Abstract
Background/Objectives:
Despite the effectiveness of bariatric surgery, there is still substantial variability in long-term weight outcomes and few factors with predictive power to explain this variability. Neuroimaging may provide a novel biomarker with utility beyond other commonly used variables in bariatric surgery trials to improve prediction of long-term weight loss outcomes. The purpose of this study was to evaluate the effects of sleeve gastrectomy (SG) on reward and cognitive control circuitry post-surgery and determine the extent to which baseline brain activity predicts weight loss at 12-months post-surgery.
Subjects/Methods:
Using a longitudinal design, behavioral, hormone, and neuroimaging data (during a desire for palatable food regulation paradigm) were collected from 18 patients undergoing SG at baseline (<1 month prior) and 12-months post-SG.
Results:
SG patients lost an average of 29.0% of their weight (% total weight loss, %TWL) at 12-months post-SG, with significant variability (range: 16.0–43.5%). Maladaptive eating behaviors (uncontrolled, emotional, and externally-cued eating) improved (p<0.01), in parallel with reductions in fasting hormones (acyl ghrelin, leptin, glucose, insulin; p<0.05). Brain activity in the nucleus accumbens (NAcc), caudate, pallidum, and amygdala during desire for palatable food enhancement vs. regulation decreased from baseline to 12-months [p(FWE)<0.05]. Dorsolateral and dorsomedial prefrontal cortex activity during desire for palatable food regulation (vs. enhancement) increased from baseline to 12-months [p(FWE)<0.05]. Baseline activity in the NAcc and hypothalamus during desire for palatable food enhancement was significantly predictive of %TWL at 12-months [p(FWE)<0.05], superior to behavioral and hormone predictors, which did not significantly predict %TWL (p>0.10). Using stepwise linear regression, left NAcc activity accounted for 54% of the explained variance in %TWL at 12-months.
Conclusions:
Consistent with previous obesity studies, reward-related neural circuit activity may serve as an objective, relatively robust predictor of post-surgery weight loss. Replication in larger studies is necessary to determine true effect sizes for outcome prediction.
Introduction
Bariatric surgery results in significant excess weight loss and health benefits, solidifying this procedure as the most effective current treatment for morbid obesity1–6. In parallel, advances in laparoscopic techniques and increased utilization of procedures such as sleeve gastrectomy (SG) have decreased complication and mortality rates7. However, more detailed analysis reveals substantial variability in weight loss and long-term outcomes, with % total weight loss (%TWL) ranging from 5–55% at 3 years post-surgery3. Investigators in the largest multisite study on bariatric surgery (Longitudinal Assessment of Bariatric Surgery, LABS) recently examined the association between over 100 baseline variables and weight loss at 3 years post-surgery, but reported that few factors emerged as conferring predictive value. For those that did meet significance, effects were small, explaining only 14% of the variance in %TWL8. A recent National Institutes of Health (NIH)-sponsored symposium focused on long-term outcomes of bariatric surgery highlighted identification of predictors as one of the top priorities in this field9. Given the serious nature of lifestyle changes required to adjust to the effects of bariatric surgery, a more precise understanding of the mechanisms behind variability in outcomes, and identification of specific baseline biomarkers of weight loss, would allow for improved long-term outcome prediction.
The recent emergence of a new subfield, prevention neuroscience, dovetails with the ongoing search for unbiased, modifiable baseline biomarkers of bariatric surgery outcomes. Supported by findings indicating that neuroimaging variables are significantly associated with health outcomes, the prevention neuroscience approach holds promise in translating neuroimaging findings into prognostic indicators and treatment options10–12. A growing number of studies have demonstrated the predictive capacity of brain activity, most commonly assessed with blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI), in informing treatment outcomes for major depressive disorder13, social anxiety14, epilepsy15, drug addiction16, and behavioral lifestyle weight-loss programs in obesity17. These trends extend previous reports documenting treatment-induced brain plasticity in psychiatric disorders18, diabetes19, and obesity20, including brain changes following weight loss surgery21–26.
Indeed, profound short-term effects (<12 months) of bariatric surgery on the brain have been noted in recent years. Among longitudinal studies, the most consistent findings suggest significant reductions from baseline to 1- to 6-months post-surgery in Roux-en-Y gastric bypass (RYGB)23–25 and laparoscopic adjustable gastric banding (LAGB)21 patients in response to high-calorie food images in the insula21, 24, medial frontal gyrus21, 24, and mesolimbic regions [putamen23, 25, ventral tegmental area (VTA)22, 23], the latter of which was more prominent in RYGB than SG patients at 6-months post-surgery22. Attenuated activity in food reward regions following bariatric surgery (vs. untreated groups or those treated non-surgically) has been documented in studies measuring brain activity at 9- to 36-months post-surgery26–28. Post-surgical changes in cortical regions associated with inhibition and cognitive control [inferior frontal gyrus (IFG), dorsolateral prefrontal cortex (DLPFC)] appear less consistent, as some studies have noted increased activity21, 28, and others have reported decreases23–25, 27. Additional reports provide evidence that some brain-related changes following treatment may be unique to the bariatric surgery (vs. behavioral intervention)20. Finally, a single report indicated that fMRI response to food cues in frontal regions at baseline was predictive of % change in body mass index (BMI) at 3- and 6-months post-LAGB, although other studies report no associations21. The majority of studies highlighted above examined short-term outcomes (1- to 6-months post-surgery) during which weight loss trajectories tend to be uniform3 and thus examining variation in outcomes may be less informative of long-term trajectories, and many focused on procedures which are now declining in use (LAGB, RYGB)20, 21, 29, 30.
Regulating responses to palatable foods is often used as part of behavioral weight loss interventions. Stemming from a rich literature on neural circuits responsible for emotion regulation, reports on regulation of neural responses to palatable food have emerged recently. In these paradigms, participants are instructed to alternately increase their desire for palatable foods or down-regulate their desire using a variety of strategies31–34. This protocol offers the advantage of simultaneously recruiting, within a single paradigm, key neural systems involved in desire for palatable food and in the regulation of this desire, which are important determinants of food choice35. Previous studies demonstrate that healthy-weight individuals exhibit differential brain activation during upregulation, cognitive reappraisal, and suppression of food desire in mesocorticolimbic circuitry (greater during upregulation) and cognitive control circuitry (greater during suppression and cognitive reappraisal)31–34. Although bariatric surgery candidates exhibit deficits in emotion regulation36, the neural mechanisms underlying these deficits are unknown. It is also unknown whether cognitive control circuitry improves post-surgery, as is observed in other cognitive domains37 and whether baseline neural activity improves prediction of long-term weight outcomes.
