Abstract
Genetic engineering of T cells with a T cell receptor (TCR) targeting tumor antigen is a promising strategy for cancer immunotherapy. Inefficient expression of the introduced TCR due to TCR mispairing may limit the efficacy and adversely affect the safety of TCR gene therapy. Here, we evaluated the safety and therapeutic efficiency of an optimized single-chain TCR (scTCR) specific for an HLA-A2.1-restricted (non-mutated) p53(264–272) peptide in adoptive T cell transfer (ACT) models using our unique transgenic mice expressing human p53 and HLA-A2.1 that closely mimic the human setting. Specifically, we showed that adoptive transfer of optimized scTCR-redirected T cells does not induce on-target and off-target autoimmunity. Furthermore, ACT resulted in full tumor protection and led to a long-lived effective, antigen-specific memory T cell response in syngeneic and xenograft models. Taken together, the study demonstrated that our scTCR specific for the broadly expressed tumor-associated antigen p53(264–272) can eradicate p53+A2.1+ tumor cells without inducing off-target or self-directed toxicities in mouse models of ACT. These data strongly support the improved safety and therapeutic efficacy of high-affinity p53scTCR for TCR-based immunotherapy of p53-associated malignancies.
Keywords: gene therapy, T cell receptor, tumor model, autoimmunity
Although T cell receptor (TCR)-gene transfer has shown clinical responses in cancer immunotherapy, inefficient expression of the introduced TCR due to TCR mispairing may limit the efficacy and adversely result in autoimmunity. Here, Echchannaoui et al. report on the improved safety and efficacy of a single-chain TCR specific for human p53.
Introduction
The adoptive transfer of tumor-reactive T cells is a promising approach in the treatment of cancer, but the challenge of isolating T cells with high avidity for tumor antigens in each patient has limited its widespread application. The transfer into T cells of T cell receptor (TCR) genes encoding high-affinity TCRs recognizing defined tumor-associated antigens (TAAs), by retroviral gene transduction, could potentially circumvent this obstacle.1 We have demonstrated that TCR gene transfer can be used to circumvent self-tolerance of autologous T cells to TAAs by transferring a humanized high-affinity MDM2- and p53-specific TCR selected from A2.1 transgenic mice.2, 3, 4, 5 We have previously reported that retroviral expression of a mouse CD8-independent, p53(264–272)-specific TCR (hereafter referred to as p53TCR) into human T cells imparted the CD8+ T lymphocytes with broad tumor-specific cytotoxic T lymphocyte (CTL) activity and turned CD4+ T cells into potent tumor-reactive, p53A2.1-specific Th cells.2 However, expression of recombinant TCR in αβ T cells may result in mixed heterodimer formation with naturally expressed endogenous TCR chains and subsequent potential self-reactivity (off-target autoimmunity).6, 7 Therefore, further improvements to optimize tumor-specific TCR constructs are needed for the clinical application of TCR gene therapy. We have shown that sequence modifications of the introduced TCR constant chains, as well as the introduction of an additional inter-chain disulfide bond between the TCR α and β chain constant domains,8 the use of human-murine hybrid TCR,9 or the inversion of amino acid residues in the constant region of the TCR α and β chains at the TCR interface,10 can reduce, but not entirely eliminate, mixed TCR chain pairing. Different strategies have been developed to knock down endogenous TCR genes, i.e., by RNAi11 or by gene editing using zinc-finger nucleases (ZFNs)12, 13 or the CRISPR and Cas9 nuclease (CRISPR/Cas9)14 and transcription activator-like effector nucleases (TALENs),15 the latest being applied in a clinical setting.16 To overcome TCR mispairing, we designed a p53 single-chain TCR (scTCR) by covalently linking the variable TCRα (Vα) and TCRβ (Vβ) domains with a short glycine-serine-rich peptide linker (Li)17 coexpressed with a truncated constant TCRα (Cα) domain. This strategy resulted in an scTCR construct that mediated efficient antitumor reactivity in human T lymphocytes in vitro and in vivo.18 To further enhance preferential TCR pairing, and thus improve surface expression and TCR function, additional cysteines8 were introduced and TCR sequences were codon optimized19 and cloned into one single 2A-based retroviral vector.20
Here, we evaluated the safety and efficacy of p53TCR gene transfer-associated on- and off-target toxicities in adoptive T cell transfer (ACT) by taking advantage of HupkiCyA2Kb (abbreviated as HupkiA2) mice, which express human wild-type TP53 exons 4–9 in place of the homologous murine TP53 DNA sequences21 and chimeric human leukocyte antigen (HLA)-A*0201Kb. We found that genetic engineering of the conventional full-length TCR could not prevent TCR-mediated lethal off-target autoimmunity in vivo. In sharp contrast, T cells engrafted with a modified scTCR did not induce graft versus host disease (GvHD). Importantly, scTCR gene transfer displayed antitumor response without on-target off-tumor toxicity against normal tissues. Taken together, our data strongly support the improved safety and therapeutic efficacy of a high-affinity scTCR specific for a non-mutated HLA-A2-restricted antigen as a potentially broad-spectrum TCR for cancer immunotherapy.
Results
Retroviral Expression of p53 scTCR and Functional Characterization
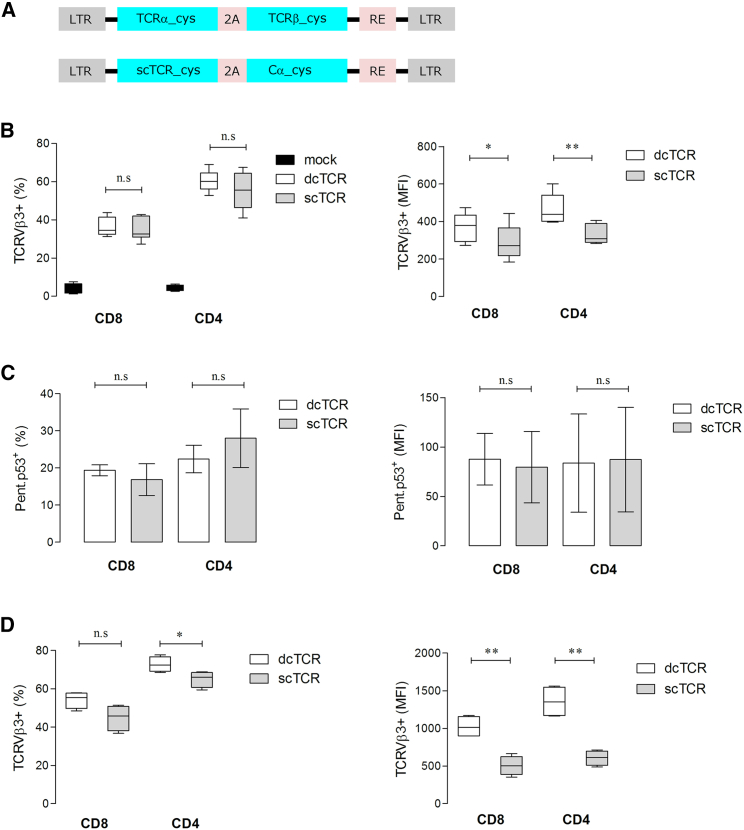
In the present study, genetic engineering approaches were used to modify a full-length, high-affinity p53TCR2 and the derived scTCR version.18 To favor matched TCR chain pairing, and thus enhancing TCR cell-surface expression, both TCRs were modified by optimization of TCR-encoding nucleotide sequences, introduction of an additional inter-chain disulfide bond (Cys) between the TCR α and β chain constant domains,8 and expression of the TCR transgene cassette in 2A virus peptide-based retroviral vectors (Figure 1A). Staining of mouse T cells with a monoclonal antibody (mAb) specific for the mouse Vβ3 subfamily domain of the p53TCR revealed comparable mean transduction efficiencies in the range of 30%–45% for CD8+ and 45%–65% for CD4+ T cells transduced with scTCR and double-chain TCR (dcTCR) (Figure 1B, left graph). However, TCR expression levels, as determined by the mean fluorescence intensity (MFI), were slightly lower in scTCR- versus dcTCR-modified CD8+ (MFI: 288 versus 367; *p = 0.0251) and CD4+ (MFI: 333 versus 464; **p = 0.0090) T cells (Figure 1B, right graph). Both T cell subsets transduced with scTCR or dcTCR showed equivalent binding frequency of 15%–18% for CD8+ and 22%–25% for CD4+ T cells (Figure 1C, left graph) and MFI values to pentameric p53A2.1 complexes for CD8+ and CD4+ T cells (Figure 1C, right graph) 24 hr after transduction. Notably, culture of scTCR- and dcTCR-transduced T cells for 1 week resulted in comparable fractions of TCRVβ3+ T cells in scTCR- and dcTCR-modified CD8+ T cells. In contrast, a lower frequency of TCRVβ3+ T cells was observed in the CD4+ T cell subset (65% versus 73% for scTCR and dcTCR, respectively; *p = 0.0406) (Figure 1D, left graph). MFI values showed a 2-fold lower TCRVβ3+ expression in scTCR- versus dcTCR-modified CD8+ (505 versus 1,024; **p = 0.0011) and CD4+ (607 versus 1,355; **p = 0.0012) T cells (Figure 1D, right graph). Moreover, scTCR- and dcTCR-T cells showed comparable antigen-specific granzyme B production (Figure S1A), tumor necrosis factor alpha (TNF-α) secretion (Figure S1B), a high avidity for the cognate antigen (within the nM range; Figure S1C), and were able to specifically lyse T2 targets pulsed with p53264–272 peptide, but not T2 cells loaded with an irrelevant FluM158–66 peptide (Figure S1D).
