Abstract
Objectives
To assess safety, efficacy and follow-up results of transcatheter closure of ventricular septal defect (VSD) using Nit-Occlud_ Lê VSD Coil (pfm medical, KÖln, Germany).
Background
Transcatheter VSD closure has achieved encouraging results but more follow-up studies are needed.
Patients and methods
Between January 2012 and December 2013 in the cardiology department, Tanta University Hospital, Tanta, Egypt, 80 patients underwent percutaneous VSD closure using Nit-Occlud_ Lê VSD Coil. Early and mid- term follow-up was done for 3 years, follow-up was concluded in 2016.
Results
The mean age of patients was 5.34 ± 3 years, and their mean weight was 17.24 ± 8.17 kg. Overall, 77 of 80 patients had perimembranous VSD with aneurysmal tissue; eight had multiple right ventricular exits, 14 had deficient aortic rim, two had high outlet muscular, and one had Gerbode defect. The procedure was successful in 98.75% of patients, and was aborted in one patient because of the development of complete heart block and the coil had to be removed. The mean procedure time was 104.98 ± 9.50 minutes. The mean fluoroscopy time was 30.58 ± 2.79 minutes. The immediate complete occlusion rate was 62%, which increased to 82.3% on the second day, and 94.9% by the 3rd month, and 97.5% by 1 year. There was a significant decrease in mitral incompetence after 6 months of follow-up (p = 0.002), and only one patient had trivial aortic incompetence prior to the procedure that remained the same during follow-up period.
Conclusion
Using Nit-Occlud_ Lê VSD-Coil to close VSD is safe and feasible in VSDs with various morphology.
keywords: Ventricular septal defect, Transcatheter closure, Nit-Occlud® Lê VSD Coil
Abbreviations
- ADO I
Amplatzer Duct Occluder I
- AR
Aortic Regurgitation
- AV loop
Arterio-Venous loop
- BSA
Body Surface Area
- CHB
Complete Heart Block
- ECG
Electrocardiogram
- LV
Left Ventricle
- LV Angiography
Left Ventricular Angiography
- LVOT
Left Ventricular Outflow Tract
- MR
Mitral Regurgitation
- m VSD
Muscular VSD
- Pm VSD
Perimembranous VSD
- RBBB
Right Bundle Branch Block
- RV
Right Ventricle
- SVC
Superior Vena Cava
- TEE
Transesophageal Echocardiography
- TR
Tricuspid Regurgitation
- TTE
Transthoracic Echocardiography
- VSD
ventricular septal defect
1. Introduction
Ventricular septal defect (VSD) is a very common congenital heart problem [1], [2]. Patients with hemodynamically significant VSD require its closure [3]. Surgery was the sole modality for VSD closure up to 1988 when the first trail for percutaneous closure was made. Then, Amplatzer (AGA Medical Corp., Golden Valley, MN, USA) developed different generations of devices to close VSD percutaneously with acceptable results [4]. However, transcatheter closure is confirmed as the standard treatment for muscular VSD (mVSD), but surgery for perimembranous VSD (pmVSD) was considered the better modality for treatment as percutaneous closure of pmVSD induced a high rate of heart block [1], [2]. Surgical closure is associated with potential risks of complete heart block (CHB) (about 2.9–5.7%), wound infection, and cardiopulmonary bypass complications (e.g., myocardial injury, pulmonary complications, electrolyte disturbance, acute kidney injury, and coagulopathy) [5], [6], [7]. Thereafter, the off-label use of many devices to close pmVSD became widespread [1], [8], [9]. Heart block has been one of the major concerns after pmVSD closure either by surgical or percutaneous means [10], [11]. Successful percutaneous closure of Gerbode defect has also been reported [12], [13].
Follow-up after VSD closure revealed varying incidence rates of tricuspid valve regurgitation (TR), aortic valve regurgitation (AR), and heart block [14]. The Nit-Occlud Lê VSD coil (pfm medical, KÖln, Germany.) has been manufactured for VSD percutaneous closure [8], [9].
A well-formed aneurysmal tissue around a VSD represents an excellent site for device implantation because it places the coil away from conductive tissue and from the aortic valve [15].
In this study, we report our mid-term results of VSD closure with Nit-Occlud Lê VSD coils in the Cardiology Department of Tanta University Hospital, Tanta, Egypt.
2. Materials and methods
2.1. Patients
This retrospective study was conducted at the Cardiology Department of Tanta University Hospital. Many of the procedures were performed in the presence of a proctor as recommended by the company training protocol, from January 2012 to December 2013 including 80 patients selected for percutaneous VSD closure with Nit-Occlud Lê VSD coils. The indications for VSD closure were as follows: clinical symptoms of significant left-to-right shunt (e.g., recurrent lower respiratory tract infections and failure to thrive manifestations despite adequate medical treatment) and/or echocardiographic evidence of significant left-to-right shunt (left ventricular and left atrial dilation). Follow-up was performed using electrocardiogram (ECG) and transthoracic echocardiography (TTE) at 24 hours prior to hospital discharge, 3 months, 6 months, and annually thereafter for 3 years. Follow-up was concluded in 2016.