The current study examined the neural substrates of the desire for palatable food and the regulation of this desire in vertical sleeve gastrectomy patients at baseline and 12-months post-SG, with weight outcomes at 12-months post-SG. We sought to examine three hypotheses: 1) At 12-months post-surgery, compared to baseline, activity in mesolimbic reward circuitry in response to desire for palatable food enhancement will be decreased, and activity in cognitive control circuitry during regulation of the desire for palatable food will be increased; 2) Baseline brain activity in these systems will predict weight loss outcomes at 12-months post-surgery; 3) Brain activity will serve as a more robust predictor than other baseline (behavioral and hormonal) variables.
Subjects and Methods
Subjects
Sleeve gastrectomy candidates were recruited from the Brigham and Women’s Hospital (BWH) Center for Metabolic and Bariatric Surgery and Massachusetts General Hospital Weight Loss Center. Eligibility criteria included 21–55 years of age, BMI 35–60, and ability communicate in English. Exclusion criteria included type 2 diabetes, neurological disease, major psychiatric illness, current illicit drug use, previous bariatric surgery, treatment with investigational medications/devices, females currently pregnant/nursing, claustrophobia, weight of >550 lbs. or >75 in. body circumference, or MRI scanning contraindications. A total of 20 candidates were recruited, completed baseline visit procedures, and underwent SG procedures between October 2013 and June 2015. Of these, 18 participants also completed 12-month visit procedures; fMRI data from 2 participants at 12-months were excluded due to excessive motion. This resulted in 18 participants with fMRI data at baseline and 16 participants with fMRI data at baseline and 12-months. All procedures were approved by the Partners Healthcare Human Research Committee.
Design and Procedure
Following phone screening to assess eligibility criteria, participants completed assessments within one month prior to bariatric surgery (baseline visit) and 12 months following bariatric surgery (12-month visit). Prior to each visit, participants were instructed to fast overnight (12 hours). After giving informed consent, participants completed a fasting blood draw and pre-scan appetite ratings, and an introduction to the fMRI task. They were then escorted to the MRI suite for the fMRI session. Participants were provided a non-standardized lunch (either a protein shake or a mixed meal self-selected from the patient cafeteria), and completed appetite ratings (pre- and post-meal), mood/behavior questionnaires, and anthropometric measurements.
Appetite Ratings and Clinical/Behavioral Questionnaires
Appetite ratings were collected using visual analogue scales (VAS). Mood/behavior questionnaires were also administered, including: Emotion Regulation Questionnaire (ERQ38),Dutch Eating Behavior Scale (DEBQ39), Three-Factor Eating Questionnaire (TFEQ40), Power of Food Scale (PFS41) Beck Depression Inventory (BDI-II42), Spielberger State-Trait Anxiety Inventory (STAI43).
Hormone Analysis
Fasting blood samples were drawn at 8:00 am, immediately spun, and stored at −80° C. Leptin was measured using a radioimmunoassay [Millipore, Billerica, MA; intra-assay coefficient of variation (CV): 3.4–8.3%; inter-assay CV: 3.6–6.2%] at the BWH Research Assay Core (BRAC). Ghrelin samples were drawn on ice and stored at −80° C in plastic tubes containing Pefabloc. Acylated ghrelin levels were measured in duplicate using an enzyme-linked immunosorbent assay (Millipore; intra-assay CV: 1.6–3.6%; inter-assay CV: 3.6–6.6%) at BRAC. Glucose and insulin were measured in duplicate via commercial assay kits at LabCorp (New Raritan, NJ).
fMRI Paradigm
In preparation for the fMRI paradigm, participants underwent a short (task-related) interview and were introduced to the task. The desire for palatable food regulation paradigm was adapted from previous publications33, 34 and consisted of 2 conditions (Enhance, Regulate). Each trial began with either an Enhance or Regulate visual cue. Next, an image of a sweet (e.g., ice cream) or savory (e.g., hamburger) highly palatable food was displayed and participants were instructed to utilize the Enhance or Regulate strategy. At the end of each trial, participants rated their desire for the food (“How much do you want this?”) on a 4-point Likert-type scale. At the end of each run, participants rated their ability to utilize the strategies using a 4-point Likert-type scale. Participants completed 5 runs consisting of 20 trials each, for 100 total trials (see Supplementary Methods and Supplementary Figure 1 for further details).
Image Acquisition
Whole-brain functional imaging was performed at the BWH MRI Research Center on a Siemens 3T Skyra using a 20-channel head coil. A T1-weighted 3D MPRAGE was acquired (176 sagittal 1.0mm slices, TR/TE=1800/2.19ms, flip angle=7°, FOV=256×256mm, voxel size =1.0×1.0×1.0mm), followed by a field map matched to the EPI sequence. For each functional run, a gradient-echo EPI pulse sequence was acquired (39 oblique-axial 3.1mm slices, TR/TE=2000/27ms, flip angle=90°, FOV=200×200mm, voxel size=3.1×3.1×3.1mm, 212 volumes/run).
fMRI Data Analysis
fMRI data were analyzed using Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging). Volumes were realigned and unwarped with phase correction provided from the fieldmap, normalized to the Montreal Neurological Institute MNI152 brain template, and smoothed with a 6 mm Gaussian kernel, then re-sampled to 3 mm isotropic. Following initial preprocessing, outliers in global mean image time series (threshold: 3.5 SD) and movement (threshold: 0.8 mm, measured as scan-to-scan movement) were detected using an artifact detection toolbox (ART; http://www.nitrc.org/projects/artifact_detect/) and entered as nuisance regressors in the single-subject level GLM. Masks excluding voxels outside the brain were applied to ensure that voxels in regions with signal dropout were not arbitrarily excluded. For the event-related design, each trial was modeled using a boxcar function convolved with a canonical hemodynamic response function. Contrasts of interest [Enhance > Regulate; Regulate > Enhance] from the single-subject analysis were tested using linear contrasts and SPM t-maps, then submitted to second level random effect group analysis.