Figure 1.
Retroviral Bicistronic Expression of p53TCR
(A) Schematic representation of the pMx retroviral vector-encoding p53-specific TCR. Double-chain (dc) and single-chain (sc) constructs are depicted. Flow cytometry analysis of the expression of the dc and sc p53TCRs in mouse T cells 24 hr after transduction by cell-surface staining with anti-TCRVβ3 mAb (B) as a mean of p53TCR expression, or with pentamer p53(264–272) staining (C). The TCR transgene expression 1 week after antigen-specific stimulation was assessed by anti-TCRVβ3 staining (D). Boxes represent interquartile range and median; whiskers represent minimum to maximum (n = 4–8 biological replicates) showing the transduction efficiency as percent of cells harboring the TCRVβ3 transgene (B–D, left panels) and the corresponding expression level as mean fluorescence intensity (MFI) (B–D, right panels) in live gated CD8+ and CD4+ T cells. As a control, mouse T cells were transduced with a mock (empty) retroviral vector. *p < 0.05; **p < 0.01. 2A, foot-and-mouth disease virus 2A peptide sequence; cys, additional cysteine residue in the constant (C) domain of the TCRα and TCRβ chains; LTR, long terminal repeat; n.s., not significant.
Lethal TCR Gene Transfer-Mediated Autoimmunity Does Not Occur in Mice Infused with p53 scTCR-Modified T Cells
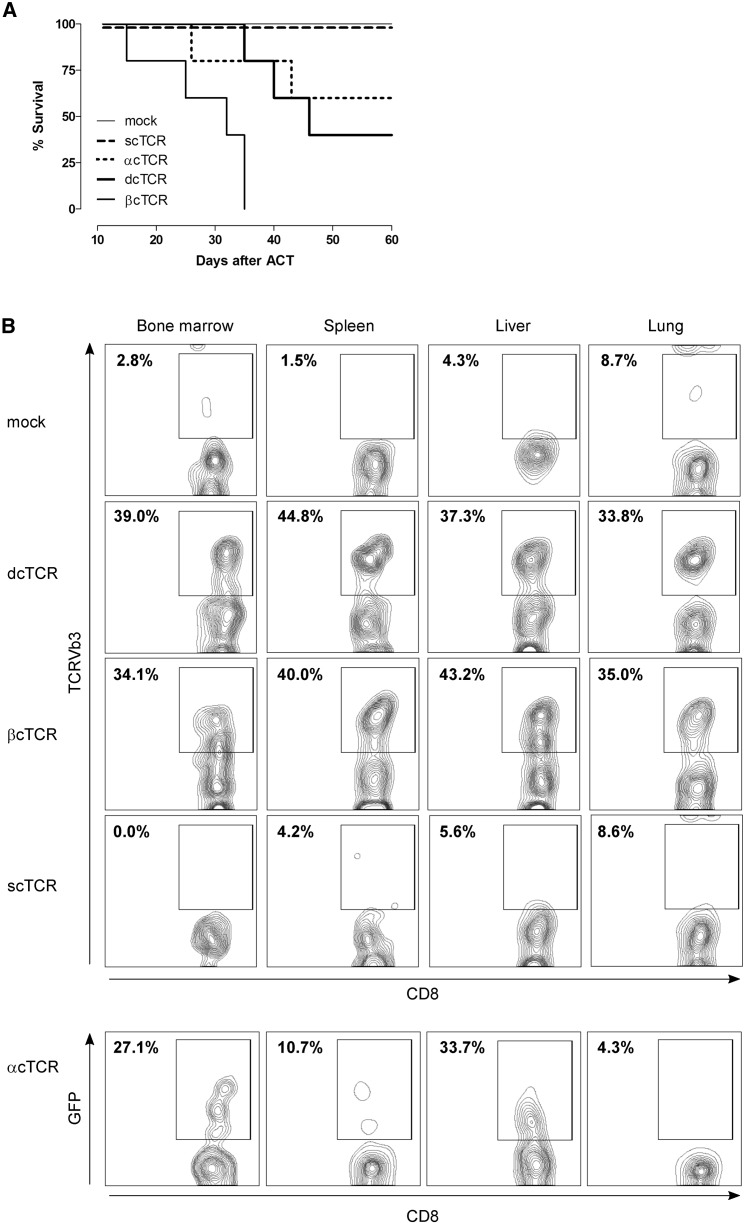
To address the safety of transduced optimized p53TCRs, we performed adoptive transfer experiments in humanized mice lacking the mouse p53 gene. Optimized dcTCR-T cells did not prevent GvHD, because GvHD symptoms occurred in 60% of infused animals after T cell transfer. In contrast, mice injected with scTCR- or mock-T cells remained GvHD-free (Figure 2A). To investigate whether GvHD induction was mediated by TCR mispairing, we generated constructs expressing either the optimized TCR α or β chain only. Retroviral transduction of TCR α or β chain (αc and βc, respectively) alone resulted in strong TCR expression as determined by flow cytometry (data not shown). Adoptive transfer of αc or βc TCR-T cells induced similar or accelerated onset of the pathology to that of dcTCR-T cells (40% and 0% survival, respectively) (Figure 2A). Mice were euthanized when they become moribund (generally 20–50 days post-T cell transfer), and target organs were harvested and examined by flow cytometry for TCR-specific T cell infiltration. Concordantly, recipients treated with scTCR- or mock-modified T cells showed no or poor CD8+Vβ3+ T cell infiltration in the bone marrow (0% versus 2.78%, respectively), spleen (4.17% versus 1.49%, respectively), liver (5.60% versus 4.26%, respectively), and lung (8.60% versus 8.7%, respectively). In contrast, mice adoptively transferred with dcTCR- or βcTCR-modified T cells exhibited a strong and similar infiltration rate (34%–44.8%) in the target organs (Figure 2B). Increased infiltration was also observed in mice infused with αcTCR-T cells as documented by the presence of CD8+GFP+ T cell infiltrates, in particular in the bone marrow (27.1%) and liver (33.7%), whereas the lung and the spleen showed a low infiltration (Figure 2B). Similar results were observed with CD4+ T cell infiltrates (data not shown). Histopathology of the spleen as a representative target organ confirmed that mice suffering from GvHD displayed prominent infiltrates of T cells compared with GvHD-free animals (Figure S2). Taken together, these results indicate that 40%–100% of mice infused with dcTCR-, βcTCR-, or αcTCR-modified T cells developed severe GvHD as a consequence of tissue damage, whereas recipient animals treated with scTCR-T cells remained GvHD free.
Figure 2.
Lethal TCR Gene Transfer-Mediated Autoimmunity Does Not Occur in Mice Infused with p53 scTCR-Modified T Cells
(A) Kaplan-Meier survival plot showing 100% survival in mice receiving 1 × 106 mock- or p53 scTCR-T cells compared with mice injected with p53 dcTCR, βcTCR, or αcTCR T cells (40%, 0%, and 60% survival, respectively; n = 5–6 mice per group). (B) Flow cytometry analysis of tissue-infiltrating T cells in recipient mice following adoptive transfer of TCR-modified T cells. Representative target organs harvested from moribund mice are shown (bone marrow, spleen, liver, and lung). Representative flow plots show live gated CD8+ T cells. Frequency of tissue-infiltrating infused T cells is expressed as percent of CD8+TCRVβ3+ or CD8+GFP+ T cells in organs from recipient mice receiving mock, dcTCR, βcTCR, scTCR, or αcTCR, respectively.