2.2. Device
The Nit-Occlud Lê VSD coil (pfm medical, KÖln, Germany) is made of nitinol wires with polyester fibers added to the left-sided parts of the loops. The device consists of a distal coil representing the larger left-sided cone and a smaller right-sided proximal cone. The device is available in different sizes: 8/6, 10/6, 12/6, 12/8, 14/8, and 16/8 mm; the first number represents the diameter of the distal coil and the second number represents the diameter of the proximal one. Coils are premounted on their delivery catheter [8].
2.3. Procedure
Our study was conducted with approval from the Ethics Committee of the Medical Science of Tanta University and complied with the principles of the Declaration of Helsinki in 1964. After explaining the benefits and the risks of the procedure and obtaining informed consent from legal guardians, all procedures were performed under general endotracheal anesthesia under fluoroscopic and transesophageal echocardiography (TEE) guidance (Figure 1, Figure 2). Amoxicillin (50–100 mg/kg, IV) was given to all patients 30–60 minutes prior to the procedure. Both femoral vein (5F) and artery (4F) were accessed percutaneously. Patients received heparin (100 IU/kg). Left ventricular (LV) angiography with a pigtail catheter in (60° left anterior oblique/20° cranial projection) was performed to define the site and size of the VSD. The LV and right ventricular (RV) sides of the VSD were measured (in millimeters) at the maximum diastolic phase using the software incorporated within the catheterization laboratory system and compared to VSD size measured by TEE in mid esophageal long axis view (110–120°) both by two-dimensional imaging and color flow mapping. No haemodynamic testing was done at implant procedures.