Two sets of group-level analyses on fMRI data were completed. First, fMRI changes from baseline to 12-month visits for the Enhance > Regulate and Regulate > Enhance contrasts were assessed using separate paired t-tests, controlling for the average absolute difference between Enhance and Regulate palatable food desire ratings at each timepoint (entered as a covariate of no interest). This covariate approach was used to avoid confounding the brain-related activity results with differential differences (between timepoints) on subjective ratings of desire for palatable foods under each condition, which allows for identification of (more objective) brain activity related to each condition exclusive of behavioral ratings, which are subjective responses. Secondly, to test the hypothesis regarding whether fMRI-related activity at baseline predicted weight loss at 12 months, multiple regression models (separately for Enhance > Regulate and Regulate > Enhance contrasts) were used, with %TWL as the outcome variable; each model controlled for the average absolute difference between Enhance and Regulate palatable food desire ratings.
For each set of analyses, region of interest (ROI) analyses were performed using small volume correction, implemented through the WFU PickAtlas SPM toolbox44. Multiple comparisons were controlled using a combination of cluster extent (k>6) and p<0.05 FWE-corrected threshold. ROI masks for the Enhance > Regulate contrast were defined anatomically (based on a manually segmented MNI-152 brain template): VTA, hypothalamus (Hypo), nucleus accumbens (NAcc), caudate, putamen, globus pallidus, amygdala, anterior insula. ROI masks for the Regulate > Enhance contrast were defined functionally, based on results for the Regulate > Enhance contrast reported in previous studies31, 34. Spheres (12 mm diameter) were drawn around the maximum voxel of activation reported in these studies in the DLPFC and DMPFC. Average parameter estimates [percent signal change (psc)] within each ROI for each participant were extracted using the Region of Interest Extraction Toolbox (REX45) and exported to SPSS (v19, Chicago, IL) for post-hoc analyses. Retrospective power analyses based on these data were computed using G*Power version 3.1.9.246 (two-tailed, α error probability=0.05).
Behavioral Data Analysis
Behavioral data were analyzed using SPSS v19. Demographic, clinical, and behavioral changes from baseline to 12 months were analyzed using paired t-tests. Appetite ratings and self-assessment ratings during the fMRI desire for palatable food regulation task were analyzed using repeated measures analysis of variance (ANOVA). For all statistical tests, a p<0.05 (2-tailed) was used; variance was similar between baseline and 12-month follow-up; thus, reported statistics reflect test values under the assumption of equal variance. Weight change from baseline to 12 months was calculated as %TWL.
Results
Demographic, Clinical, and Behavioral Data
Participants were primarily non-Hispanic, Caucasian females; over half of the participants (66.7%) had a college education. Demographic variables at baseline are presented in Table 1.
Table 1.
Baseline Demographic Characteristics
| n | 18 |
|---|---|
| Age (M±SD) | 38.4 ± 10.1 |
| Gender (F/M) | 16/2 |
| Race (%) | |
| Caucasian | 83.3 |
| African American | 5.6 |
| Other | 11.1 |
| Ethnicity (%) | |
| Hispanic | 16.7 |
| Non-Hispanic | 83.3 |
| Education (%) | |
| High school/GED | 16.7 |
| Some college | 16.7 |
| Bachelor’s degree | 38.9 |
| Master’s degree | 22.2 |
| Doctoral degree | 5.6 |
As expected, weight and BMI decreased significantly post-surgery (p<0.01), with an average %TWL of 29.0% (range: 16.0 to 43.5%; see Table 2). Maladaptive eating behaviors (TFEQ, DEBQ, PFS) also decreased significantly post-surgery (see Table 2). Depressive symptoms (BDI) were lower at 12 months than baseline, while emotion regulation scores (ERQ) and state anxiety (STAI) showed no changes over time (see Table 2).
Table 2.
Clinical, Behavioral, and Hormonal Characteristics at Baseline and 12-months
| Baseline (M±SD) | 12-months (M±SD) | t | p | |
|---|---|---|---|---|
| Weight (lbs.) | 256.6 ± 36.2 | 181.0 ±34.2 | 14.7 | <0.001 |
| BMI | 41.8 ± 4.5 | 29.6 ± 4.0 | 14.5 | <0.001 |
| % Total Weight Loss | 29.0 ± 7.7 | |||
| TFEQ | ||||
| Cognitive Restraint of Eating | 48.8 ± 24.4 | 65.4 ± 26.1 | −2.2 | 0.041 |
| Uncontrolled Eating | 59.7 ± 19.6 | 14.2 ± 4.9 | 11.8 | <0.001 |
| Emotional Eating | 60.2 ± 24.8 | 27.5 ± 22.4 | 6.0 | <0.001 |
| DEBQ | ||||
| Emotional Eating | 3.2 ± 0.7 | 1.9 ± 0.9 | 6.7 | <0.001 |
| Restrained Eating | 3.1 ± 0.7 | 2.8 ± 1.0 | 1.5 | 0.162 |
| External Eating | 3.4 ± 0.5 | 2.3 ± 0.8 | 5.3 | <0.001 |
| PFS | ||||
| Food available | 3.0 ± 0.9 | 1.5 ± 0.7 | 10.3 | <0.001 |
| Food present | 3.7 ± 0.9 | 2.3 ± 1.2 | 6.3 | <0.001 |
| Food tasted | 3.3 ± 0.9 | 2.4 ± 0.9 | 5.2 | <0.001 |
| ERQ* | ||||
| Cognitive Reappraisal | 18.8 ± 9.3 | 16.2 ± 7.7 | 1.1 | 0.284 |
| Expressive Suppression | 17.7 ± 5.8 | 20.4 ± 5.8 | −1.9 | 0.074 |
| BDI | 10.4 ± 6.7 | 2.8 ± 3.1 | 4.6 | <0.001 |
| STAI Trait Anxiety | 40.8 ± 15.1 | 42.0 ± 10.9 | −0.28 | 0.781 |
| Palatable Food Desire Rating during Enhance* | 3.2 ± 0.4 | 2.6 ± 0.7 | 4.5 | <0.001 |
| Palatable Food Desire Rating during Regulate* | 1.7 ± 0.4 | 1.7 ± 0.4 | 0.2 | 0.806 |
| Acyl ghrelin (pg/mL)** | 254.9 ± 126.5 | 79.7 ± 87.9 | 4.76 | <0.001 |
| Leptin (ng/mL)** | 29.4 ± 18.7 | 19.3 ± 10.6 | 2.08 | 0.050 |
| Glucose (mg/dL) | 96.9 ± 18.8 | 80.1 ± 5.5 | 3.76 | 0.002 |
| Insulin (uIU/mL)** | 18.2 ± 10.8 | 6.1 ± 3.2 | 5.30 | <0.001 |
Data missing for 1 subject at 12-months
Data missing for 1 subject at baseline and 2 subjects at 12-months
For self-reported hunger, there was a main effect of time [F(2, 17)=100.23, p<0.01], with slight increases from pre-scan to pre-meal, and decreases post-meal, but no effect of visit [F(1,17)=2.88, p>0.10] (see Supplementary Figure 2). For fullness, there was a main effect of time [F(2,17)=142.17, p˂0.01], with increased fullness pre-meal to post-meal, a main effect of visit [F(1,17)=4.87, p<0.05], driven by increased fullness at 12 months, and an interaction effect [F(1,17)=6.69, p<0.01]. Fullness increased more from pre-meal to post-meal at the 12 month visit, compared to baseline (see Supplementary Figure 2).