Expansion of Adoptively Transferred p53 scTCR-T Cells in Preconditioned Recipients Does Not Lead to Depletion of the Hematopoietic Compartment
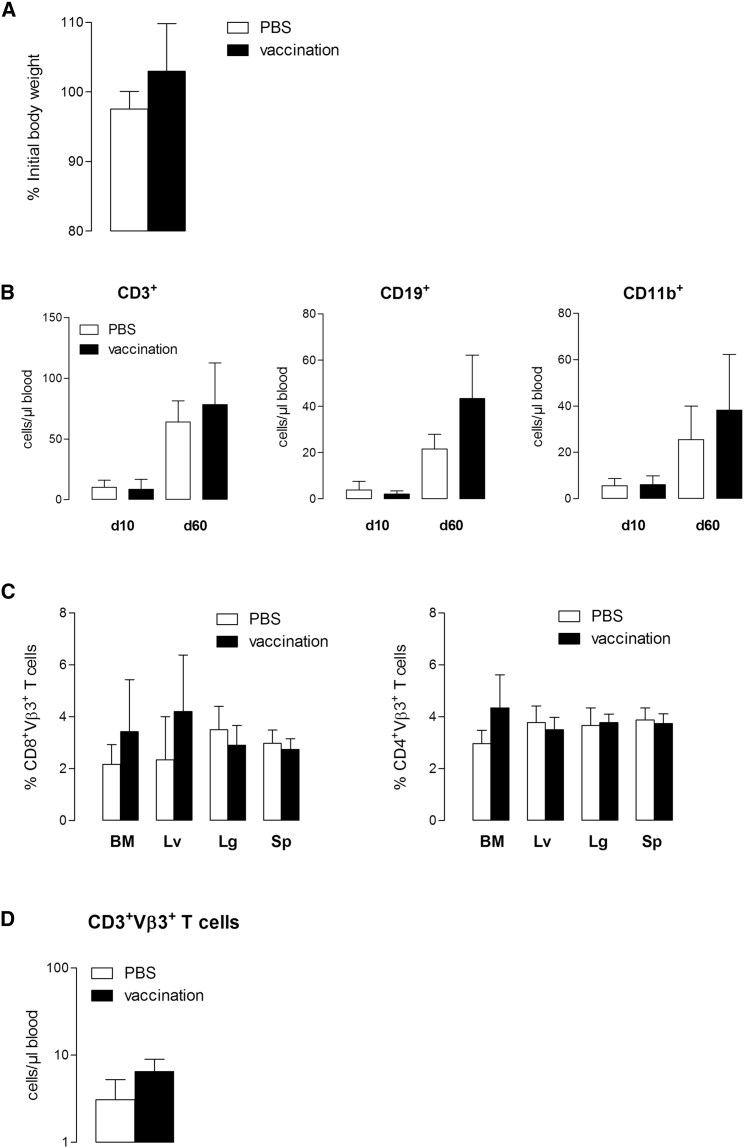
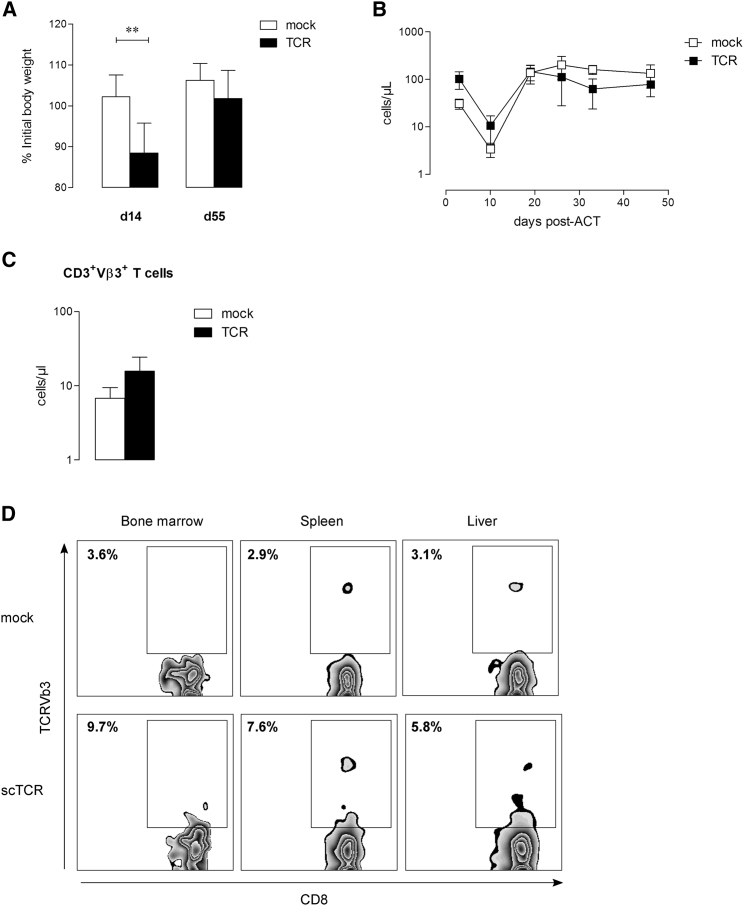
Because expression of p53 in normal tissues may raise the concern of potential on-target toxicity, we performed ACT experiments in HupkiA2 mice. In vitro, scTCR-modified CD8+ T cells were able to specifically lyse p53-overexpressing while ignoring p53 null A2.1+ tumor cells (Figure S3A), mouse embryonic fibroblasts (MEFs), and activated T cells from HupkiA2 mice (Figure S3B). These observations further indicated that fratricide killing does not occur among scTCR-T cells. Mice received a lymphodepleting chemotherapy regimen prior to T cell transfer and were given 2.5 × 106 p53TCR-transduced syngeneic T cells, vaccinated with p53(256–282) polypeptide together with anti-CD40 and CpG or PBS at day 1 after T cell transfer. Vaccination-induced expansion of TCR-specific T cells did not induce signs of toxicity as reflected by stable body weight (Figure 3A). To determine whether TCR-gene transfer could result in a depletion of hematopoietic cells, we assessed a detailed examination of hematopoietic reconstitution by analyzing the absolute white blood cell (WBC) counts, including T and B lymphocytes and monocytes in peripheral blood. Drop of the absolute T cell (CD3+), B cell (CD19+), and monocyte (CD11b+) numbers shortly after lymphodepletion (day 10) was similarly reconstituted in vaccinated and PBS-control groups by day 60 (Figure 3B). A detailed analysis of tissues showed any infiltration of p53TCR-T cells in vital organs (Figure 3C). Accordingly, the persistence of TCRVβ3+ T cells in the peripheral blood was transient as numbers dropped to steady-state levels 60 days post-transfer (Figure 3D). Because we cannot exclude that ACT-mediated on-target toxicity may correlate with the dose of infused T cells, TBI pre-conditioned HupkiA2 mice were injected with 20 × 106 and 10 × 106 p53TCR- or mock-transduced T cells at 1-week intervals and treated with interleukin-2 (IL-2). Under these conditions, T cell expansion (Figure 4A) was associated with a transient weight loss in mice transferred with specific T cells compared with control recipients (Figure 4B). TCR-treated animals recovered their initial body weight and did not develop signs of toxicity. The transient persistence of transferred T cells in the peripheral circulation (Figure 4C) and the absence of organ-infiltrating CD8+ TCRVβ3+ T cells (Figure 4D) strongly suggest that adoptive transfer of scTCR-specific T cells does not result in on-target toxicity.
Figure 3.
Expansion of Adoptively Transferred p53-Specific scTCR-Modified T Cells in Chemotherapy-Preconditioned Recipients Does Not Induce Depletion of the Hematopoietic Compartment
HupkiA2 recipient mice were infused (i.v.) with 2.5 × 106 p53 scTCR-modified HupkiA2 T cells and received a single dose (s.c.) of p53-peptide vaccination or PBS 24 hr after T cell infusion (n = 5 mice per group). (A) Percentage of initial body weight at day 60 after adoptive T cell transfer. (B) Peripheral blood cell counts at days 10 and 60 after adoptive transfer: CD3+ T cells, CD19+ B cells, and CD11b+ monocytes. (C) Flow cytometry analysis of tissue-infiltrating TCRVβ3+ CD8+ (left panel) and CD4+ (right panel) T cells in bone marrow (BM), liver (Lv), lung (Lg), and spleen (Sp) at day 60 after adoptive transfer. Results are expressed as the percentage of TCRVβ3+ cells per CD8 or CD4 subset. (D) Peripheral blood counts of CD3+TCRVβ3+-specific T cells at day 60 after adoptive transfer. Data represent mean numbers ± SEM from five mice in each group. n.s., not significant.
