Abstract
Background
Severe putamen dopamine depletion characterizes Parkinson’s disease (PD) and multiple system atrophy (MSA). The extent of the depletion is greater than can be accounted for by loss of nigrostriatal dopaminergic terminals alone. We used putamen tissue levels and ratios of cysteinyl and parent catechols to explore possible denervation-independent abnormalities of dopamine synthesis and fate in PD and MSA. 5-S-Cysteinyldopa (Cys-DOPA) is produced from spontaneous oxidation of DOPA and 5-S-cysteinyldopamine (Cys-DA) from spontaneous oxidation of DA.
Methods
Post-mortem putamen tissue samples from 17 PD and 25 MSA patients and 30 controls were assayed for endogenous catechols including DA, its cytoplasmic metabolites (Cys-DA, 3,4-dihydroxyphenylacetic acid, 3,4-dihydroxyphenylethanol, and 3,4-dihydroxyphenylacetaldehyde), and tyrosine hydroxylation products proximal to DA (DOPA and Cys-DOPA).
Results
The PD and MSA groups did not differ in mean values of parent or cysteinyl catechols, and the data for the two groups were lumped. In the patients an index of vesicular storage of DA (the ratio of DA to the sum of its cytoplasmic metabolites) averaged 54% of control (p = 0.001), and an index of L-aromatic-amino-acid decarboxylase (LAAAD) activity (the ratio of DA and the sum of its cytoplasmic metabolites to the sum of DOPA + Cys-DOPA) averaged 21% of control (p < 0.0001). An index of innervation (the sum of DOPA + Cys-DOPA) averaged 63% of control (p = 0.01).
Interpretation
Based on patterns of parent and cysteinyl catechols in putamen, PD and MSA involve decreased vesicular uptake and decreased LAAAD activity in the residual dopaminergic terminals. The combination seems to contribute importantly to dopamine depletion in these diseases.
Keywords: Cysteinyl-dopamine, Cysteinyl-DOPA, Parkinson’s disease, Multiple system atrophy
Graphical abstract
The parkinsonian movement disorder in Parkinson’s disease (PD) and multiple system atrophy (MSA) is associated with profound depletion of the catecholamine, dopamine (DA), in the putamen [1]. It is widely presumed that this deficiency directly and solely reflects loss of nigrostriatal neurons; however, the extent of putamen DA depletion in PD (more than 90% in most studies) is greater than can be accounted for by the loss of nigral dopaminergic neurons or striatal dopaminergic terminals alone (about 60–80%) [2], [3].
The difference could reflect denervation-independent abnormalities of DA storage and synthesis in the residual neurons. Recent studies have supported the view that both PD and MSA involve a decreased ability to retain catecholamines in vesicles [4], [5], and essentially all of putamen DA content is in the vesicles. In addition, PD is associated with decreased activity of L-aromatic-amino-acid decarboxylase (LAAAD) [6]. Since LAAAD is required for DA synthesis from DOPA, LAAAD deficiency could also contribute to DA depletion.
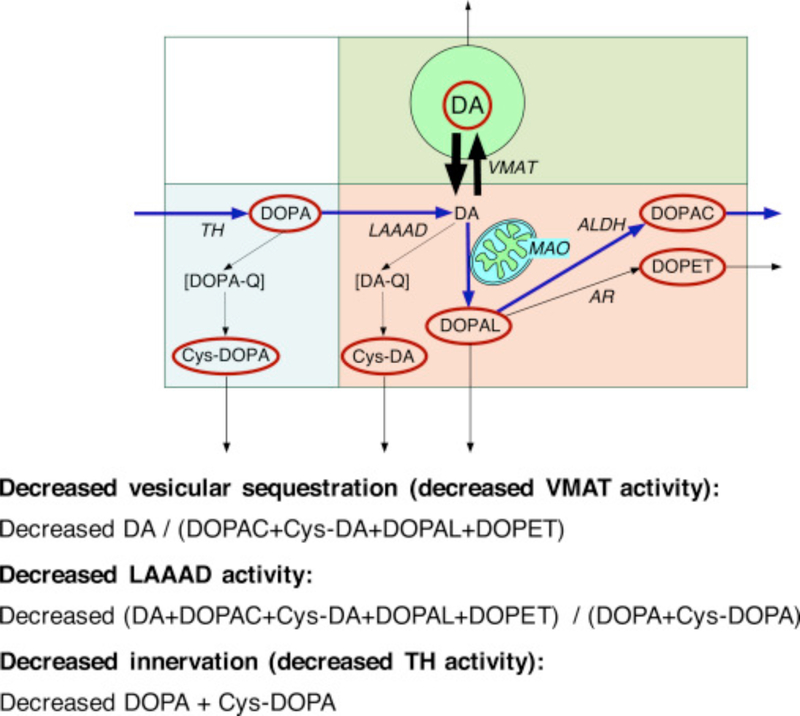
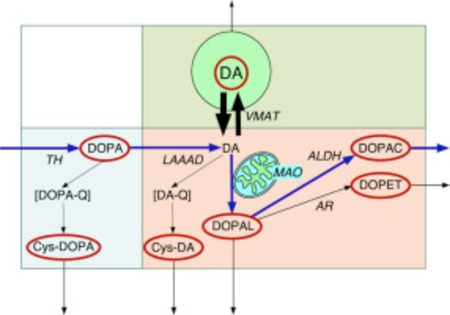