On the desire for palatable food ratings during the task, there was a main effect of visit [F(1,15)=47.11, p<0.001], a main effect of condition [F(1,15)=23.91, p<0.001], and a visit x condition interaction [F(1,15)=9.26, p<0.01]. Post-hoc comparisons showed no changes from baseline to 12 months post-surgery in desire for palatable foods during the Regulate condition (see Table 2). Desire for palatable food during the Enhance condition decreased at 12 months. Ratings on the self-assessment of use of emotion regulation strategies during the food desire regulation task are reported in Supplementary Figure 3.
Hormone Data
Appetite-regulatory hormone levels changed markedly post-surgery. Fasting levels of acylated ghrelin (p<0.001, Cohen’s d=1.31), leptin (p=0.05, d=0.58), glucose (p<0.05, d=1.18), and insulin (p<0.001, d=1.93) decreased from baseline to 12 months (see Table 2).
fMRI Data
Change in Brain Activity from Baseline to 12 months
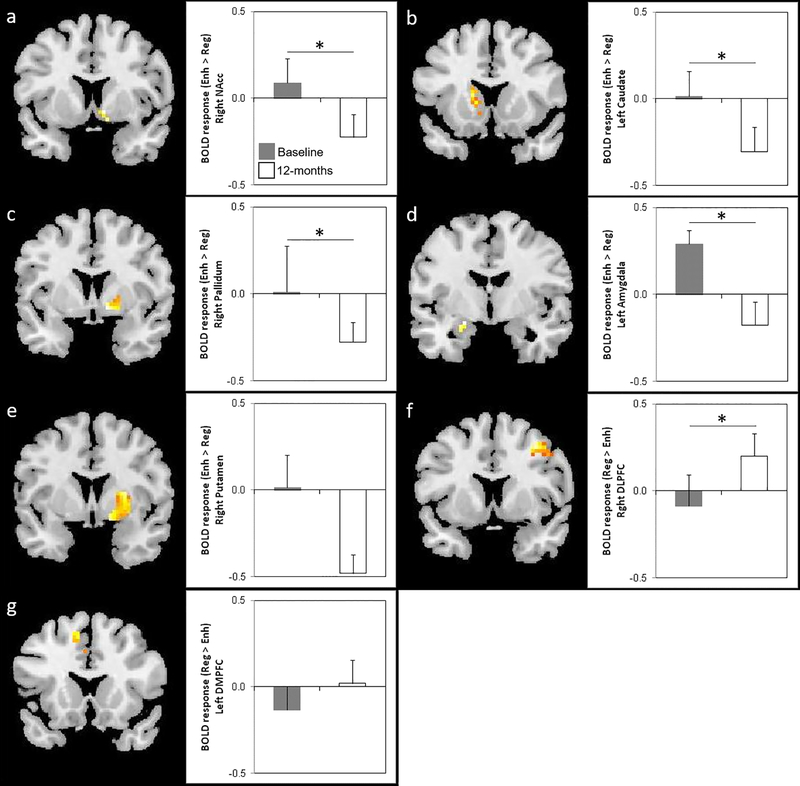
For Hypothesis 1, analyses demonstrate that from baseline to 12 months post-surgery, fMRI activity in response to palatable food stimuli under the Enhance > Regulate contrast decreased in the right NAcc, left caudate, right pallidum, and left amygdala at the pFWE<0.05 level (see Table 3 and Figure 1). Under the Regulate > Enhance contrast, fMRI activity increased in the right DLPFC at the pFWE<0.05 level (see Table 3 and Figure 1).
Table 3.
Change in BOLD Response to Palatable Food for Enhance vs. Regulate Conditions at Baseline and 12-months Post-Surgery
| Condition | ROI | Hemisphere | k(E) | x | y | z1 | Z-score | Uncorrected p-value2 |
Voxel-level PFWE-corr3 |
|---|---|---|---|---|---|---|---|---|---|
| Enhance > Regulate: Baseline > 12-months | |||||||||
| NAcc | R | 14 | 12 | 5 | −11 | 2.73 | 0.003 | 0.05 | |
| Caudate | L | 110 | −15 | 2 | 22 | 3.41 | <0.001 | 0.04 | |
| Pallidum | R | 34 | 12 | 5 | −5 | 3.09 | 0.001 | 0.04 | |
| Putamen | R | 176 | 15 | 11 | −8 | 3.22 | 0.001 | 0.09 | |
| Amygdala | L | 14 | −21 | −4 | −14 | 3.07 | 0.001 | 0.05 | |
| Regulate > Enhance: 12-months > Baseline | |||||||||
| DLPFC | R | 73 | 36 | 11 | 49 | 3.56 | <0.001 | 0.04 | |
| DMPFC | L | 27 | −12 | 20 | 49 | 3.29 | <0.001 | 0.08 | |
Coordinates are presented in MNI space
Voxel-wise Z-score significance level p<0.05 uncorrected for multiple comparisons within a hypothesized ROI; ROIs listed represent regions of significantly activated clusters within the a priori hypothesized ROI
FWE rate (family-wise error rate) used for SVC (small volume correction): Voxel-level significance level (FWE-corrected within the search volume of interest); p values for ROIs reaching p(FWE-corrected)<0.05 are bolded
Figure 1. Change in Neural Response to Enhance vs. Regulate Conditions before and 12-months after SG.
For each ROI, figures show SPM maps of brain activity for the Enhance > Regulate contrast at pFWE<0.05 in the (a) right NAcc, (b) left caudate, (c) right pallidum, (d) left amygdala, and at pFWE<0.10 in the (e) right putamen, and for the Regulate > Enhance contrast at pFWE<0.05 in the (f) right DLPFC and at pFWE<0.10 in the (g) left DMPFC. Bar graphs on the right of each figure visually present the mean BOLD response (±SEM) to the Enhance vs. Regulate condition within a 3mm sphere drawn around the peak voxel (see Table 3 for MNI coordinates) for the baseline (gray bar) vs. 12-month post-surgery (white bar) comparison. * = p<0.05, FWE-corrected.