Figure 4.
Adoptive Transfer of High Numbers of p53 scTCR-T Cells Does Not Induce p53 Autoimmunity
HupkiA2 recipient mice were infused (i.v.) at a 1-week interval with 20 × 106 and 10 × 106 mock or TCR-modified HupkiA2 T cells and received an IL-2 injection (i.p.) at days 10–12 after T cell infusion (n = 5 mice per group). (A) Percentage of initial body weight at days 14 and 55 after adoptive T cell transfer. (B) Expansion of CD3+ T cell counts in peripheral blood after adoptive transfer. (C) Peripheral blood counts of CD3+TCRVβ3+-specific T cells at day 55 after adoptive transfer. Data represent mean numbers ± SEM from five mice in each group. (D) Flow cytometry analysis of tissue-infiltrating TCRVβ3+ CD8+ T cells in bone marrow (BM), spleen (Sp), and liver (Lv) at day 83 after adoptive transfer. **p < 0.01. n.s., not significant.
Antigen-Specific scTCR-T Cells Exert Antitumor Reactivity In Vivo
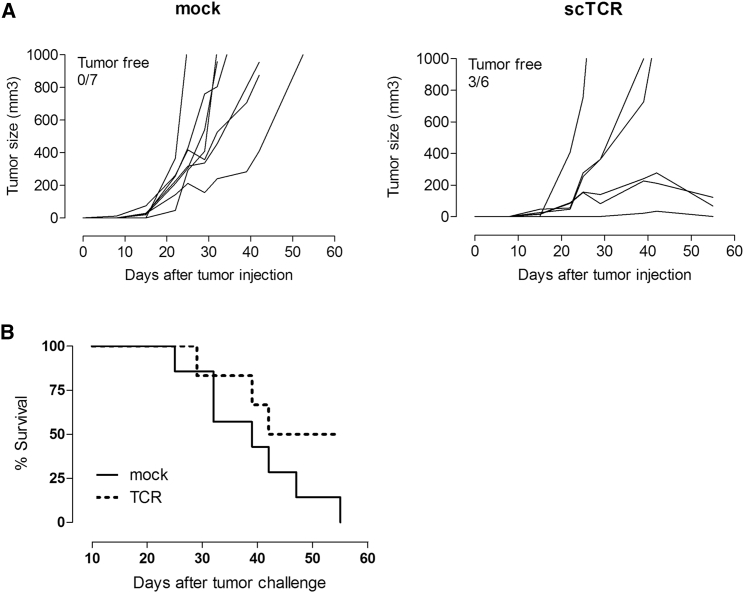
To assess the in vivo antitumor efficacy of scTCR-redirected T cells, TBI-preconditioned HupkiA2 mice were given 6 × 106 mock- or TCR-modified syngeneic T cells and simultaneously injected with 0.2 × 106 A2Kb p53 mutant MEF/R172H tumor cells. To further expand infused T cells, we vaccinated all mice (subcutaneously [s.c.]) with a long-mer p53 peptide along with anti-CD40 and the Toll-like receptor agonist imiquimod (Aldara; Meda, Sweden). Mock-modified T cells did not exert antitumor effect because all inoculated mice showed rapid tumor growth (Figure 5A, left panel), whereas 50% of mice treated with scTCR-modified T cells could eradicate tumors (Figure 5A, right panel), which results in a prolonged tumor-free survival (Figure 5B) after one single infusion of TCR-specific T cells only. Furthermore, mice transferred with TCR-modified T cells developed an antigen-specific memory response as demonstrated by the presence of functional p53 scTCR CD8+ T cells in the spleen of treated mice at day 97 post-T cell transfer (Figure S4).
Figure 5.
Adoptive Transfer of p53 scTCR-Modified Mouse T Cells Displays Antitumor Response in a Syngeneic Tumor Model
HupkiA2 recipient mice were injected (s.c.) with MEF/R172H tumor cells and infused (i.v.) with mock or p53 scTCR-T cells as described in the Materials and Methods (n = 6–7 mice per group). Representative data from two independent experiments are shown. (A and B) Growth and size of tumors after adoptive transfer of mock- (A, left panel; n = 7) or p53 scTCR-T cells (A, right panel; n = 6) and the corresponding Kaplan-Meier survival plot (B).
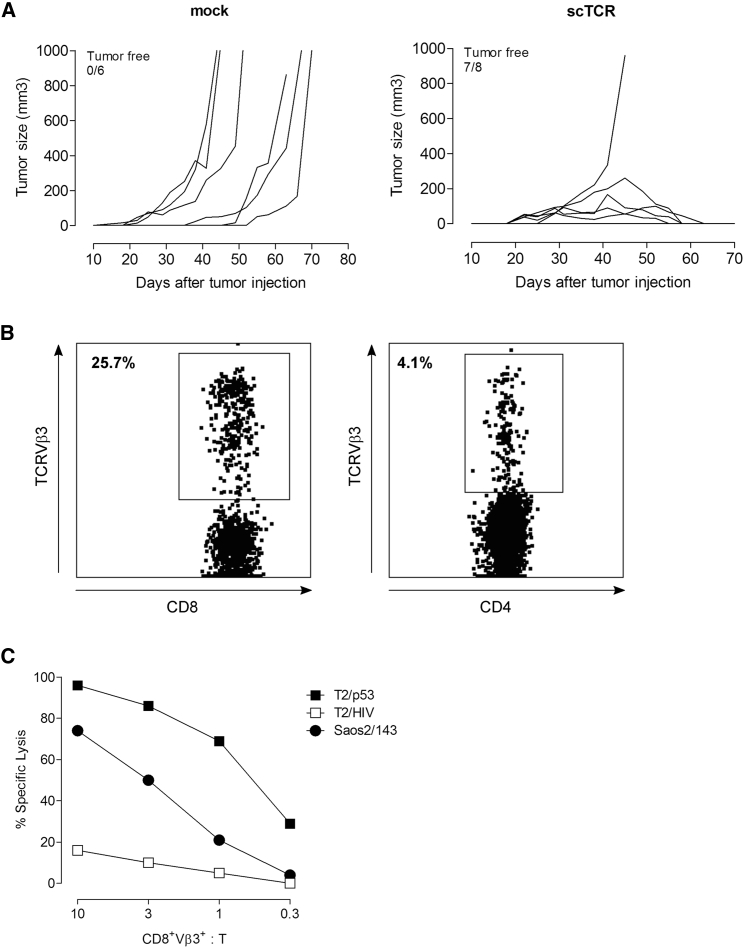
The therapeutic significance of scTCR-mediated antitumor responses in vivo was further evaluated in a xenograft model of osteosarcoma. We first demonstrated that scTCR-modified human T cells exhibit avidities (dissociation constant [KD]) within the nanomolar (nM) range as assessed by binding to pentameric p53A2.1 complexes (Figure S5A). High avidity of the scTCR construct translated into efficient cytolysis of the p53+A2.1+ Saos2/143 osteosarcoma cell line (Figure S5B). We then assessed the capacity of TCR-redirected human T cells to eradicate established osteosarcoma tumors. NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were injected (s.c.) with Saos2/143 cells in the flank and infused with mock- or scTCR-modified bulk human T cells via the tail vein 7 days later. All mice received IL-2 (intraperitoneally [i.p.]) on the day of T cell transfer and at day 7 to induce T cell expansion. Mice transferred with scTCR-T cells showed complete eradication of tumors (Figure 6A, right panel), whereas mock control animals developed large growing tumors (Figure 6A, left panel). Moreover, mice transferred with scTCR-T cells developed a long-lived antigen-specific response as demonstrated by the persistence of TCRVβ3+ CD8+ and CD4+ T cells in the spleen at day 178 after transfer (Figure 6B). These ex-vivo antigen-specific memory T cells showed functional activity in a cytolytic assay against parental Saos2/143 tumor cells, as well as T2 cells pulsed with p53264–272 (Figure 6C). These results demonstrate that transfer of high-avidity p53scTCR-modified human T cells in tumor-bearing NSG mice results in a potent eradication of tumors and leads to long-lived effective antigen-specific memory T cell response.