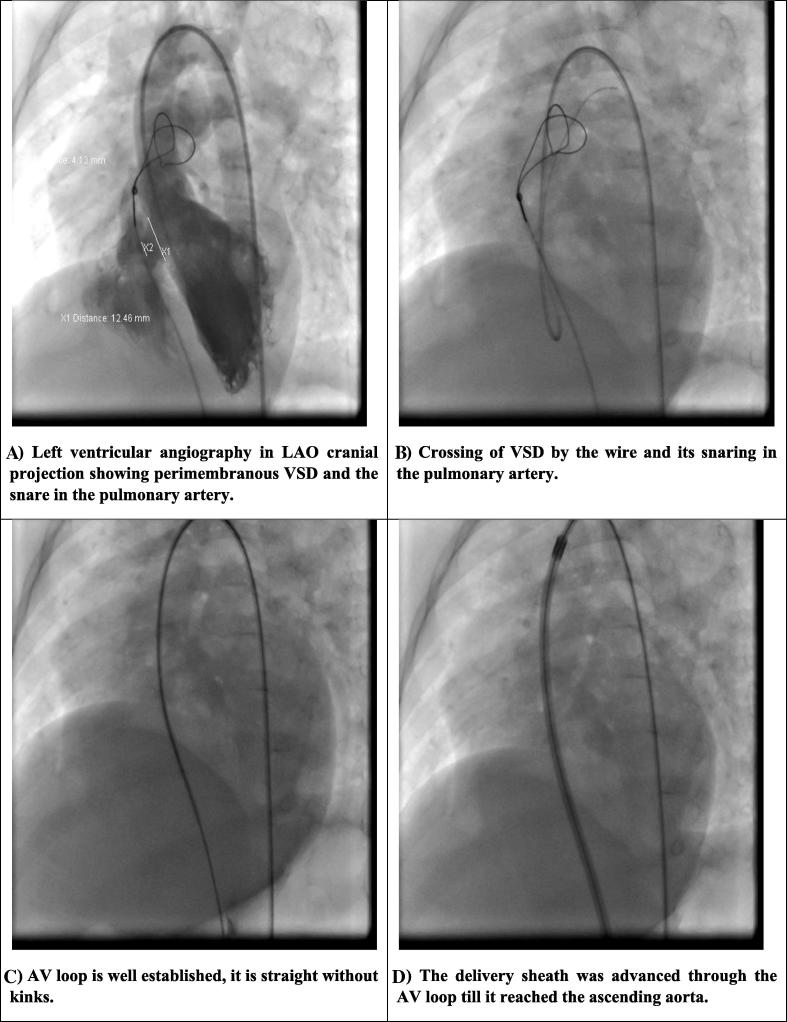
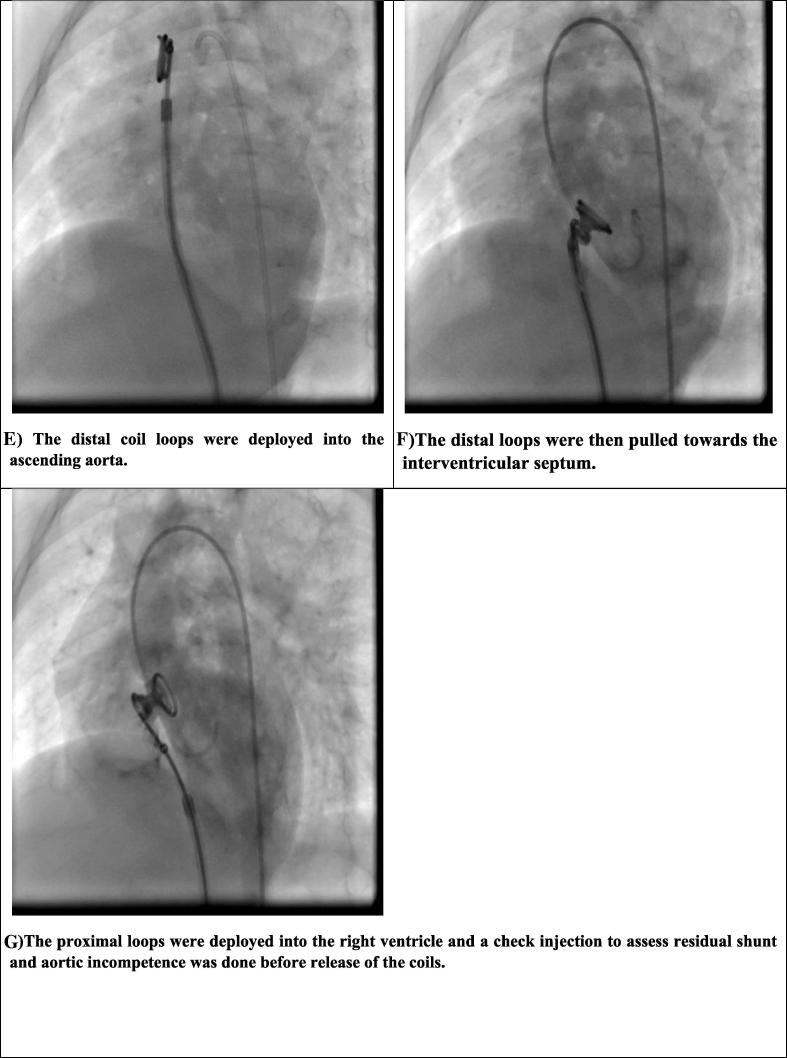
Figure 1.
The procedure of VSD closure. (A) Left ventricular angiography in LAO cranial projection showing perimembranous VSD and the snare in the pulmonary artery. (B) Crossing of VSD by the wire and its snaring in the pulmonary artery. (C) AV loop is well established; it is straight without kinks. (D) The delivery sheath was advanced through the AV loop until it reached the ascending aorta. (E) The distal coil loops were deployed into the ascending aorta. (F) The distal loops were then pulled toward the interventricular septum. (G) The proximal loops were deployed into the right ventricle and a check injection to assess residual shunt and aortic incompetence was done prior to release of the coils. AV = arteriovenous; LAO = left anterior oblique; VSD = ventricular septal defect.
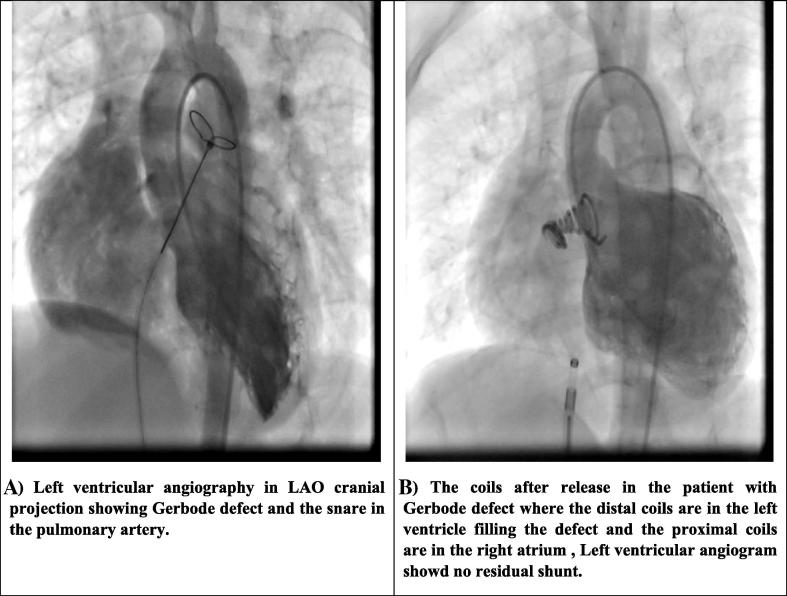
Figure 2.
Gerbode defect closure. (A) Left ventricular angiography in LAO cranial projection showing Gerbode defect and the snare in the pulmonary artery. (B) The coils after release in the patient with Gerbode defect where the distal coils are in the left ventricle filling the defect and the proximal coils are in the right atrium; left ventricular angiogram showed no residual shunt. LAO = left anterior oblique; VSD = ventricular septal defect.