We used simultaneous measurements of parent and cysteinyl catechols to examine these possibilities. Inspection of the concept diagram in Fig. 1 helps understand the indices chosen to assess vesicular storage, LAAAD activity, and denervation. DOPA is decarboxylated enzymatically to form DA, and DA is deaminated enzymatically to form the intermediate metabolite 3,4-dihydroxyphanylacetaldehyde (DOPAL), followed by enzymatic conversion to 3,4-dihydroxyphenylacetic acid (DOPAC) or to the minor metabolite 3,4-dihydroxyphenylethanol (DOPET). A small proportion of cytoplasmic DOPA undergoes spontaneous oxidation to form DOPA-quinone and then 5-S-cysteinyl-DOPA (Cys-DOPA); and a small proportion of cytoplasmic DA undergoes spontaneous oxidation to form 5-S-cysteinyl-dopamine (Cys-DA). Cys-DOPA is not a substrate for LAAAD [7].
Fig. 1. Concept diagram about sources and metabolic fate of dopamine in putamen tissue.
Tyrosine hydroxylase (TH) catalyzes the conversion of tyrosine to DOPA, and L-aromatic-amino-acid decarboxylase (LAAAD) converts DOPA to dopamine (DA). Most of the DA in putamen tissue is in vesicles, due to uptake mediated by the vesicular monoamine transporter (VMAT). Cytoplasmic DA can be metabolized by monoamine oxidase (MAO) in the outer mitochondrial membrane to form 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is metabolize by aldehyde dehydrogenase (ALDH) to form 3,4-dihydroxyphenylacetic acid (DOPAC) or by aldehyde/aldose reductase (AR) to form 3,4-dihydroxyphenylethanol (DOPET). Cytoplasmic DA can oxidize spontaneously to form DA-quinone (DA-Q) and then 5-S-cysteinyl-DA (Cys-DA), and cytoplasmic DOPA can oxidize spontaneously to form DOPA-quinone (DOPA-Q) and then 5-S-cysteinyl-DOPA (Cys-DOPA). The rectangle in aqua corresponds to products of TH proximal to DA; in pink to cytoplasmic DA metabolites; and in green to vesicular DA.
From inspection of Fig. 1, if there were vesicular storage defect, then the ratio of DA, in green, to the sum of its cytoplasmic metabolites (i.e., DA/(DOPAC + Cys-DA + DOPAL + DOPET)), in pink, would be decreased. If there were attenuated LAAAD activity, then the ratio of DA and its metabolites, in pink and green, to the sum of the tyrosine hydroxylase products proximal to DA (i.e., DOPA + Cys-DOPA), in aqua, would be decreased. Finally, if there were denervation, then the local concentration of TH activity would be expected to be decreased, and so the sum of DOPA + Cys-DOPA, in aqua, which are two products of TH acting on tyrosine proximal to the LAAAD step, would be decreased.
1. Methods
1.1. Patient material
Post-mortem brain tissue was obtained from 17 patients with neuropathologically confirmed sporadic PD, 21 patients with MSA, and 25 control subjects, most of whom were autopsied at the University of Miami Brain Endowment Bank. The study was conducted with approval of the Human Subjects Research Office (M809) of the University of Miami. Post-mortem intervals (duration between death and brain freezing) were recorded and in all subjects were ≤24 h. Lewy bodies and glial cytoplasmic inclusions were identified with antibodies to alpha-synuclein. The control subjects were selected to have similar mean age and post-mortem intervals as the PD and MSA groups and did not have Alzheimer-related pathology.
1.2. Assays of catechols in tissue
The putamen samples were matched at the same posterior level and subregional localization. The samples were stored at −70 °C or colder until thawed for catechol assays in our laboratory [8].