Figure 6.
Adoptive Transfer of p53 scTCR-Modified Human T Cells Eradicates Established Tumors in a Xenograft Model of Osteosarcoma
NSG mice were injected (s.c.) with Saos2/143 tumor cells and infused (i.v.) with mock or scTCR-modified human bulk T cells as described in the Materials and Methods. All mice received IL-2 injection (i.p.) at days 7 and 14 after tumor challenge. (A) Growth and size of tumors after adoptive transfer of mock (left panel, n = 6 mice) or TCR-specific (right panel, n = 8) T cells. Representative data from three independent experiments are shown. (B) Representative flow plots show the frequency of spleen-infiltrating TCR-specific T cells as percent of TCRVβ3+ T cells (CD8+, left panel; CD4+, right panel) at day 178 after adoptive T cell transfer. (C) Cytolytic activity of ex-vivo antigen-specific memory T cells (described in B) in response to T2 cells pulsed with 10 μM p53264–272 or irrelevant HIV476–484 peptide and Saos2/143 tumor cell line at the indicated CD8+Vβ3+-to-target ratio.
Discussion
Several studies have shown that aberrant p53 expression is often associated with poor prognosis in human cancers.22 However, neither high steady-state levels of p53 nor elevated p53 synthesis alone has been shown to be a prerequisite for tumor regression by p53-specific CTLs. Instead, p53 turnover appeared to be an important factor governing the susceptibility of malignant cells to CTLs.23 Both overexpression and slow turnover of mutant p53 protein, as well as aberrant expression and high turnover of wild-type (WT) p53 protein, result in presentation of high copy numbers of WT p53 peptide-major histocompatibility complex (MHC) class I complexes on tumor cells as compared with low copy numbers on normal cells.5, 23, 24 Consistent with these findings, clinical studies have reported increasing frequencies of WT p53(264–272) peptide-specific CD8+ T cells in the peripheral circulation of patients with squamous cell carcinoma of the head and neck (SCCHN).25, 26 Accordingly, the prioritization of cancer antigens ranked non-mutant p53 antigens in the top 10 candidates,27 making p53 a promising broad-spectrum tumor antigen4, 28 for cancer immunotherapy. However, we and others5, 29 have demonstrated that selected p53 mutations can affect cleavage of the flanking peptides 264–272 and its subsequent presentation by A*0201. As a consequence, tumor cells that overexpress these mutations are not recognized by A*0201-restricted CTL specific for the flanking p53 epitope, 264–272, making our scTCR(264–272) inefficient for targeting these types of tumors. Adoptive TCR-gene therapy is currently challenged by a low therapeutic efficacy and the risk of on- and off-target toxicity. In the present study we addressed the therapeutic safety challenges, using a high-avidity mouse scTCR directed against a human HLA-A2.1-restricted non-mutant p53 epitope in pre-clinical mouse models.
We first genetically modified p53TCR constructs to minimize TCR mispairing. We have shown that cysteine modifications are able to increase the expression of the introduced TCR due to preferential chain pairing,8 but fail to entirely prevent mispairing in human T cells.30 Of note, the TCR α and β chain genes used in these studies were encoded by a two-retroviral vector system. Here, p53TCR transgene cassettes were further codon-optimized and cloned into a single 2A-based retroviral vector that, in addition to enhancing TCR expression, may limit the potential risk of oncogene transformation31 compared with a dual-retroviral vector system. Our in vitro results showed that compared with dcTCR, scTCR-modified CD4+ and CD8+ T cells displayed equivalent high avidity and were able to mediate specific lysis of p53 mutant A2.1+ tumor cells. Although genetic modifications preserved the antigen specificity, the optimized dcTCR was more likely still prone to mispairing as retroviral transduction of modified TCR α or β chain alone resulted in strong mixed TCR dimer expression. Here, we asked whether TCR mispairing observed in vitro with optimized dcTCR could result in off-target toxicity in pre-clinical mouse models of adoptive transfer using as recipients p53 null A2.1Kb mice to preclude any potential p53-dependent on-target effect. Adoptive transfer of TCR α or β chain-transduced T cells induced similar onset of lethal GvHD to that of dcTCR-modified T cells, suggesting that the pathology was likely mediated by self-reactive mixed TCR dimers, in line with the occurrence of TCR gene transfer-associated lethal GvHD observed by Bendle and colleagues.6 However, in contrast with this study, genetic modifications of the p53 dcTCR did not prevent autoimmunity because GvHD occurred in the majority of infused animals, suggesting that genetic engineering is not equally applicable to all TCRs as a strategy to reduce mixed TCR dimers-associated toxicity. As opposed to dcTCR, no signs of GvHD were observed in mice injected with scTCR-modified T cells, resulting in long-term overall survival post-ACT. Concordantly, detailed examination of target tissues, including bone marrow, spleen, and liver, did not show infiltration of scTCR-modified T cells, suggesting that although residual mispairing was observed with the scTCR in vitro,32 this does not, in contrast with a dcTCR, lead to off-target autoimmunity in vivo.
The choice of target tumor antigens for cancer immunotherapy is of central importance because the transfer of high-avidity T cells may cause severe on-target toxicity, as reported for an allo-restricted TCR against hyaluronan-mediated motility receptor (HMMR) in a humanized mouse model.33 The first clinical trial using TCR-engineered T cells targeting MART-1 has demonstrated the feasibility of this approach to mediate cancer regression in metastatic melanoma patients.34 The lack of on-target toxicity in this trial was most likely because T cells were engrafted with a medium-affinity TCR. These results contrasted with the dangerous antigen-specific toxicity reported in patients infused with T cells engineered to express high-avidity TCRs against MART-1 and gp100,35 carcinoembryonic antigen (CEA),36 or MAGE-A3.37 To date, two clinical trials without on-target-associated toxicity were reported with high-affinity TCRs targeting cancer-testis (CT) antigen NY-ESO-1 in melanoma38 and myeloma.39 Objective clinical responses without apparent on-target off-tumor toxicity might be because of the absence of NY-ESO-1 expression in normal tissues. In addition, the clinical efficacy of high-affinity TCR may be limited because of MHC-restricted fratricide, as shown for a survivin-specific TCR.40 However, these results contrasted with another high-affinity survivin-specific TCR without toxicity against hematopoietic progenitors.41 Discrepancy between these studies may be explained by the fact that the first TCR was isolated from an allo-restricted repertoire (absence of thymic education), whereas the second TCR was selected from an autologous repertoire. As opposed to survivin, p53 autoimmunity is unlikely to occur with p53TCR-transduced T cells because we and others did not observe reactivity against normal A2.1+ cells, including hepatocytes, fibroblasts, or activated T cells.2, 42 In the present study, we assessed the capacity of an optimized high-affinity p53 scTCR to cause on-target toxicity in vivo, using A2Kb mice expressing the human p53 protein. TCR-associated fratricide was not observed after adoptive transfer in vivo, because scTCR-specific T cells were able to expand following vaccination. Importantly, preconditioning regimens plus vaccination-induced expansion of transferred TCR-specific T cells did not result in on-target toxicity for hematopoietic progenitor cells, because mice recovered normal WBC counts after ACT and survived without apparent signs of toxicity. A detailed analysis of several tissues revealed no infiltrating scTCR-specific T cells and the absence of tissue damage. Furthermore, we performed a more potent regimen consisting of lymphodepletion before ACT along with repetitive injections of a high T cell number and observed the same outcome, strongly suggesting that adoptive transfer of high-affinity p53scTCR-T cells does not mediate on-target toxicity.
Importantly, TCR-encoding sequences were codon optimized while keeping the CDR3 region unmodified to avoid potential fatal toxicity caused by TCR cross-reactivity as recently observed with affinity-enhanced T cells in myeloma and melanoma patients.43, 44 One of the key findings in the present study is that optimization of scTCR to improve safety did not alter the TCR expression level and the T cell function in vitro. It has been demonstrated that increasing TCR expression has a profound effect on in vivo antitumor activity.45 Yet, other factors such as the differentiation and phenotype of T cells can also impact the outcome of ACT therapy.46, 47 In mice, adoptive transfer of naive T cells has demonstrated superior in vivo expansion, persistence, and antitumor reactivity compared with effector memory T cell and effector T cell subsets.48 In clinical trials, transfer of a less differentiated T cell subset correlated with objective clinical responses.49 Here, we have shown that the co-transfer of less differentiated CD4+ and CD8+ T cells, high-dose IL-2, and vaccination following lymphodepleting regimens resulted in effective antitumor T cell responses in a syngeneic mouse tumor model. Using a xenograft model of osteosarcoma, we could demonstrate that transfer of high-avidity TCR-modified human T cells resulted in a complete eradication of established tumors and was associated with long-lived effective memory T cell response.
