu W., Harmon S., Giles J.R., Wenz B. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawler S.E., Speranza M.C., Cho C.F., Chiocca E.A. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017;3:841–849. doi: 10.1001/jamaoncol.2016.2064. [DOI] [PubMed] [Google Scholar]
- 19.Fountzilas C., Patel S., Mahalingam D. Review: Oncolytic virotherapy, updates and future directions. Oncotarget. 2017;8:102617–102639. doi: 10.18632/oncotarget.18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichty B.D., Breitbach C.J., Stojdl D.F., Bell J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 21.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J., Fernandez E. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017;170:1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma P., Allison J.P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twyman-Saint Victor C., Rech A.J., Maity A., Rengan R., Pauken K.E., Stelekati E., Benci J.L., Xu B., Dada H., Odorizzi P.M. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G., Liu D., Cooper T.K., Kimchi E.T., Qi X., Avella D.M., Li N., Yang Q.X., Kester M., Rountree C.B. Successful chemoimmunotherapy against hepatocellular cancer in a novel murine model. J. Hepatol. 2017;66:75–85. doi: 10.1016/j.jhep.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Wang P., Li H., Du X., Liu M., Huang Q., Wang Y., Wang S. The Efficacy of Oncolytic Adenovirus Is Mediated by T-cell Responses against Virus and Tumor in Syrian Hamster Model. Clin. Cancer Res. 2017;23:239–249. doi: 10.1158/1078-0432.CCR-16-0477. [DOI] [PubMed] [Google Scholar]
- 28.Benencia F., Courrèges M.C., Fraser N.W., Coukos G. Herpes virus oncolytic therapy reverses tumor immune dysfunction and facilitates tumor antigen presentation. Cancer Biol. Ther. 2008;7:1194–1205. doi: 10.4161/cbt.7.8.6216. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulos K.P., Gluck L., Martin L.P., Olszanski A.J., Tolcher A.W., Ngarmchamnanrith G., Rasmussen E., Amore B.M., Nagorsen D., Hill J.S., Stephenson J., Jr. First-in-Human Study of AMG 820, a Monoclonal Anti-Colony-Stimulating Factor 1 Receptor Antibody, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017;23:5703–5710. doi: 10.1158/1078-0432.CCR-16-3261. [DOI] [PubMed] [Google Scholar]
- 30.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Rutkowski P., Grob J.J., Cowey C.L., Lao C.D., Wagstaff J., Schadendorf D., Ferrucci P.F. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellmann M.D., Callahan M.K., Awad M.M., Calvo E., Ascierto P.A., Atmaca A., Rizvi N.A., Hirsch F.R., Selvaggi G., Szustakowski J.D. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell. 2018;33:853–861.e4. doi: 10.1016/j.ccell.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson A.C., Joller N., Kuchroo V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 35.Lewis C.E., Harney A.S., Pollard J.W. The Multifaceted Role of Perivascular Macrophages in Tumors. Cancer Cell. 2016;30:18–25. doi: 10.1016/j.ccell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asgharzadeh S., Salo J.A., Ji L., Oberthuer A., Fischer M., Berthold F., Hadjidaniel M., Liu C.W., Metelitsa L.S., Pique-Regi R. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J. Clin. Oncol. 2012;30:3525–3532. doi: 10.1200/JCO.2011.40.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hambardzumyan D., Gutmann D.H., Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016;19:20–27. doi: 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steidl C., Lee T., Shah S.P., Farinha P., Han G., Nayar T., Delaney A., Jones S.J., Iqbal J., Weisenburger D.D. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N. Engl. J. Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ries C.H., Hoves S., Cannarile M.A., Rüttinger D. CSF-1/CSF-1R targeting agents in clinical development for cancer therapy. Curr. Opin. Pharmacol. 2015;23:45–51. doi: 10.1016/j.coph.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Pyonteck S.M., Akkari L., Schuhmacher A.J., Bowman R.L., Sevenich L., Quail D.F., Olson O.C., Quick M.L., Huse J.T., Teijeiro V. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J., Escamilla J., Mok S., David J., Priceman S., West B., Bollag G., McBride W., Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruffell B., Chang-Strachan D., Chan V., Rosenbusch A., Ho C.M., Pryer N., Daniel D., Hwang E.S., Rugo H.S., Coussens L.M. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zamarin D., Holmgaard R.B., Subudhi S.K., Park J.S., Mansour M., Palese P., Merghoub T., Wolchok J.D., Allison J.P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishio N., Diaconu I., Liu H., Cerullo V., Caruana I., Hoyos V., Bouchier-Hayes L., Savoldo B., Dotti G. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 2014;74:5195–5205. doi: 10.1158/0008-5472.CAN-14-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou W., Sampath P., Rojas J.J., Thorne S.H. Oncolytic Virus-Mediated Targeting of PGE2 in the Tumor Alters the Immune Status and Sensitizes Established and Resistant Tumors to Immunotherapy. Cancer Cell. 2016;30:108–119. doi: 10.1016/j.ccell.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z., Ravindranathan R., Kalinski P., Guo Z.S., Bartlett D.L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 2017;8:14754. doi: 10.1038/ncomms14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ricca J.M., Oseledchyk A., Walther T., Liu C., Mangarin L., Merghoub T., Wolchok J.D., Zamarin D. Pre-existing Immunity to Oncolytic Virus Potentiates Its Immunotherapeutic Efficacy. Mol. Ther. 2018;26:1008–1019. doi: 10.1016/j.ymthe.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathios D., Kim J.E., Mangraviti A., Phallen J., Park C.K., Jackson C.M., Garzon-Muvdi T., Kim E., Theodros D., Polanczyk M. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci. Transl. Med. 2016;8:370ra180. doi: 10.1126/scitranslmed.aag2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park H.J., Park J.S., Jeong Y.H., Son J., Ban Y.H., Lee B.H., Chen L., Chang J., Chung D.H., Choi I., Ha S.J. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J. Immunol. 2015;194:5801–5811. doi: 10.4049/jimmunol.1401936. [DOI] [PubMed] [Google Scholar]
- 52.Yan D., Kowal J., Akkari L., Schuhmacher A.J., Huse J.T., West B.L., Joyce J.A. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment-mediated therapeutic resistance in gliomas. Oncogene. 2017;36:6049–6058. doi: 10.1038/onc.2017.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeNardo D.G., Brennan D.J., Rexhepaj E., Ruffell B., Shiao S.L., Madden S.F., Gallagher W.M., Wadhwani N., Keil S.D., Junaid S.A. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peranzoni E., Lemoine J., Vimeux L., Feuillet V., Barrin S., Kantari-Mimoun C., Bercovici N., Guérin M., Biton J., Ouakrim H. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc. Natl. Acad. Sci. USA. 2018;115:E4041–E4050. doi: 10.1073/pnas.1720948115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butowski N., Colman H., De Groot J.F., Omuro A.M., Nayak L., Wen P.Y., Cloughesy T.F., Marimuthu A., Haidar S., Perry A. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro-oncol. 2016;18:557–564. doi: 10.1093/neuonc/nov245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tseng W., Leong X., Engleman E. Orthotopic mouse model of colorectal cancer. J. Vis. Exp. 2007;(10):484. doi: 10.3791/484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng Z., Jensen S.M., Messenheimer D.J., Farhad M., Neuberger M., Bifulco C.B., Fox B.A. Multispectral Imaging of T and B Cells in Murine Spleen and Tumor. J. Immunol. 2016;196:3943–3950. doi: 10.4049/jimmunol.1502635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.