Baseline Behavioral Characteristics and Biomarkers as Predictors of Weight Loss at 12 months
For Hypotheses 2 and 3, results indicate no statistically-significant associations between baseline behavioral/clinical characteristics (DEBQ, TFEQ, PFS, ERQ, BDI, STAI scores or appetite ratings) and %TWL at 12 months (mean |r|=0.18, range: 0.01–0.39; mean p=0.50, range: 0.99–0.11; see Supplementary Figure 4 for a representative scatterplots). Baseline hormone levels were unrelated to %TWL (mean |r|=0.17, range: 0.01–0.38; mean p=0.58, range: 0.96–0.13; see Supplementary Figure 4).
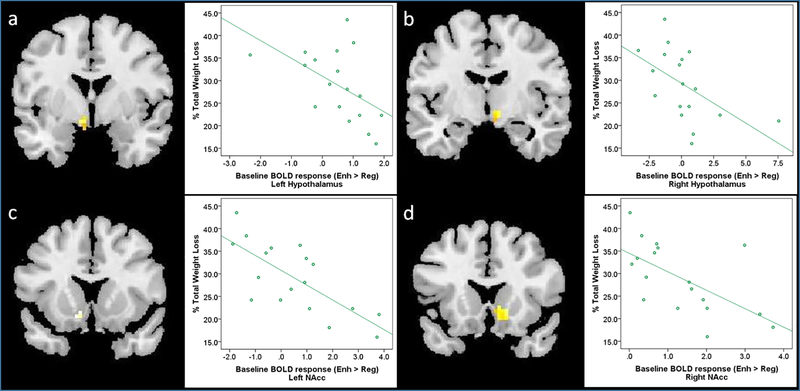
However, there were significant negative relationships between fMRI activity during the Enhance > Regulate contrast at baseline and %TWL at 12 months. fMRI activity in the bilateral hypothalamus and bilateral NAcc was negatively related to %TWL at 12-months (see Table 4 and Figure 2). This suggests that participants with elevated fMRI activity in these regions during enhanced desire (vs. regulation of desire) for palatable foods at baseline demonstrated lower weight loss 12 months post-surgery. These relationships remained significant after accounting for variables (sex, age, baseline BMI) most consistently linked to weight outcomes8. No regions showed positive relationships between baseline fMRI activity during the Enhance > Regulate contrast and %TWL. There were no regions meeting significance for the relationship between baseline fMRI activity during the Regulate > Enhance contrast and %TWL. Collectively, these findings indicate stronger relationships between baseline brain activity and %TWL, compared to baseline behavioral, clinical, or hormonal variables and %TWL.
Table 4.
Relationship between Baseline BOLD Response to Palatable Food for Enhance vs. Regulate Conditions and % Total Weight Loss at 12-months Post-Surgery
| Condition | ROI | Hemisphere | k(E) | x | y | z1 | Z-score | Uncorrected p-value2 | Voxel-level pFWE-corr3 | Pearson Partial Correlation Coefficient, r (p)4 |
|---|---|---|---|---|---|---|---|---|---|---|
| Enhance: Positive Relationship | ||||||||||
| none | ||||||||||
| Enhance: Negative Relationship | ||||||||||
| Hypothalamus | L | 19 | −6 | −10 | −5 | 2.95 | 0.002 | 0.04 | −0.52 (0.032) | |
| R | 13 | 9 | −10 | −5 | 2.85 | 0.002 | 0.05 | −0.57 (0.018) | ||
| NAcc | L | 6 | −12 | 14 | −11 | 2.41 | 0.008 | 0.09 | −0.75 (0.001) | |
| R | 20 | 15 | 20 | −8 | 2.87 | 0.002 | 0.04 | −0.63 (0.006) | ||
| Regulate: Positive Relationship | ||||||||||
| none | ||||||||||
| Regulate: Negative Relationship | ||||||||||
| none | ||||||||||
Coordinates are presented in MNI space
Voxel-wise Z-score significance level p<0.05 uncorrected for multiple comparisons within a hypothesized ROI; ROIs listed represent regions of significantly activated clusters within the a priori hypothesized ROI
FWE rate (family-wise error rate) used for SVC (small volume correction): Voxel-level significance level (FWE-corrected within the search volume of interest); p values for ROIs reaching p(FWE-corrected)<0.05 are bolded
Controlling for mean desire for palatable food rating difference at Baseline; p values for ROIs reaching p<0.05 are bolded
Figure 2. Neural Response to Enhance vs. Regulate before SG Predicts Weight Loss at 12-months after SG.
For each ROI, figures show SPM maps of significant relationships between brain activity during the Enhance > Regulate contrast at baseline and % total weight loss (%TWL) at 12-months after SG at pFWE<0.05 in the (a) left hypothalamus, (b) right hypothalamus, and (c) left NAcc, and at pFWE<0.10 in the (d) right NAcc. Scatterplots on the right of each figure visually present the relationship between %TWL and BOLD response for the Enhance > Regulate contrast at a 3mm sphere drawn around the peak voxel (see Table 4 for MNI coordinates) at baseline.
To examine the relative predictive strength of each baseline ROI which had a significant r and p value (L NAcc, R NAcc, L Hypo, R Hypo), stepwise linear regression was used. This analysis demonstrated that baseline activity in only one ROI (L NAcc) met selection criterion for inclusion in the final model [F(1,16)=18.80, p<0.002, R2=0.54]. Individual differences in BOLD activity in the L NAcc accounted for 54% of the explained variance in %TWL at 12 months. Based on a median split of fMRI activity in the L NAcc (Enhance > Regulate contrast), individuals exhibiting lower activity in the L NAcc at baseline achieved, on average, 7% greater weight loss (32.6%) than those exhibiting higher L NAcc activity (25.5%).
A retrospective power analysis based on these data revealed effect sizes (ES) of 1.17–1.59 for the change from baseline to 12 months post-SG in BOLD activity during Enhance > Regulate in the NAcc, caudate, putamen, pallidum, and amygdala, and achieved power of 99%. With ES of 0.52–0.75 for the relationship between %TWL at 12 months and baseline BOLD activity during Enhance > Regulate (n=16 patients) in the hypothalamus and NAcc, achieved power was 62–98%.