In conclusion, our study provided evidence that an optimized high-affinity scTCR specific for the broadly expressed (non-mutant) p53(264–272) antigen can eradicate p53+A2.1+ tumor cells in vivo without inducing off-target or self-directed toxicities in humanized mouse models. To our knowledge, this is the first time that safety aspects with respect to mispairing and TCR-mediated on-target toxicity and therapeutic efficacy of TCR-gene transfer are addressed in vivo.
The present results demonstrated the improved safety and potency of an optimized p53scTCR construct as a potential therapeutic TCR for immunotherapy of p53-associated malignancies.
Materials and Methods
Peptides, Antibodies, and Pentameric pA2.1 Complexes
HLA.A2.1-restricted p53(264–272),4, 5 p53(256–282) polypeptides, FluM1(58–66), and gp100(280–288) peptides were synthesized by Biosyntan (Berlin, Germany) and HIV(476–484) peptide from Affina Immuntechnik (Berlin, Germany). Phycoerythrin (PE)-conjugated anti-HLA-A2.1, allophycocyanin (APC) anti-human CD8, and fluorescein isothiocyanate (FITC) anti-human CD4 were obtained from BD Biosciences. The monoclonal anti-mouse antibodies were PE anti-CD11b, APC anti-CD3, PE-Cy5/peridinin chlorophyll protein complex (PerCP)-Cy5.5 anti-CD4 (eBioscience), PE anti-TCRVβ3, PE anti-CD19, APC anti-CD8, FITC anti-CD11b (BD Biosciences), FITC anti-TNF-α (BD Biosciences), and FITC anti-granzyme B (eBioscience). PE-labeled HLA-A2.1 p53(264–272) Pentamer were obtained from ProImmune. For absolute cell counts, sulfate latex beads 4% 5 μm (Invitrogen/Molecular Probes) were used.
Mice and Cells
Eight- to 12-week-old C57BL/6 and NOD.Cg-PrkdcscidIL2rgtm1Wjl/SzJ (NSG) mice were obtained from the central animal facility of the Johannes Gutenberg University Mainz, Germany. TP53 null A2Kb50 and HupkiA2 mice, generated by crossing Hupki21 and CyA2Kb mice (generated by Cytel Corporation), were used as a source of donor cells and recipients for adoptive immunotherapies. NSG mice were used as recipients for engraftment of tumor cell lines and adoptive transfer of human T cells. All mice were housed under normal conditions according to the guidelines for animal care of the animal facility of the Johannes Gutenberg University. Experimental procedures were performed according to German regulations for the use of laboratory animals. Human A2.1+ cell lines were p53 null osteosarcoma line Saos2 and its p53-transfectant, Saos2/143,24 Jurkat (JA2), and transporters associated with antigen processing (TAP)-deficient cell line T2 (American Type Culture Collection [ATCC]). Human cell lines were maintained in RPMI (GIBCO) supplemented with 10% fetal calf serum (FCS), 1% glutamine, and 1% penicillin-streptomycin. MEFs isolated from either p53 null A2Kb or HupkiA2 mice were immortalized and transformed by E1A/H-ras oncogenes, and subsequently transduced with retroviral vectors encoding the mouse mutant p53R172H and R270H plus the human HLA.A2.1-restricted p53(264–272) epitope (provided by Moshe Oren, Israel). MEF tumor cell lines were maintained in DMEM (GIBCO) supplemented with 10% FCS, 25 mM HEPES-buffer, 1% glutamine, and 1% penicillin-streptomycin. Primary T cells were isolated from freshly harvested spleens of p53 null A2Kb or HupkiA2 mice. Separation of spleen T cell subsets (CD4+ and CD8+ T cells) was performed with magnetic-activated cell sorting (MACS) cell separation beads (Miltenyi Biotec) according to the manufacturer’s protocol. Mice were sacrificed by cervical dislocation, and freshly isolated tumor cells were dissociated by mincing the tissue with scalpels into 0.5-mm small pieces. Dissociated tissue was further triturated and filtered through a 100-μm cell strainer to obtain single-cell suspension. The retroviral packaging cell line Phoenix-Ampho was obtained from the ATCC.
Cloning of TCR Genes and Retroviral Transduction of T Lymphocytes
Full-length mouse TCR α and β chain cDNAs encoding a high-affinity p53TCR were generated and described earlier.2 The original scTCR version17 was described earlier.18 TCR constructs chains were modified by the insertion of an additional inter-chain disulfide bond (Cys) between the TCR α and β chain constant domains,8 and TCR sequences were codon modified (Invitrogen GeneArt, Regensburg, Germany) and cloned into one single 2A-based retroviral vector.20 We used the pGMP or pMx-IRES-GFP retroviral vector backbones to express full-length dcTCRs and scTCRs or TCRα and TCRβ chains, respectively. Mouse splenocytes were retrovirally transduced as described51 with some modifications. In brief, retroviral constructs (10 μg of plasmid DNA encoding TCR αβ chains) were used to transfect Phoenix-Ampho packaging cells using Fugene6 (Roche) with the pCL-Eco construct (7 μg DNA). Where stated, an RFP-control vector was included. T cells were cultivated and activated as described earlier.50 Viral supernatants were collected 48 hr post-transfection and used to transduce 4 × 106 activated splenocytes by coculture on retronectin-coated (Takara-Bio) 24-well plates in the presence of 4 μg/mL polybrene. TCR-modified T cells were cultivated and expanded by weekly restimulation with irradiated peptide-loaded Jurkat-A2.1 (JA2) cells and C57BL/6 spleen feeder cells in the presence of 10% T cell growth factor (TCGF). Retroviral transduction, selection, and expansion of human bulk CD8+/CD4+ T cells were performed as described earlier.18
Mouse Models of ACT and Vaccination
For the syngeneic mouse model, CyA2Kb mice were pre-conditioned with 5.5 Gy of total-body irradiation (TBI) 1 day before ACT. For the “off-target” model, p53 null A2Kb mice received 1 × 106 scTCR- or mock-transduced syngeneic T cells by tail-vein injection. For the “on-target” model, HupkiA2 mice were given 20 × 106 and 10 × 106 p53TCR- or mock-transduced syngeneic T cells at days 1 and 7 after TBI, respectively. In both models, mice were treated with 7.2 × 105 IU of IL-2 (Proleukin, Novartis) i.p. twice daily at days 10, 11, and 12 after adoptive transfer. In a second “on-target” model, HupkiA2 mice received a different lymphodepleting regimen prior to T cell transfer: injection (i.p.) of 2 mg of cyclophosphamide at days −5 and −4, and 2 mg of fludarabine at days −3, −2, and −1. At day 0, mice were given 2.5 × 106 p53TCR-transduced syngeneic T cells and injected (s.c.) with 100 μg of p53(256–282) polypeptide together with 40 μg of anti-CD40 (Alexis) and 20 μg of CpG or PBS at day 1 after T cell transfer. Mice were monitored daily for clinical symptoms of GvHD (i.e., weight loss, posture, activity, fur texture, and skin integrity) using a scoring system developed by Cooke et al.52 to quantitate disease grade and progression. Mice were euthanized when they become moribund, and target organs were analyzed by flow cytometry and histopathologic assessment of GvHD. Bone marrow cells were harvested from the femurs and tibiae. Single-cell suspension of bone marrow, liver, lung, and spleen cell suspensions were prepared by gently mincing the tissues and filtered through a 100-μm cell strainer. For the syngeneic tumor model, HupkiA2 mice were irradiated with 5.5 Gy and 24 hr later injected (s.c.) with 0.2 × 106 MEF/R172H tumor cells in 100 μL of PBS in the right flank and given 6 × 106 p53 scTCR- or mock-transduced syngeneic T cells by tail vein. One day after adoptive transfer, mice were injected (s.c.) with 100 μg of p53(256–282) peptide, 40 μg of anti-CD40, and received a topical application (62 mg) of imiquimod cream in the right flank. In the xenograft tumor model of osteosarcoma, NSG mice were injected (s.c.) with 3 × 106 Saos2/143 tumor cells in 100 μL of PBS in the right flank and given (by tail vein) 5 × 106 p53 scTCR- or mock-transduced human bulk T cells 7 days later, when tumors were palpable. All mice received a first injection of 7.2 × 105 IU of IL-2 (i.p.) on day of T cell transfer and a second dose 7 days later. In both models, tumor sizes were measured using digital calipers twice a week and calculated as the product of (length × width2). Mice were sacrificed when tumor size was 1 cm3.