The proper size of the coil was selected; its distal end was equal to or 1–2 mm larger than the VSD diameter as measured from the LV side, and at least twice the smallest exit on the RV side. In case the defect had deficient aortic rim, the coil was selected aiming to fit into aneurysm without protrusion into left ventricular outflow tract (LVOT) [16].
A 10–15 mm goose neck snare (Ev3 Endovascular, Inc., Plymouth, MN, USA) was advanced through the venous access into the pulmonary artery and the snare was left there. A 4F Judkin’s right coronary catheter or a 4F cut pigtail catheter were used to cross the VSD from the LV side. A noodle wire (St. Jude Medical, St. Paul, MN, USA) or Terumo wire (Terumo Corporation, Tokyo, Japan) were advanced to either branch pulmonary artery or superior vena cava (SVC). The wire was snared and exteriorized out the right femoral vein. This established an arteriovenous wire loop (AV loop) which had to be straight without any incorporation to any of the tricuspid tissue indicted by any kink or resistance. The delivery system was introduced through the femoral vein and advanced to the ascending aorta through the kissing technique. Then, the arterial catheter was exchanged for a pigtail catheter over the guide wire and an aortogram was done to delineate the aortic valve. The pigtail was pulled down in the descending aorta for fear of entangling with the coil [16].
The tip of the delivery catheter was kept away from the tip of the long sheath. All loops of the coil except the last two were deployed in the ascending aorta. Both the delivery catheter and the long sheath were carefully pulled back across the aortic valve into the left ventricle. Once the coil was pulled back into the defect, it adapted to the shape of the defect. Check LV angiography was done, and then the remaining loops were positioned on the RV side of the defect. In Gerbode defect, the remaining coils were positioned in the right atrium (Fig. 2). After complete implantation, tricuspid valve, aortic valve, and residual shunt were assessed by TEE and another LV angiography prior to release [16].
Urine color was observed in all patients to detect haemolysis. Antiplatelet therapy with 5 mg/kg/d aspirin orally for 6 months and endocarditis prophylaxis were prescribed (also for 6 months) and continued in case there was a presence of persistent residual shunt [17].
2.4. Statistical analysis
Data are expressed as a frequency or percentage for nominal variables and as the mean ± standard deviation for continuous variables. Data were analyzed using IBM SPSS software package, version 20.0 (IBM Corp., Armonk, NY, USA). Chi-square test was used for qualitative periods. Pearson’s correlation test was used for linear correlation between two variables. It has a value between +1 and −1, where 1 = total positive linear correlation, 0 = no linear correlation, and −1 = total negative linear correlation. Statistical significance of the obtained results was judged at the 5% level.
3. Results
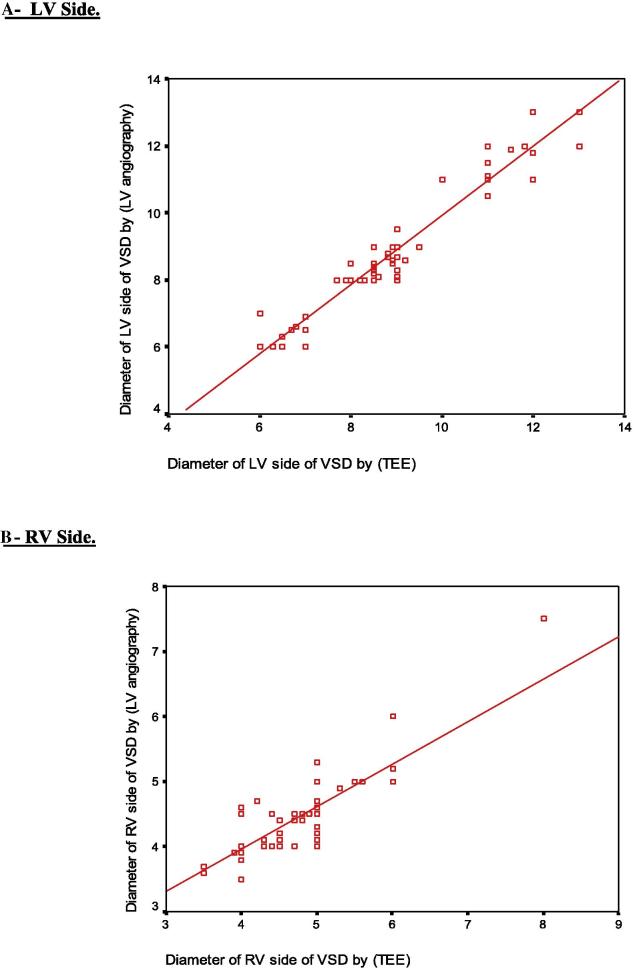
Eighty patients [50 females (62.5%) and 30 males (37.5%)] underwent transcatheter VSD closure. Their mean age was 5.34 ± 3 (range 1.5–13.0) years, and their mean weight was 17.24 ± 8.17 (range 7.8–44.0) kg (Table 1). Seventy-seven out of 80 patients had pmVSD with aneurysmal tissue. Multiple RV exits were present in eight patients, and aortic rim was deficient (<3 mm) in 14 patients. The coils were selected to fill the aneurysm in those with deficient aortic rim. Two patients had high outlet muscular defect and one had Gerbode defect (direct type) (Table 2, Table 3). The mean VSD diameter as measured by TEE from the LV side was 8.83 ± 1.68 (range 6.0–13.0) mm and that measured using LV angiography was 8.71 ± 1.80 (range 6.0–13.0) mm. The mean VSD diameter as measured by TEE from the RV side was 4.83 ± 0.69 (range 3.5–8) mm and that measured using LV angiography was 4.50 ± 0.58 (range 3.5–7.5) mm (Table 4). There was a significant positive linear correlation between VSD diameters measured by both TEE and LV angiography (r = 0.965 for the LV side and r = 0.784 for the RV side) (p = 0.001) (Table 5 and Fig. 3).