Discussion
This study tested the effects of sleeve gastrectomy on behavioral, hormonal, and neural outcomes at 12 months. The larger goal was to develop an early model for outcome prediction to be tested in larger future studies. Towards these aims, data from the current study provided three main results. First, significant decreases in weight, maladaptive eating behaviors, depressed mood, and appetite-regulatory hormones were observed at 12 months post-surgery. Secondly, desire for palatable food was reduced at 12 months, at behavioral and neural levels, with attenuation of mesolimbic reward circuitry activity during enhancement of palatable food desire in tandem with increased recruitment of frontal cognitive control regions. Finally, we identified brain activity in the NAcc, but not behavioral or hormone data, as a predictor of weight loss post-SG at 12 months. Collectively, these findings contribute to the knowledge base on 1 year outcomes following SG, provide an early proof-of-concept model for use of neuroimaging to predict outcomes in bariatric surgery, and potentially spur development of innovative techniques designed to modify brain activity prior to surgery35.
As expected, patients lost significant body weight, endorsed fewer problematic eating behaviors, and exhibited lower levels of appetite-regulatory hormones following surgery. The average of 29% TWL at the 12 month follow-up was similar to that reported in larger studies47, and suggests that the current sample is generally representative in terms of the primary surgical outcome. Further, self-report of emotional eating and external eating declined at 12 months. Along with improvement in mood and modest changes in restraint-related eating, these effects mirror those found in other SG populations48.
Although the desire for palatable food is an almost universal human experience, intense and frequent desire for specific palatable foods occurs at a higher rate in individuals with obesity49 and has been linked prognostically to poor dieting success50, 51. Reduced desire for palatable food has been reported in SG patients following surgery48, and current data replicate this finding, implying a robust effect of SG on desire for palatable food. This is supported by our neuroimaging results, which demonstrate decreases from pre- to post-SG in several mesolimbic regions (NAcc, pallidum, caudate, amygdala) during food desire enhancement, similar to previous studies noting reward circuitry activity in response to passive viewing of palatable foods declines post-surgery22, 23, 25, 26. Collectively, these data imply a profound impact of bariatric surgery on regions linking food reward and anticipation of intake of palatable food. The mechanism for this effect is unclear, but may involve gut-brain pathways such as the vagus nerve, changes in the gut microbiome, or the influence of hormones (ghrelin, PYY, leptin, GLP-1) on hypothalamic neuron activity, with downstream effects on mesoaccumbal circuitry, given normalization of these hormones after SG52 and evidence of effects of these hormones on VTA53 dopamine neurons.
Though less widespread than the effect on mesolimbic regions, current analyses suggest heightened activity from pre- to post-SG in cognitive inhibitory regions (DLPFC, DMPFC) during reappraisal-induced regulation of food desire. Dorsolateral and dorsomedial prefrontal cortex have been implicated in voluntary control of responses to negative and positive valence stimuli54, and recruitment of these regions during cognitive reappraisal of rewarding images has been interpreted as key to the integration between working memory and motor responses in the context of motivated, self-relevant behavior55. In relation to this study, this might involve inhibiting movement toward a rewarding stimulus (palatable food) in order to achieve the desired outcome (not eating the palatable food), as would be expected for a patient who is attempting to regulate food desire in order to achieve maximal benefits of bariatric surgery. Enhanced DLPFC activity has been reported in RYGB patients who were defined as “more successful” at weight loss, when assessed one-year post-surgery using a similar paradigm28. Together, these findings suggest that although DLPFC/DMPFC activity may be attenuated post-surgery during passive viewing of palatable foods, volitional engagement under the command to inhibit food desire is associated with increased activity in the DLPFC/DMPFC post-surgery.
Despite these post-operative changes, closer attention to the data reveals substantial variation in responses, as not all patients achieved maximal weight loss. The prevalence of suboptimal outcomes, a pattern recently highlighted in mainstream media56, along with recommendations from the NIH-sponsored workshop on bariatric surgery9, underscores the need to improve identification of baseline predictors of weight loss outcomes. Towards this goal, our analyses revealed NAcc/hypothalamus activity during enhancement of food palatable desire predicted %TWL at 12 months post-surgery. This suggests that individuals with elevated activity in these regions at baseline are at an increased risk of poor weight loss outcomes. Moreover, brain activity appeared to be a better predictor than subjective (self-report of eating behaviors) and other objective (appetite-regulatory hormone) variables. As such, these data are supportive of the utility of neuroimaging for prediction of outcomes related to health, as in drug addiction and depression57 (for review, see10). For example, Marhe and colleagues used stepwise regression to examine the relative capacity of self-report of cocaine craving severity, attentional bias task behavioral data, and brain activity in predicting cocaine use following treatment, reporting that craving in the preceding week and activity in the dorsal anterior cingulate cortex explained 45% of the variance in the number of days of cocaine use in the three months following treatment, with dACC independently explaining 22%16. We found substantial variance in %TWL was accounted for by baseline brain activity in the left NAcc.
Overall, our data support two primary clinically-relevant directions. First, in keeping with previously-mentioned trends of increased DLPFC activity during the Regulate (vs. Enhance) condition, results provide evidence that cognitive therapy focused on individually-based value appraisal and behavior modification might enhance weight loss if included as an adjunctive therapy to surgery. The focus of this approach would be on an individual’s personal values and goals for weight loss, and behavior change around these values, similar to methods followed under our Regulate strategy, rather than more simple instructions to “restrain” one’s desire to consume highly palatable foods. This type of an approach resulted in better weight loss outcomes (cognitive reappraisal-focused therapy) when compared to traditional lifestyle intervention in a non-surgical context58, but we are not aware of any studies that have tested this as adjunctive therapy for weight loss in bariatric surgery. Future investigations should examine whether early post-surgery intervention with individually-focused reappraisal strategies enhances surgical weight loss long term via modification of food intake behavior.
Secondly, the baseline NAcc results under the Enhance condition introduce a new target for modification in pre-operative brain stimulation-based interventions. For example, recent findings suggest brain stimulation techniques (such as transcranial magnetic stimulation and transcranial direct current stimulation) may produce reductions in desire for palatable foods35, 59–61. We propose that similar tools may offer an avenue to explore the effects of brain activity modification in bariatric surgery patients, with the goal of normalizing mesolimbic region activity in vulnerable individuals prior to surgery to maximize weight loss outcomes.