Flow Cytometry and T Cell Assays
Samples were stained with antibodies against indicated cell-surface markers, fixed, and permeabilized. Intracellular staining of TNF-α and granzyme B was performed using the BD Cytofix/Cytoperm Kit (BD Biosciences) according to the manufacturer’s protocol. Flow cytometry acquisitions were performed on a FACSCalibur instrument (BD Biosciences) or FACSCanto II (BD Biosciences), and data were analyzed with FlowJo software (Tree Star). Standard 51Cr-release assays were performed at the indicated effector-to-target (E:T) ratio in duplicate wells as described earlier.32 Equilibrium peptide-MHC pentamer binding data were plotted as Scatchard plot of MFI/concentration of pentamer against MFI. The avidity or KD is calculated as KD = −1/slope.2, 18 The avidity and the half-maximal stimulatory concentration (EC50) of the different TCR constructs were determined with Prism version 3.0 (GraphPad Software). Standard IFN-γ ELISPOT assay of ex-vivo tumor-antigen-specific mouse T cells and p53TCR-specific T cell line against T2 pulsed with the cognate peptide was performed in duplicate wells as reported previously.2
Histology
Mice were sacrificed by cervical dislocation and spleens were removed by dissection, immediately processed with Optimal Cutting Temperature medium (OCT Tissue Tek; Miles), snap-frozen in liquid nitrogen, and stored at −80°C. Immunohistochemical CD4 staining was performed on 2- to 4-μm frozen sections using clone YTS191.1 antibody (1:300 dilution; Lifespan Biosciences). A secondary anti-rat IgG biotin-conjugated antibody (1:300; eBioscience) and a streptavidin-peroxidase polymer (1:1,000; Sigma) were used to amplify the signal. CD4-positive cells were visualized with diaminobenzidine (DAB) chromogenic substrate (3, 3-diaminobenzidine tetrahydrochloride; Carl Roth). Sections were counterstained with H&E for tissue morphology.
Statistical Analysis
Two-tailed Student’s t test was used to compare differences between groups. p < 0.05 was considered significant.
Author Contributions
H.E. designed and performed the research, analyzed and interpreted the data, and wrote the paper; J.P. and E.A.F. performed the research, and analyzed and interpreted the data; B.H., C.L.-J., and R.-H.V. analyzed and interpreted the data; and M.T. designed the research, analyzed and interpreted the data, and wrote the paper.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We thank Dr. Markus Radsak (University Medical Center, Mainz) for providing CpG. This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (DFG; KFO183 TP1 to H.E., R.-H.V., and M.T.; GK1043 to M.T.), Collaborative Research Center 1292 (CRC 1292 TP06 to M.T.), Deutsche Krebshilfe (Project 111546 to M.T.), and the German Consortium for Translational Cancer Research (DKTK) Frankfurt/Mainz location.
Footnotes
Supplemental Information includes five figures and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.11.006.
Supplemental Information
References
- 1.Schumacher T.N. T-cell-receptor gene therapy. Nat. Rev. Immunol. 2002;2:512–519. doi: 10.1038/nri841. [DOI] [PubMed] [Google Scholar]
- 2.Kuball J., Schmitz F.W., Voss R.H., Ferreira E.A., Engel R., Guillaume P., Strand S., Romero P., Huber C., Sherman L.A., Theobald M. Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity. 2005;22:117–129. doi: 10.1016/j.immuni.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Stanislawski T., Voss R.H., Lotz C., Sadovnikova E., Willemsen R.A., Kuball J., Ruppert T., Bolhuis R.L., Melief C.J., Huber C. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat. Immunol. 2001;2:962–970. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- 4.Theobald M., Biggs J., Dittmer D., Levine A.J., Sherman L.A. Targeting p53 as a general tumor antigen. Proc. Natl. Acad. Sci. USA. 1995;92:11993–11997. doi: 10.1073/pnas.92.26.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theobald M., Ruppert T., Kuckelkorn U., Hernandez J., Häussler A., Ferreira E.A., Liewer U., Biggs J., Levine A.J., Huber C. The sequence alteration associated with a mutational hotspot in p53 protects cells from lysis by cytotoxic T lymphocytes specific for a flanking peptide epitope. J. Exp. Med. 1998;188:1017–1028. doi: 10.1084/jem.188.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendle G.M., Linnemann C., Hooijkaas A.I., Bies L., de Witte M.A., Jorritsma A., Kaiser A.D., Pouw N., Debets R., Kieback E. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 2010;16:565–570. doi: 10.1038/nm.2128. 1p following 570. [DOI] [PubMed] [Google Scholar]
- 7.van Loenen M.M., de Boer R., Amir A.L., Hagedoorn R.S., Volbeda G.L., Willemze R., van Rood J.J., Falkenburg J.H., Heemskerk M.H. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc. Natl. Acad. Sci. USA. 2010;107:10972–10977. doi: 10.1073/pnas.1005802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuball J., Dossett M.L., Wolfl M., Ho W.Y., Voss R.H., Fowler C., Greenberg P.D. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen C.J., Zhao Y., Zheng Z., Rosenberg S.A., Morgan R.A. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voss R.H., Willemsen R.A., Kuball J., Grabowski M., Engel R., Intan R.S., Guillaume P., Romero P., Huber C., Theobald M. Molecular design of the Calphabeta interface favors specific pairing of introduced TCRalphabeta in human T cells. J. Immunol. 2008;180:391–401. doi: 10.4049/jimmunol.180.1.391. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto S., Mineno J., Ikeda H., Fujiwara H., Yasukawa M., Shiku H., Kato I. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res. 2009;69:9003–9011. doi: 10.1158/0008-5472.CAN-09-1450. [DOI] [PubMed] [Google Scholar]
- 12.Mastaglio S., Genovese P., Magnani Z., Ruggiero E., Landoni E., Camisa B., Schiroli G., Provasi E., Lombardo A., Reik A. NY-ESO-1 TCR single edited stem and central memory T cells to treat multiple myeloma without graft-versus-host disease. Blood. 2017;130:606–618. doi: 10.1182/blood-2016-08-732636. [DOI] [PubMed] [Google Scholar]
- 13.Provasi E., Genovese P., Lombardo A., Magnani Z., Liu P.Q., Reik A., Chu V., Paschon D.E., Zhang L., Kuball J. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 2012;18:807–815. doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eyquem J., Mansilla-Soto J., Giavridis T., van der Stegen S.J., Hamieh M., Cunanan K.M., Odak A., Gönen M., Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berdien B., Mock U., Atanackovic D., Fehse B. TALEN-mediated editing of endogenous T-cell receptors facilitates efficient reprogramming of T lymphocytes by lentiviral gene transfer. Gene Ther. 2014;21:539–548. doi: 10.1038/gt.2014.26. [DOI] [PubMed] [Google Scholar]
- 16.Qasim W., Zhan H., Samarasinghe S., Adams S., Amrolia P., Stafford S., Butler K., Rivat C., Wright G., Somana K. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017;9:eaaj2013. doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 17.Robinson C.R., Sauer R.T. Optimizing the stability of single-chain proteins by linker length and composition mutagenesis. Proc. Natl. Acad. Sci. USA. 1998;95:5929–5934. doi: 10.1073/pnas.95.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voss R.H., Thomas S., Pfirschke C., Hauptrock B., Klobuch S., Kuball J., Grabowski M., Engel R., Guillaume P., Romero P. Coexpression of the T-cell receptor constant alpha domain triggers tumor reactivity of single-chain TCR-transduced human T cells. Blood. 2010;115:5154–5163. doi: 10.1182/blood-2009-11-254078. [DOI] [PubMed] [Google Scholar]
- 19.Scholten K.B., Kramer D., Kueter E.W., Graf M., Schoedl T., Meijer C.J., Schreurs M.W., Hooijberg E. Codon modification of T cell receptors allows enhanced functional expression in transgenic human T cells. Clin. Immunol. 2006;119:135–145. doi: 10.1016/j.clim.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Szymczak A.L., Workman C.J., Wang Y., Vignali K.M., Dilioglou S., Vanin E.F., Vignali D.A. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat. Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 21.Luo J.L., Yang Q., Tong W.M., Hergenhahn M., Wang Z.Q., Hollstein M. Knock-in mice with a chimeric human/murine p53 gene develop normally and show wild-type p53 responses to DNA damaging agents: a new biomedical research tool. Oncogene. 2001;20:320–328. doi: 10.1038/sj.onc.1204080. [DOI] [PubMed] [Google Scholar]