Table 1.
Baseline characteristics of patients (n = 80).
| Median | Mean ± SD | Range | |
|---|---|---|---|
| Age (y) | 5.0 | 5.34 ± 3.02 | 1.5–13.0 |
| Weight (kg) | 15.0 | 17.24 ± 8.17 | 7.8–44.0 |
| Height (cm) | 105.75 | 107.37 ± 20.35 | 75.0–156.0 |
| BSA | 0.67 | 0.71 ± 0. 24 | 0. 40–1.39 |
BSA = body surface area; SD = standard deviation.
Table 2.
Types of VSD (n = 80).
| VSD type | N | % |
|---|---|---|
| Perimembranous | 77 | 96.25 |
| Gerbode | 1 | 1.25 |
| Muscular (high outlet) | 2 | 2.5 |
VSD = ventricular septal defect.
Table 3.
Morphological features of perimembranous VSD (n = 77).
| Perimembranous VSD | Yes |
No |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Aneurysm | 77 | 100 | 0 | 0 |
| Multiple RV exits | 8 | 10.4 | 69 | 89.6 |
| Deficient aortic rima | 14 | 18.2 | 63 | 81.8 |
VSD = ventricular septal defect.
Deficient aortic rim: <3 mm [mean ± standard deviation (range)], 3.69 ± 1.36 mm (0–6 mm).
Table 4.
VSD diameters.
| Item | VSD diameter by TEE (mm) |
VSD diameter by LV angiogram (mm) |
||
|---|---|---|---|---|
| LV side | RV side | LV side | RV side | |
| Median | 8.5 | 5 | 8.3 | 4.5 |
| Mean ± SD | 8.83 ± 1.68 | 4.83 ± 0.69 | 8.71 ± 1.80 | 4.50 ± 0.58 |
| Range | 6–13 | 3.5–8 | 6–13 | 3.5–7.5 |
LV = left ventricular; RV = right ventricular; SD = standard deviation; TEE = transesophageal echocardiography; VSD = ventricular septal defect.
Table 5.
Correlation between VSD size measured using TEE and LV angiography (LV Angiography).
| Diameter of LV side of VSD by TEE | ||
| r | p | |
| Diameter of LV side of VSD by LV angiography | 0.965 | 0.001* |
| Diameter of RV side of VSD by TEE |
||
| r | p | |
| Diameter of RV side of VSD by LV angiography | 0.784 | 0.001* |
LV = left ventricular; RV = right ventricular; TEE = transesophageal echocardiography; VSD = ventricular septal defect.
Statistically significant at p ≤ 0.05.
Figure 3.
Correlation between VSD size measured by transesophageal echocardiography (TEE) and left ventricular angiography. (A) Left ventricular side. (B) Right ventricular side.
The procedure was successful (closure of VSD without complications) in 79 patients (98.75%). The procedure was aborted in one patient aged 12 months. In this patient, the defect was immediately subaortic with good aneurysm and two RV exits; the LV side of the defect was 14 mm and the larger RV exit was 8 mm, so the coil size selected was 16 × 8. After complete deployment of the device, the patient developed CHB with hemodynamic instability and with no response to steroids, and sinus rhythm was restored when the RV coils were pulled into the sheath. So, the coil had to be removed, and the defect was closed 6 months later using Amplatzer Duct Occluder I (AGA Medical Corp.) with mild to moderate residual shunt. The mean procedure time was 104.98 ± 9.50 (range 86–130) minutes. The mean fluoroscopy time was 30.58 ± 2.79 (range 26–39) minutes (Table 6).
Table 6.