1.3. Data analysis and statistics
Tissue catechol concentrations were expressed as fmol/mg wet weight. For statistical tests individual neurochemical data were log transformed. This is a commonly used and appropriate approach when compared groups differ substantially not only in mean values but also in standard deviations and the standard deviations vary directly with the mean values.
Most of the samples had been assayed previously, before we had developed methodology for simultaneous measurements of cysteinyl and parent catechols. Re-thawing of the samples decreased DOPAL contents. Therefore, DOPAL data from the first set of assays were used.
Catechol concentrations in PDA and MSA vs. control groups were compared by factorial analyses of variance with Fisher’s PLSD post-hoc test. Pearson correlation coefficients relating neurochemical values across subjects were calculated using Kaleidagraph 4.0 (Synergy Software).
2. Results
2.1. Patient demographics and post-mortem intervals
The mean age of the MSA group was 63 ± (SEM) 2 years (range 46–72 years), with 11 men and 14 women; 22 were Caucasian, 2 Hispanic, and 1 Greek. The mean age of the PD group was 76 ± 1 years (range 66–85 years), with 13 men and 4 women; all were Caucasian. The mean age of the control group was 60 ± 4 years (range 41–91 years), with 17 men and 13 women; 19 were Caucasian, 4 Hispanic, 3 Black, and 4 of mixed ethnic background. Mean post-mortem intervals did not differ among the groups.
2.2. Putamen contents of catechols
Compared to controls, putamen DA was decreased by 96% and DOPAC by 97% in MSA and by 93% and 95% in PD (p < 0.0001 each; Table 1). Mean DOPAL was decreased in both MSA (by 94%) and PD (by 84%, p < 0.0001 each). DOPAL/DA ratios were increased in both MSA (2.4 times control, p = 0.02) and PD (3.5 times control, p = 0.009). In both the MSA and PD groups, the mean putamen DOPAC/DOPAL ratio was decreased from that in the control group (by 61% and 74%; p = 0.03, p = 0.0007). Since the MSA and PD groups did not differ in mean values for any of the measured catechols or in catechol concentration ratios, the data in the 2 groups were lumped for comparisons of the patient group with the control group.
Table 1. Putamen catechols and catechol ratios in patients with Parkinson’s disease or multiple system atrophy and in control subjects.
Mean values expressed ±SEM. P values are for independent means t−tests.
| Parameter | Patients | Controls | % of Control | p |
|---|---|---|---|---|
| DA | 2.17 ± 0.73 | 18.43 ± 2.07 | 11.8% | 0.0001 |
| DOPAC | 0.42 ± 0.16 | 2.47 ± 0.42 | 16.8% | <0.0001 |
| DOPAL | 0.07 ± 0.04 | 0.48 ± 0.11 | 14.0% | <0.0001 |
| DOPET | 0.006 ± 0.003 | 0.028 ± 0.007 | 20.3% | <0.0001 |
| Cys-DA | 0.031 ± 0.008 | 0.326 ± 0.041 | 9.6% | <0.0001 |
| Vesicular Storage | ||||
| DA/(DOPAC + Cys-DA + DOPAL + DOPET) | 4.41 ± 0.55 | 8.15 ± 1.29 | 54.2% | 0.001 |
| DA/Cys-DA | 47.1 ± 6.1 | 63.3 ± 5.5 | 74.4% | 0.005 |
| DA/DOPAL | 39.1 ± 9.7a | 74.8 ± 15.4 | 52.3% | 0.002 |
| DA/DOPAC | 6.2 ± 0.8 | 19.7 ± 8.3 | 31.2% | 0.001 |
| LAAAD Activity | ||||
| (DA + DOPAC + Cys-DA + DOPAC + DOPAL + DOPET)/(DOPA + Cys-DOPA) | 4.17 ± 1.55 | 20.06 ± 3.17 | 20.8% | <0.0001 |
| Cys-DA/Cys-DOPA | 0.92 ± 0.28 | 6.40 ± 1.06 | 14.4% | <0.0001 |
| Cys-DA/DOPA | 0.05 ± 0.01 | 0.32 ± 0.05 | 14.7% | <0.0001 |
| DOPAC/DOPA | 0.60 ± 0.23 | 2.11 ± 0.35 | 28.4% | <0.0001 |
| TH Activity | ||||
| DOPA + Cys-DOPA | 1.18 ± 0.31 | 1.87 ± 0.37 | 63.1% | 0.01 |
| DOPA | 1.13 ± 0.31 | 1.80 ± 0.36 | 63.1% | 0.01 |
| Cys-DOPA | 0.05 ± 0.01 | 0.07 ± 0.01 | 70.9% | 0.08 |
| LAAAD and Vesicular Storage | ||||
| DA/Cys-DOPA | 77.5 ± 29.4 | 350.2 ± 47.9 | 22.1% | p< 0.0001 |
| DA/DOPA | 3.6 ± 1.4 | 18.5 ± 3.2 | 19.6% | p< 0.0001 |
Data for 2 outliers excluded.