- 22.Robles A.I., Harris C.C. Clinical outcomes and correlates of TP53 mutations and cancer. Cold Spring Harb. Perspect. Biol. 2010;2:a001016. doi: 10.1101/cshperspect.a001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vierboom M.P., Zwaveling S., Bos G.M.J., Ooms M., Krietemeijer G.M., Melief C.J., Offringa R. High steady-state levels of p53 are not a prerequisite for tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. Cancer Res. 2000;60:5508–5513. [PubMed] [Google Scholar]
- 24.Kuckelkorn U., Ferreira E.A., Drung I., Liewer U., Kloetzel P.M., Theobald M. The effect of the interferon-gamma-inducible processing machinery on the generation of a naturally tumor-associated human cytotoxic T lymphocyte epitope within a wild-type and mutant p53 sequence context. Eur. J. Immunol. 2002;32:1368–1375. doi: 10.1002/1521-4141(200205)32:5<1368::AID-IMMU1368>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Albers A.E., Qian X., Kaufmann A.M., Mytilineos D., Ferris R.L., Hoffmann T.K., DeLeo A.B. Phenotype of p53 wild-type epitope-specific T cells in the circulation of patients with head and neck cancer. Sci. Rep. 2018;8:10716. doi: 10.1038/s41598-018-29067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann T.K., Donnenberg A.D., Finkelstein S.D., Donnenberg V.S., Friebe-Hoffmann U., Myers E.N., Appella E., DeLeo A.B., Whiteside T.L. Frequencies of tetramer+ T cells specific for the wild-type sequence p53(264-272) peptide in the circulation of patients with head and neck cancer. Cancer Res. 2002;62:3521–3529. [PubMed] [Google Scholar]
- 27.Cheever M.A., Allison J.P., Ferris A.S., Finn O.J., Hastings B.M., Hecht T.T., Mellman I., Prindiville S.A., Viner J.L., Weiner L.M., Matrisian L.M. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theobald M., Offringa R. Anti-p53-directed immunotherapy of malignant disease. Expert Rev. Mol. Med. 2003;5:1–13. doi: 10.1017/S1462399403006173. [DOI] [PubMed] [Google Scholar]
- 29.Shamalov K., Levy S.N., Horovitz-Fried M., Cohen C.J. The mutational status of p53 can influence its recognition by human T-cells. OncoImmunology. 2017;6:e1285990. doi: 10.1080/2162402X.2017.1285990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas S., Xue S.A., Cesco-Gaspere M., San José E., Hart D.P., Wong V., Debets R., Alarcon B., Morris E., Stauss H.J. Targeting the Wilms tumor antigen 1 by TCR gene transfer: TCR variants improve tetramer binding but not the function of gene modified human T cells. J. Immunol. 2007;179:5803–5810. doi: 10.4049/jimmunol.179.9.5803. [DOI] [PubMed] [Google Scholar]
- 31.Recchia A., Bonini C., Magnani Z., Urbinati F., Sartori D., Muraro S., Tagliafico E., Bondanza A., Stanghellini M.T., Bernardi M. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T cells. Proc. Natl. Acad. Sci. USA. 2006;103:1457–1462. doi: 10.1073/pnas.0507496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knies D., Klobuch S., Xue S.A., Birtel M., Echchannaoui H., Yildiz O., Omokoko T., Guillaume P., Romero P., Stauss H. An optimized single chain TCR scaffold relying on the assembly with the native CD3-complex prevents residual mispairing with endogenous TCRs in human T-cells. Oncotarget. 2016;7:21199–21221. doi: 10.18632/oncotarget.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spranger S., Jeremias I., Wilde S., Leisegang M., Stärck L., Mosetter B., Uckert W., Heemskerk M.H., Schendel D.J., Frankenberger B. TCR-transgenic lymphocytes specific for HMMR/Rhamm limit tumor outgrowth in vivo. Blood. 2012;119:3440–3449. doi: 10.1182/blood-2011-06-357939. [DOI] [PubMed] [Google Scholar]
- 34.Morgan R.A., Dudley M.E., Wunderlich J.R., Hughes M.S., Yang J.C., Sherry R.M., Royal R.E., Topalian S.L., Kammula U.S., Restifo N.P. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson L.A., Morgan R.A., Dudley M.E., Cassard L., Yang J.C., Hughes M.S., Kammula U.S., Royal R.E., Sherry R.M., Wunderlich J.R. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parkhurst M.R., Yang J.C., Langan R.C., Dudley M.E., Nathan D.A., Feldman S.A., Davis J.L., Morgan R.A., Merino M.J., Sherry R.M. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 2011;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan R.A., Chinnasamy N., Abate-Daga D., Gros A., Robbins P.F., Zheng Z., Dudley M.E., Feldman S.A., Yang J.C., Sherry R.M. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins P.F., Morgan R.A., Feldman S.A., Yang J.C., Sherry R.M., Dudley M.E., Wunderlich J.R., Nahvi A.V., Helman L.J., Mackall C.L. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rapoport A.P., Stadtmauer E.A., Binder-Scholl G.K., Goloubeva O., Vogl D.T., Lacey S.F., Badros A.Z., Garfall A., Weiss B., Finklestein J. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leisegang M., Wilde S., Spranger S., Milosevic S., Frankenberger B., Uckert W., Schendel D.J. MHC-restricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors. J. Clin. Invest. 2010;120:3869–3877. doi: 10.1172/JCI43437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arber C., Feng X., Abhyankar H., Romero E., Wu M.F., Heslop H.E., Barth P., Dotti G., Savoldo B. Survivin-specific T cell receptor targets tumor but not T cells. J. Clin. Invest. 2015;125:157–168. doi: 10.1172/JCI75876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen C.J., Zheng Z., Bray R., Zhao Y., Sherman L.A., Rosenberg S.A., Morgan R.A. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J. Immunol. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cameron B.J., Gerry A.B., Dukes J., Harper J.V., Kannan V., Bianchi F.C., Grand F., Brewer J.E., Gupta M., Plesa G. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linette G.P., Stadtmauer E.A., Maus M.V., Rapoport A.P., Levine B.L., Emery L., Litzky L., Bagg A., Carreno B.M., Cimino P.J. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Witte M.A., Jorritsma A., Kaiser A., van den Boom M.D., Dokter M., Bendle G.M., Haanen J.B., Schumacher T.N. Requirements for effective antitumor responses of TCR transduced T cells. J. Immunol. 2008;181:5128–5136. doi: 10.4049/jimmunol.181.7.5128. [DOI] [PubMed] [Google Scholar]
- 46.Hinrichs C.S., Borman Z.A., Gattinoni L., Yu Z., Burns W.R., Huang J., Klebanoff C.A., Johnson L.A., Kerkar S.P., Yang S. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klebanoff C.A., Gattinoni L., Palmer D.C., Muranski P., Ji Y., Hinrichs C.S., Borman Z.A., Kerkar S.P., Scott C.D., Finkelstein S.E. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin. Cancer Res. 2011;17:5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinrichs C.S., Borman Z.A., Cassard L., Gattinoni L., Spolski R., Yu Z., Sanchez-Perez L., Muranski P., Kern S.J., Logun C. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl. Acad. Sci. USA. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klebanoff C.A., Gattinoni L., Restifo N.P. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J. Immunother. 2012;35:651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theobald M., Biggs J., Hernández J., Lustgarten J., Labadie C., Sherman L.A. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J. Exp. Med. 1997;185:833–841. doi: 10.1084/jem.185.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kessels H.W., Wolkers M.C., van den Boom M.D., van der Valk M.A., Schumacher T.N. Immunotherapy through TCR gene transfer. Nat. Immunol. 2001;2:957–961. doi: 10.1038/ni1001-957. [DOI] [PubMed] [Google Scholar]
- 52.Cooke K.R., Kobzik L., Martin T.R., Brewer J., Delmonte J., Jr., Crawford J.M., Ferrara J.L. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.