Procedural time and success (n = 80).
| N | % | |
|---|---|---|
| Success | ||
| No | 1 | 1.25 |
| Yes | 79 | 98.75 |
| Procedure time (min) | ||
| Range | 86–130 | |
| Mean ± SD | 104.98 ± 9.50 | |
| Median | 105 | |
| Fluoroscopy time (min) | ||
| Range | 26–39 | |
| Mean ± SD | 30.58 ± 2.79 | |
| Median | 30 | |
SD = standard deviation.
The coils used were as follows: 8 × 6 in 14 (17.5%) patients, 10 × 6 in 48 (60%) patients, 12 × 6 in 16 (20%) patients, 14 × 8 in one patient with Gerbode defect (1.25%), and 16 × 8 in one patient who developed CHB (1.25%). The mean (distal coil/LV side of VSD) ratio was 1.18 ± 0.09 (range 0.9–1.33) as the distal coil was equal to or 1–2 mm larger than the LV side of the VSD without coil oversizing to avoid development of CHB. In some cases, smaller coils were used to be hanged inside the aneurysm (Table 7).
Table 7.
Size of used coils (n = 80).
| N | % | |
|---|---|---|
| Coil size | ||
| 8 × 6 | 14 | 17.5 |
| 10 × 6 | 48 | 60 |
| 12 × 6 | 16 | 20 |
| 14 × 8 | 1 | 1.25 |
| 16 × 8 | 1 | 1.25 |
| Distal coil/LV side of VSD ratio | Range | 0.9–1.33 |
| Mean ± SD | 1.18 ± 0.09 | |
| Median | 1.2 | |
LV = left ventricular; VSD = ventricular septal defect.
3.1. Closure rate
Immediately after VSD closure, complete closure with no residual shunt was observed in 49 out of 79 patients (62%). This figure rose to 65 patients (82.3%) after 24 hours on discharge from hospital, 75 patients (94.9%) after 3 months, 77 patients (97.5%) after 1 year, and remained the same until the end of the follow-up period. Thus, residual shunt has significantly decreased during follow-up (p = 0.001).
3.2. Trivial residual shunt (foaming)
Trivial residual shunt (foaming) was present in 24 patients (30.4%) immediately after closure and regressed in 16 patients after 24 hours, then disappeared after 3 months.
3.3. Mild residual shunt
Mild residual shunt was present in six patients (7.6%) and remained the same after 24 hours, then regressed in two patients after 3 months, regressed in another two patients after 1 year, and persisted in two patients (2.5%) until the end of the follow-up period (Table 8).
Table 8.
Follow-up results (residual shunt) (n = 79).
| Item | Immediately after closure |
24 h after closure |
3 & 6 mo after closure |
1, 2, & 3 y after closure |
p | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| Residual shunt | No | 49 | 62 | 65 | 82.3 | 75 | 94.9 | 77 | 97.5 | 0.001* |
| Yes | 30 | 38 | 14 | 17.7 | 4 | 5.1 | 2 | 2.5 | ||
| Trivial | 24 | 30.4 | 8 | 10.1 | 0 | 0 | 0 | 0 | ||
| Mild | 6 | 7.6 | 6 | 7.6 | 4 | 5.1 | 2 | 2.5 | 0.513 | |
Statistically significant at p ≤ 0.05.
We have not encountered any cases of severe residual shunt or hemolysis.
3.4. Mild mitral regurgitation
Mild mitral regurgitation (MR) (assessed by measuring vena contracta and regurgitant jet area using color flow mapping) was present in 17.7% of patients prior to closure because of dilated mitral annulus and significantly decreased after 6 months of follow-up with significant p value (0.002) (Table 9).
Table 9.
Follow-up results (mitral regurgitation) (n = 79).
| Item | Mitral regurgitation |
p | |||
|---|---|---|---|---|---|
|
N |
Mild |
||||
| n | % | n | % | ||
| Prior to closure | 65 | 82.3 | 14 | 17.7 | |
| Immediately after closure | 65 | 82.3 | 14 | 17.7 | 1.0 |
| 24 h after closure | 65 | 82.3 | 14 | 17.7 | 1.0 |
| 3 mo after closure | 72 | 91.1 | 7 | 8.9 | 0.101 |
| 6 mo after closure | 77 | 97.5 | 2 | 2.5 | 0.002* |
| 1 y after closure | 78 | 98.7 | 1 | 1.3 | 0.001* |
| 2 y after closure | 79 | 100 | 5 | 6.3 | 0.001* |
| 3 y after closure | 79 | 100 | 5 | 6.3 | 0.001* |
Statistically significant at p ≤ 0.05.
3.5. Mild tricuspid regurgitation
Mild tricuspid regurgitation (TR) (assessed by measuring vena contracta, regurgitant jet area using color flow mapping, shape and density of TR Doppler signal) was observed in five patients (6.3%) prior to closure and remained the same over the 3 years of follow-up.