2.3. Indices of vesicular storage
Based on inspection of Fig. 1, several indices would be expected to be sensitive to alterations in vesicular storage. The main chosen index, corresponding to the ratio of DA to the sum of its cytoplasmic metabolites, averaged 54% of control (p = 0.0015). Other indices, such as DA/Cys-DA, DA/DOPAL, and DA/DOPAC, were also decreased, to about the same extent (Table 1).
2.4. Indices of LAAAD activity
The main chosen index of LAAAD activity corresponded to the ratio of the sum of DA and its cytoplasmic metabolites, divided by the sum of compounds proximal to the LAAAD step (DOPA + Cys-DOPA). The mean value for this index in the patient group was markedly decreased to 21% of the value in the control group (p < 0.0001). Other indices, such as the Cys-DA/Cys-DOPA, Cys-DA/DOPA, and DOPAC/DOPA ratios were also decreased to about the same extent (Table 1).
2.5. Indices of innervation (TH activity)
The main chosen index of TH activity was the sum of DOPA + Cys-DOPA. The mean value for this index in the patient group was 63% of that in the control group (p = 0.0097). The mean values for DOPA and Cys-DOPA considered individually were also decreased in the patients, to about the same extent as the sum of DOPA + Cys-DOPA (Table 1).
3. Discussion
We report post-mortem neurochemical evidence for two concurrent, denervation-independent determinants of putamen DA depletion in PD and MSA—decreased vesicular sequestration of cytoplasmic DA and decreased LAAAD activity. We estimated vesicular storage to be decreased to about 50% of normal and LAAAD activity to be decreased to about 20% of normal.
Neither decreased vesicular uptake nor decreased LAAAD activity in PD is a novel finding [4], [6]; however, previous studies did not examine the relative contributions of denervation, decreased vesicular storage, and decreased LAAAD activity to putamen dopamine depletion. The present findings point to remarkably severely decreased putamen LAAAD activity in PD and MSA.
Decreased DOPA and Cys-DOPA contents can be explained by denervation, decreased TH activity, or loss of DOPA-ergic neurons, and the present results cannot distinguish among these possibilities. Nevertheless, the extent of decrease in the sum of DOPA and Cys-DOPA contents in the present study, to 63% of control, agrees reasonably well with reports based on striatal immunoreactive TH [2] and counts of nigral dopaminergic neurons [3] in PD.
Since essentially all of putamen tissue DA is in the vesicles, and DOPA is the immediate product of the rate-limiting enzyme in cytoplasmic DA synthesis (tyrosine hydroxylase), decreased putamen DA/DOPA ratios could indicate decreased DA storage for a given amount of synthesis [8]. This application, however, does not take LAAAD activity into account, and the present data indicate that putamen LAAAD activity is decreased in PD and MSA. In the setting of low LAAAD activity, DA/DOPA ratios overestimate the extent of decrease in vesicular sequestration. The more specific index of vesicular sequestration used in the present study—the DA content divided by the sum of its cytoplasmic neuronal metabolites—led to an estimated modest decrease in vesicular sequestration, to 54% of control.
Information was not available about the timing of the last dose of medications prior to death, and possible influences of medications administration must be considered. If the patients were on levodopa/carbidopa, this would have been expected to increase the putamen tissue DOPA and Cys-DOPA concentrations, in which case the extent of denervation would be underestimated. Values for the ratio of DA to the sum of its metabolites, the chosen index for vesicular sequestration, would be unaffected. Carbidopa inhibits LAAAD but does not penetrate the blood-brain barrier, and animal experiments indicate that at clinically used doses carbidopa does not inhibit LAAAD in striatal tissue [9]. Therefore, levodopa/carbidopa treatment would not be expected to affect the values for the chosen index of putamen LAAAD activity, the ratio of DA plus the sum of its cytoplasmic metabolites to the sum of DOPA + Cys-DOPA. Monoamine oxidase inhibitors would be expected to shift the fate of cytoplasmic DA toward vesicular uptake and spontaneous oxidation [10] but would not be expected to alter values for the sum of DA and its cytoplasmic metabolites.