Along with strengths of this study noted above, we acknowledge limitations which reduce the generalization and impact of our findings. First, our sample size was relatively small, and without a control group of BMI-matched individuals, attribution of effects specifically to SG is limited. Future studies would improve the ability to conclusively link SG to these outcomes through comparison to a BMI-matched group. Additionally, the lunch provided to subjects was not standardized across subjects or visits, which may have introduced variability in appetite ratings, although variability appeared to be quite low for these ratings, and statistical tests on appetite ratings did not reveal differences in variance across timepoints or visits for these ratings. Finally, our sample was primarily female and Caucasian, and while consistent with sex and race/ethnicity distributions in bariatric surgery3, it prevents examination of potential sex differences in our outcome variables and generalization to other racial/ethnic groups.
In summary, our findings provide evidence of robust reduction in brain reward activity at 12 months post-surgery in SG patients, along with normalization of eating behaviors and appetite-regulatory hormones. Further, we present novel data demonstrating prediction of 12-month weight loss by baseline activity in the NAcc, a key mesolimbic reward region. Future investigations should seek to replicate these data in larger samples, with an eye towards refining predictive algorithms and combining with tools which capitalize on the objective, modifiable nature of brain activity biomarkers to maximize weight loss outcomes for all individuals seeking bariatric surgery.
Supplementary Material
Acknowledgements
The authors are grateful to study participants for volunteering their time, Vanessa Calderon, Mark Gorman, and Noreen Harrington for coordinating subject recruitment, and Max Curran for programming the fMRI paradigm. This work was funded by the Harvard Nutrition Obesity Research Center (P30 DK040561), Global Foundation for Eating Disorders, and the Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and affiliated academic health care centers). Support for a portion of LMH’s time was provided by NIMH K01 HD019222 and BWH BRI Fund for Research Excellence.
Sources of support:
Harvard Nutrition Obesity Research Center (P30 DK040561)
Global Foundation for Eating Disorders
The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758)
NIMH K01 HD091222
Brigham and Women’s Hospital Research Institute Fund for Research Excellence
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Supplementary Information.
Supplementary information is available at International Journal of Obesity’s website.
References
- 1.Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC et al. Health benefits of gastric bypass surgery after 6 years. JAMA 2012; 308(11): 1122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlin AM, Zeni TM, English WJ, Hawasli AA, Genaw JA, Krause KR et al. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg 2013; 257(5): 791–7. [DOI] [PubMed] [Google Scholar]
- 3.Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013; 310(22): 2416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikramuddin S, Korner J, Lee WJ, Connett JE, Inabnet WB, Billington CJ et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013; 309(21): 2240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366(17): 1567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012; 307(1): 56–65. [DOI] [PubMed] [Google Scholar]
- 7.Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman W et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009; 361(5): 445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courcoulas AP, Christian NJ, O’Rourke RW, Dakin G, Patchen Dellinger E, Flum DR et al. Preoperative factors and 3-year weight change in the Longitudinal Assessment of Bariatric Surgery (LABS) consortium. Surg Obes Relat Dis 2015; 11(5): 1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courcoulas AP, Yanovski SZ, Bonds D, Eggerman TL, Horlick M, Staten MA et al. Long-term outcomes of bariatric surgery: a National Institutes of Health symposium. JAMA Surg 2014; 149(12): 1323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabrieli JD, Ghosh SS, Whitfield-Gabrieli S. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron 2015; 85(1): 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall PA. Prevention Neuroscience: A new frontier for preventive medicine. Prev Med 2016; 86: 114–6. [DOI] [PubMed] [Google Scholar]
- 12.Nichols TE, Das S, Eickhoff SB, Evans AC, Glatard T, Hanke M et al. Best practices in data analysis and sharing in neuroimaging using MRI. Nat Neurosci 2017; 20(3): 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furey ML, Drevets WC, Hoffman EM, Frankel E, Speer AM, Zarate CA Jr. Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiatry 2013; 70(3): 280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitfield-Gabrieli S, Ghosh SS, Nieto-Castanon A, Saygin Z, Doehrmann O, Chai XJ et al. Brain connectomics predict response to treatment in social anxiety disorder. Mol Psychiatry 2016; 21(5): 680–5. [DOI] [PubMed] [Google Scholar]
- 15.van Veenendaal TM, DM IJ, Aldenkamp AP, Hofman PA, Vlooswijk MC, Rouhl RP et al. Metabolic and functional MR biomarkers of antiepileptic drug effectiveness: A review. Neurosci Biobehav Rev 2015; 59: 92–9. [DOI] [PubMed] [Google Scholar]
- 16.Marhe R, Luijten M, van de Wetering BJ, Smits M, Franken IH. Individual differences in anterior cingulate activation associated with attentional bias predict cocaine use after treatment. Neuropsychopharmacology 2013; 38(6): 1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murdaugh DL, Cox JE, Cook EW 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage 2012; 59(3): 2709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heller AS, Johnstone T, Light SN, Peterson MJ, Kolden GG, Kalin NH et al. Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. Am J Psychiatry 2013; 170(2): 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ten Kulve JS, Veltman DJ, van Bloemendaal L, Barkhof F, Drent ML, Diamant M et al. Liraglutide Reduces CNS Activation in Response to Visual Food Cues Only After Short-term Treatment in Patients With Type 2 Diabetes. Diabetes Care 2016; 39(2): 214–21. [DOI] [PubMed] [Google Scholar]
- 20.Bruce AS, Bruce JM, Ness AR, Lepping RJ, Malley S, Hancock L et al. A comparison of functional brain changes associated with surgical versus behavioral weight loss. Obesity (Silver Spring) 2014; 22(2): 337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruce JM, Hancock L, Bruce A, Lepping RJ, Martin L, Lundgren JD et al. Changes in brain activation to food pictures after adjustable gastric banding. Surg Obes Relat Dis 2012; 8(5): 602–8. [DOI] [PubMed] [Google Scholar]