3.6. Trivial aortic regurgitation
Trivial aortic regurgitation (AR) was observed in one patient prior to the procedure without aortic valve prolapse into the defect; the coil was not in contact with the aortic valve and AR remained the same during the follow-up period. We did not encounter aortic leaflet capture by the device in any of our patients. The loops of left disc were configured in the ascending aorta and then the whole ensemble was slowly retracted until the device moved across the aortic valve into the LV. We were careful to keep the tip of the coil directed upwards to prevent hooking of aortic valve. Also, keeping the long sheath next to the coil helps the valve to open by the sheath.
Right bundle branch block developed in one patient 1 year later, with no hemodynamic effect.
4. Discussion
Transcatheter closure of VSD is an attractive treatment option compared to surgery, which is associated with higher morbidity and mortality related to the procedure and the use of cardiopulmonary bypass [18].
Nowadays, various types of VSDs can be closed percutaneously; the commonly used devices are the Amplatzer family of occluders either designed for VSD closure or for other indications. The off-label use of such devices necessitates long-term follow-up [19].
Butera et al. [17] reported percutaneous pmVSD closure in 104 patients using two different Amplatzer devices (AGA Medical Corp.): the mVSD occluder and the pmVSD occluder. The procedural success rate was 96.2%, but with high incidence of developing CHB that required pacemaker implantation in six patients (5.7%; 2 in the early phase and 4 during the follow-up). The median follow-up period was 38.5 months [17].
The coil used in our work is specifically designed for VSD closure. It is funnel shaped and flexible, designed to close the defect by filling it rather than to stent and clamp it, so it does not afford radial force inside the septum or pressure on the surrounding structures [8].
Ödemiş et al. [9] reported short- and mid-term results of using Nit-Occlud Lê VSD coils to close pmVSD in 20 patients. All VSDs had aneurysmal tissue except one, all were closed successfully, three patients developed hemolysis which persisted in one of them despite implantation of a detachable coil to eliminate residual shunt, and the coil had to be removed surgically. No heart block or other valvular dysfunctions were encountered during the follow-up period of 12.3 ± 6.6 months [9].
Haas et al. [8] reported a registry from many centers in Europe, confirming very positive feedback of using Nit-Occlud Lê VSD coil to close various types of VSDs, in that the procedure was successful in 91.9% of patients. Trivial residual shunt was present in 50.0% of patients, which decreased significantly during follow-up. Hemolysis was encountered in four patients; it disappeared spontaneously in two of them, required a second device in one, and persisted after 2 years and had to be removed surgically. One patient developed transient AV block. The mean follow-up period was 31.3 months [8].
We report one case report of Gerbode defect (direct type), diagnosed by TTE and confirmed angiographically. We closed it successfully using a 14 × 8 coil with no residual shunt.
In this work, we report a successful VSD closure rate of 98.75%. All were perimembranous type with aneurysm, except for two that were of high outlet muscular type (at the junction between membranous and muscular septum) and one Gerbode defect. In one patient with CHB with hemodynamic instability, the device had to be removed and the procedure was aborted. No hemolysis, no migration, no new AR, no new TR, and complete closure with no residual shunt were observed in 62% of patients immediately after the procedure, and this rose to 82.3% at discharge, 94.9% after 3 months, and 97.5% after 1 year until the end of the 3-year follow-up period.
4.1. Limitations
This is a retrospective, single center experience. Larger, prospective, and perhaps randomized multicenter studies are needed.
5. Conclusion
The Nit-Occlud Lê VSD coil can be used safely and in a very efficient way for the transcatheter closure of VSD with excellent mid-term follow-up results.