We identified 2 MSA patients with cerebellar ataxia and no parkinsonism (i.e., no bradykinesia, rigidity, or resting tremor) during life, classified as MSA-C. Because of the low numbers of patients, we did not compare MSA-C vs. MSA-P statistically; however, it was of interest that the MSA-C patients had 5 times higher putamen dopamine content and 4 times higher DOPAC and DOPAL contents than did the MSA-P patients, findings that would fit with a more severe nigrostriatal lesion in MSA-P.
In conclusion, in addition to a loss of nigrostriatal dopaminergic innervations, decreased vesicular storage and decreased LAAAD activity in the residual terminals seem to contribute to the profound putamen DA depletion found in PD and MSA. The results of this study add to evidence that functional abnormalities in extant nigrostriatal terminals play a role in putamen DA deficiency in these diseases.
Highlights.
Putamen dopamine depletion typifies Parkinson’s disease and multiple system atrophy.
The depletion is greater than explained by nigrostriatal denervation alone.
We measured putamen cysteinyl and parent catechols in both diseases.
Evidence was obtained for decreased dopamine synthesis and vesicular uptake.
The results indicate denervation-independent abnormalities of dopamine metabolism.
Acknowledgements
The research reported here was supported by the intramural research program of the National Institute of Neurological Disorders and Stroke, project No. ZIA NS003033.
Abbreviations
- ALDH
aldehyde dehydrogenase
- DA
dopamine
- DHPG
3,4-dihydroxyphenylglycol
- DOPAC
3,4-dihydroxyphenylacetic acid
- DOPAL
3,4-dihydroxyphenylacetaldehyde
- DOPET
3,4-dihydroxyphenylethanol
- MSA
Multiple system atrophy
- NE
norepinephrine
- PD
Parkinson’s disease
- VMAT
vesicular monoamine transporter
Footnotes
Conflicts of interest
The Authors have no conflicts of interest to disclose.
References
- [1].Kish SJ, Shannak K, Hornykiewicz O Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications, N. Engl. J. Med, 318 (1988), pp. 876–880 [DOI] [PubMed] [Google Scholar]
- [2].DelleDonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, Frigerio R, Burnett M, Wszolek ZK, Uitti RJ, Ahlskog JE, Dickson DW Incidental Lewy body disease and preclinical Parkinson disease, Arch. Neurol, 65 (2008), pp. 1074–1080 [DOI] [PubMed] [Google Scholar]
- [3].Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PR, Blessing WW, Geffen LB Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease Ann. Neurol, 27 (1990), pp. 373–385 [DOI] [PubMed] [Google Scholar]
- [4].Pifl C, Rajput A, Reither H, Blesa J, Cavada C, Obeso JA, Rajput AH, Hornykiewicz O Is Parkinson’s disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human and nonhuman primate striatum, J. Neurosci, 34 (2014), pp. 8210–8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Sharabi Y, Mash DC Decreased vesicular storage and aldehyde dehydrogenase activity in multiple system atrophy, Park. Relat. Disord, 21 (2015), pp. 567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nagatsu T, Sawada M Biochemistry of postmortem brains in Parkinson’s disease: historical overview and future prospects, J. Neural Transm. Suppl (2007), pp. 113–120 [DOI] [PubMed] [Google Scholar]
- [7].Goldstein DS, Holmes C, Sullivan P, Jinsmaa Y, Kopin IJ, Sharabi Y Elevated cerebrospinal fluid ratios of cysteinyl-dopamine/3,4-dihydroxyphenylacetic acid in parkinsonian synucleinopathies, Park. Relat. Disord, 31 (2016), pp. 79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ, Sharabi Y Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease, J. Neurochem, 126 (2013), pp. 591–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jonkers N, Sarre S, Ebinger G, Michotte Y Benserazide decreases central AADC activity, extracellular dopamine levels and levodopa decarboxylation in striatum of the rat, J. Neural Transm, 108 (2001), pp. 559–570 [DOI] [PubMed] [Google Scholar]
- [10].Goldstein DS, Jinsmaa Y, Sullivan P, Holmes C, Kopin IJ, Sharabi Y Comparison of monoamine oxidase inhibitors in decreasing production of the autotoxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde in PC12 cells, J. Pharmacol. Exp. Ther, 356 (2016), pp. 484–493 [DOI] [PMC free article] [PubMed] [Google Scholar]