- 22.Faulconbridge LF, Ruparel K, Loughead J, Allison KC, Hesson LA, Fabricatore AN et al. Changes in neural responsivity to highly palatable foods following roux-en-Y gastric bypass, sleeve gastrectomy, or weight stability: An fMRI study. Obesity (Silver Spring) 2016; 24(5): 1054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochner CN, Kwok Y, Conceicao E, Pantazatos SP, Puma LM, Carnell S et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg 2011; 253(3): 502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochner CN, Laferrere B, Afifi L, Atalayer D, Geliebter A, Teixeira J. Neural responsivity to food cues in fasted and fed states pre and post gastric bypass surgery. Neurosci Res 2012; 74(2): 138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochner CN, Stice E, Hutchins E, Afifi L, Geliebter A, Hirsch J et al. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience 2012; 209: 128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014; 63(6): 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank S, Heinze JM, Fritsche A, Linder K, von Feilitzsch M, Konigsrainer A et al. Neuronal Food Reward Activity in Patients With Type 2 Diabetes With Improved Glycemic Control After Bariatric Surgery. Diabetes Care 2016; 39(8): 1311–7. [DOI] [PubMed] [Google Scholar]
- 28.Goldman RL, Canterberry M, Borckardt JJ, Madan A, Byrne TK, George MS et al. Executive control circuitry differentiates degree of success in weight loss following gastric-bypass surgery. Obesity (Silver Spring) 2013; 21(11): 2189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg 2013; 23(4): 427–36. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen NT, Nguyen B, Gebhart A, Hohmann S. Changes in the makeup of bariatric surgery: a national increase in use of laparoscopic sleeve gastrectomy. J Am Coll Surg 2013; 216(2): 252–7. [DOI] [PubMed] [Google Scholar]
- 31.Hollmann M, Hellrung L, Pleger B, Schlogl H, Kabisch S, Stumvoll M et al. Neural correlates of the volitional regulation of the desire for food. Int J Obes (Lond) 2012; 36(5): 648–55. [DOI] [PubMed] [Google Scholar]
- 32.Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A 2010; 107(33): 14811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte M, Jansen A. Fighting food temptations: the modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. Neuroimage 2012; 60(1): 213–20. [DOI] [PubMed] [Google Scholar]
- 34.Yokum S, Stice E. Cognitive regulation of food craving: effects of three cognitive reappraisal strategies on neural response to palatable foods. Int J Obes (Lond) 2013; 37(12): 1565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin 2015; 8: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zijlstra H, van Middendorp H, Devaere L, Larsen JK, van Ramshorst B, Geenen R. Emotion processing and regulation in women with morbid obesity who apply for bariatric surgery. Psychol Health 2012; 27(12): 1375–87. [DOI] [PubMed] [Google Scholar]
- 37.Handley JD, Williams DM, Caplin S, Stephens JW, Barry J. Changes in Cognitive Function Following Bariatric Surgery: a Systematic Review. Obes Surg 2016; 26(10): 2530–7. [DOI] [PubMed] [Google Scholar]
- 38.Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol 2003; 85(2): 348–62. [DOI] [PubMed] [Google Scholar]
- 39.van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord 1986; 5(2): 295–315. [Google Scholar]
- 40.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985; 29(1): 71–83. [DOI] [PubMed] [Google Scholar]
- 41.Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite 2009; 53(1): 114–8. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Steer RA, Brown G, K. Manual for the Beck Depression Inventory-II, Psychological Corporation: San Antonio, TX, 1996. [Google Scholar]
- 43.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory, Consultin Psychologists Press: Palo Alto, CA, 1970. [Google Scholar]
- 44.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19(3): 1233–9. [DOI] [PubMed] [Google Scholar]
- 45.Whitfield-Gabrieli S Region of Interest Extraction (REX) Toolbox. In. Cambridge, MA: Gabrieli Lab, MIT: Department of Brain and Cognitive Sciences, 2009. [Google Scholar]
- 46.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39(2): 175–91. [DOI] [PubMed] [Google Scholar]
- 47.Sczepaniak JP, Owens ML, Shukla H, Perlegos J, Garner W. Comparability of weight loss reporting after gastric bypass and sleeve gastrectomy using BOLD data 2008–2011. Obes Surg 2015; 25(5): 788–95. [DOI] [PubMed] [Google Scholar]
- 48.Rieber N, Giel KE, Meile T, Enck P, Zipfel S, Teufel M. Psychological dimensions after laparoscopic sleeve gastrectomy: reduced mental burden, improved eating behavior, and ongoing need for cognitive eating control. Surg Obes Relat Dis 2013; 9(4): 569–73. [DOI] [PubMed] [Google Scholar]
- 49.Chao A, Grilo CM, White MA, Sinha R. Food cravings, food intake, and weight status in a community-based sample. Eat Behav 2014; 15(3): 478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buscemi J, Rybak TM, Berlin KS, Murphy JG, Raynor HA. Impact of food craving and calorie intake on body mass index (BMI) changes during an 18-month behavioral weight loss trial. J Behav Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meule A, Richard A, Platte P. Food cravings prospectively predict decreases in perceived self-regulatory success in dieting. Eat Behav 2017; 24: 34–38. [DOI] [PubMed] [Google Scholar]
- 52.Mans E, Serra-Prat M, Palomera E, Sunol X, Clave P. Sleeve gastrectomy effects on hunger, satiation, and gastrointestinal hormone and motility responses after a liquid meal test. Am J Clin Nutr 2015; 102(3): 540–7. [DOI] [PubMed] [Google Scholar]
- 53.van der Plasse G, van Zessen R, Luijendijk MC, Erkan H, Stuber GD, Ramakers GM et al. Modulation of cue-induced firing of ventral tegmental area dopamine neurons by leptin and ghrelin. Int J Obes (Lond) 2015; 39(12): 1742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex 2014; 24(11): 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Staudinger MR, Erk S, Walter H. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cereb Cortex 2011; 21(11): 2578–88. [DOI] [PubMed] [Google Scholar]
- 56.Kolata G After weight-loss surgery, a year of joys and disappointments. New York Times: 2016. December 27. [Google Scholar]
- 57.Goldstein-Piekarski AN, Korgaonkar MS, Green E, Suppes T, Schatzberg AF, Hastie T et al. Human amygdala engagement moderated by early life stress exposure is a biobehavioral target for predicting recovery on antidepressants. Proc Natl Acad Sci U S A 2016; 113(42): 11955–11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stice E, Yokum S, Burger K, Rohde P, Shaw H, Gau JM. A pilot randomized trial of a cognitive reappraisal obesity prevention program. Physiol Behav 2015; 138: 124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gluck ME, Alonso-Alonso M, Piaggi P, Weise CM, Jumpertz-von Schwartzenberg R, Reinhardt M et al. Neuromodulation targeted to the prefrontal cortex induces changes in energy intake and weight loss in obesity. Obesity (Silver Spring) 2015; 23(11): 2149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall PA, Vincent CM, Burhan AM. Non-invasive brain stimulation for food cravings, consumption, and disorders of eating: A review of methods, findings and controversies. Appetite 2017. [DOI] [PubMed] [Google Scholar]
- 61.Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW et al. What goes up, can come down: Novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res 2015; 1628(Pt A): 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.