Acknowledgments
We greatly appreciate the help and guidance of Professor Ziyad M. Hijazi in the preparation of the manuscript, and Professor Trong-Phi Lê, who helped us in practical work in most of cases in the catheterization laboratory.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Rodríguez F.B., Sparano A., Robles Y., Urbano E., Hermanni M., Garcia C. Percutaneous transcatheter closure of perimembranous ventricular septal defects in one working group, long-term follow-up. J Pediatr Neonatal Care. 2016;5:00168. [Google Scholar]
- 2.Tianjin Z., Yaling L.U.O., Xueqing Z. Transcatheter closure vs surgical closure of ventricular septal defect in China: a metaanalysis. Cross-Cult Commun. 2015;11:57–65. [Google Scholar]
- 3.Liu J., Wang Z., Gao L., Tan H., Zheng Q., Zhang M. A large institutional Study on Outcomes and complications after transcatheter closure of a perimembranous-type ventricular septal defect in 890 cases. Acta Cardiol Sin. 2013;29:271–276. [PMC free article] [PubMed] [Google Scholar]
- 4.Carminati M., Butera G., Chessa M., De Giovanni J., Fisher G., Gewillig M. Transcatheter closure of congenital ventricular septal defects: results of the European Registry. Eur Heart J. 2007;28:2361–2368. doi: 10.1093/eurheartj/ehm314. [DOI] [PubMed] [Google Scholar]
- 5.Liu S., Chen F., Ding X., Zhao Z., Ke W., Yan Y. Comparison of results and economic analysis of surgical and transcatheter closure of perimembranous ventricular septal defect. Eur J Cardiothorac Surg. 2012;42:157–162. doi: 10.1093/ejcts/ezs519. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Q., Zhao Z., Zuo J., Yang J., Wang H., Yu S. A comparative study: early results and complications of percutaneous and surgical closure of ventricular septal defect. Cardiology. 2009;114:238–243. doi: 10.1159/000232405. [DOI] [PubMed] [Google Scholar]
- 7.Yang J., Yang L., Yu S., Liu J., Zuo J., Chen W. Transcatheter versus surgical closure of perimembranous ventricular septal defects in children. J Am Coll Cardiol. 2014;63:1159–1168. doi: 10.1016/j.jacc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Haas N.A., Kock L., Bertram H., Boekenkamp R., De Wolf D., Ditkivskyy I. Interventional VSD-Closure with the Nit-Occlud® Lê VSD-Coil in 110 patients: early and midterm results of the EUREVECO-Registry. Pediatr Cardiol. 2017;38:215–227. doi: 10.1007/s00246-016-1502-8. [DOI] [PubMed] [Google Scholar]
- 9.Ödemiş E., Saygi M., Guzeltas A., Tanidir I.C., Ergul Y., Ozyilmaz I. Transcatheter closure of perimembranous ventricular septal defects using Nit-Occlud® Lê VSD Coil: early and mid-term results. Pediatr Cardiol. 2014:817–823. doi: 10.1007/s00246-013-0860-8. [DOI] [PubMed] [Google Scholar]
- 10.Yang R., Kong X.Q., Sheng Y.H., Zhou L., Xu D., Yong Y.H. Risk factors and outcomes of post-procedure heart blocks after transcatheter device closure of perimembranous ventricular septal defect. JACC Cardiovasc Interv. 2012;5:422–427. doi: 10.1016/j.jcin.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Yip W.C.L., Zimmerman F., Hijazi Z.M. Heart block and empirical therapy after transcatheter closure of perimembranous ventricular septal defect. Catheter Cardiovasc Interv. 2005;66:436–441. doi: 10.1002/ccd.20512. [DOI] [PubMed] [Google Scholar]
- 12.Dangol A., Bansal M., Al-Khatib Y. Transcatheter closure of acquired left ventricle-to-right atrium shunt: first case report in an infant and review of the literature. Pediatr Cardiol. 2013;34:1258–1260. doi: 10.1007/s00246-012-0372-y. [DOI] [PubMed] [Google Scholar]
- 13.Otaigbe B.E., Orubide D. Case report rare presentation of gerbode defect in a 4-month-old Nigerian and a review of the literature. Case Rep Cardiol. 2013;2013:4–7. doi: 10.1155/2013/564786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahmath M.R.K., Numan M., Dilawar M. Medium to long-term echo follow-up after ventricular septal defect device closure. Asian Cardiovasc Thorac Ann. 2016;24:422–427. doi: 10.1177/0218492316645746. [DOI] [PubMed] [Google Scholar]
- 15.Ergene O., Kahya Eren N., Nazli C., Duygu H., Kocabas U. Percutaneous closure of perimembranous ventricular septal defects associated with septal aneurysm in adults. Turk Kardiyol Dern Ars. 2015;43:699–704. doi: 10.5543/tkda.2015.50945. [DOI] [PubMed] [Google Scholar]
- 16.Lê T.-P. Ventricular septal defect closure: closure of perimembranous VSD using PFM coil. In: Sievert H., Qureshi S.A., Wilson N., Hijazi Z.M., editors. Interventions in structural, valvular, and congenital heart disease. 2nd ed. Taylor & Francis Group; Broken Sound Parkway NW, Suite: 2015. p. 15. [Google Scholar]
- 17.Butera G., Carminati M., Chessa M., Piazza L., Micheletti A., Negura D.G. Transcatheter closure of perimembranous ventricular septal defects, early and long-term results. J Am Coll Cardiol. 2007;50:1189–1195. doi: 10.1016/j.jacc.2007.03.068. [DOI] [PubMed] [Google Scholar]
- 18.Chungsomprasong P., Durongpisitkul K., Vijarnsorn C., Soongswang J., Lê T.P. The results of transcatheter closure of VSD using Amplatzer® device and NIT Occlud® Lê coil. Catheter Cardiovasc Interv. 2011;78:1032–1040. doi: 10.1002/ccd.23084. [DOI] [PubMed] [Google Scholar]
- 19.Yang J., Yang L., Wan Y., Zuo J., Zhang J., Chen W. Transcatheter device closure of perimembranous ventricular septal defects: mid-term outcomes. Eur Heart J. 2010;31:2238–2245. doi: 10.1093/eurheartj/ehq240. [DOI] [PMC free article] [PubMed] [Google Scholar]