Abstract
Background:
Consistent with nigrostriatal dopamine depletion, low cerebrospinal fluid (CSF) concentrations of 3,4-dihydroxyphenylacetic acid (DOPAC), the main neuronal metabolite of dopamine, characterizes Parkinson’s disease (PD) even in recently diagnosed patients. Whether low CSF levels of DOPAC or DOPA, the precursor of dopamine, identify pre-clinical PD in at- risk healthy individuals has been unknown.
Methods:
Participants in the intramural NINDS PDRisk study entered information about family history of PD, olfactory dysfunction, dream enactment behavior, and orthostatic hypotension at a protocol-specific website. After at least 3 risk factors were confirmed by on-site screening, 26 subjects had CSF sampled for cerebrospinal fluid levels of catechols and were followed for at least 3 years.
Results:
Of 26 PDRisk subjects, 4 were diagnosed with PD (Pre-Clinical PD group); 22 risk-matched (mean 3.2 risk factors) subjects remained disease-free after a median of 3.7 years (No-PD group). The Pre-Clinical PD group had lower initial DOPA and DOPAC levels than did the No-PD group (p=0.0302, p=0.0190). All 3 subjects with both low DOPA (<2.63 pmol/mL) and low DOPAC (<1.22 pmol/mL) levels, based on optimum cut-off points using the minimum distance method, developed PD, whereas none of 14 subjects with both normal DOPA and DOPAC levels did so (75% sensitivity at 100% specificity, p=0.0015 by 2-tailed Fisher’s exact test).
Conclusions:
In people with multiple PD risk factors, those with low CSF DOPA and low CSF DOPAC levels develop clinical disease during follow-up. We suggest that neurochemical biomarkers of central dopamine deficiency identify the disease in a pre-clinical phase.
Keywords: Parkinson’s disease, Biomarkers, Catecholamines, DOPAC, DOPA
By the time Parkinson’s disease (PD) manifests with motor signs, substantial loss of nigrostriatal dopaminergic neurons has already occurred. There is great interest in identifying cerebrospinal fluid (CSF) biomarkers of the neurodegenerative process, both for early diagnosis and to track effects of putative disease-modifying treatment or prevention strategies.
Consistent with profound striatal dopamine depletion, CSF concentrations of 3,4-dihydroxyphenylacetic acid (DOPAC), the main neuronal metabolite of dopamine, are decreased in PD [1, 2], even in patients with a recent diagnosis [3]. CSF levels of endogenous 3,4-dihydroxyphenylalanine (DOPA), the precursor of dopamine, are also decreased in levodopa-untreated patients [2] however, whether low CSF DOPAC or low CSF DOPA provides a neurochemical biomarker of pre-clinical PD in at-risk individuals has been unknown.
Here we report data from the PDRisk prospective cohort study of the National Institute of Neurological Disorders and Stroke (NINDS) that are relevant to this issue. In the PDRisk study, a small group of individuals with multiple PD risk factors (at least 3 of: family history, olfactory dysfunction [4], dream enactment behavior [5], orthostatic hypotension [6]) but without motor symptoms suggestive of PD, have been intensively and comprehensively studied and then reevaluated at about 1.5-year intervals. The PDRisk subjects reported here underwent lumbar puncture for assays of CSF DOPAC and DOPA and were followed for at least 3 years.
METHODS
Subjects in this study gave written informed consent to participate in intramural protocols approved by the NINDS institutional review board (IRB). Consent for the PDRisk study was in two forms—electronic at a protocol-specific, IRB-approved website and in writing at the time of evaluation at the Clinical Center of the National Institutes of Health (NIH).
Inclusion Criteria
There were 4 categories of risk—genetic, olfactory, symptoms of RBD, and OH. With respect to genetics, a positive family history (one immediate or more than one non-immediate family member with PD) or positive genetic testing (e.g., LRRK2, alpha-synuclein, glucocerebrosidase) satisfied this criterion. Olfactory dysfunction reported at the protocol-specific website satisfied the criterion. To satisfy the RBD risk factor criterion, the individual or member of the individual’s household must have reported movements of the body or limbs associated with dreaming and at least one of the following: potentially harmful sleep behavior, dreams that appeared to be acted out, and sleep behavior that disrupted sleep continuity. To satisfy the OH risk factor criterion, the individual must have reported symptoms of OH or having OH as defined by consensus criteria.
Exclusion Criteria
People younger than 18 years old or older than 70 years old were excluded. A candidate subject was excluded if there was a disqualifying condition or clinical considerations required continued treatment with a drug likely to interfere with the scientific results.
Study Design
The primary outcome measure was a diagnosis of PD by a neurologist who was blinded to the results of the catecholaminergic biomarkers testing. Data collection has been ongoing since 2009.
Recruitment was by advertisement using a Google ad referring to a protocol-specific, IRB-approved web page. Candidate participants who were flagged as reporting at least 3 risk factors were pre-screened by a Research Nurse, to confirm eligibility criteria.
The screening examination at the NIH Clinical Center was to obtain consent for further participation in the study, verify at least 3 risk factors, and perform clinical and laboratory tests (e.g., autonomic function tests described below). Subjects with at least 3 confirmed risk factors underwent inpatient biomarkers testing.
For follow-up testing subjects were re-evaluated at about 1.5-year intervals, for neurological examinations and neuroimaging reassessments. Participants who started on exclusionary drugs after enrollment were not brought back for follow-up inpatient testing; however, they could be contacted by phone, mail, or secure e-mail, to learn of their clinical status.
PD was diagnosed according to accepted clinical criteria such as bradykinesia, rigidity, resting tremor, and imbalance.
Identification of OH
Each subject was evaluated in a dedicated patient testing room in the NIH Clinical Center. After at least 15 minutes with the subject at supine rest, the subject was tilted head-up at 90 degrees for 5 minutes. OH was defined as a decrease in systolic blood pressure of at least 20 mmHg or in diastolic pressure of at least 10 mmHg between lying supine and head-up tilting for at least 3 minutes.
CSF Collection
To obtain CSF, subjects underwent lumbar puncture by a neuroradiologist under fluoroscopic guidance. A total of 12 1-mL aliquots of CSF were obtained in cold 1.5-mL plastic sample tubes that were placed immediately in dry ice. We report data about CSF catechols in the 6th aliquot. CSF levels of DOPA and DOPAC were measured in our laboratory by batch alumina extraction followed by liquid chromatography with series electrochemical detection, as described previously [7].
18F-DOPA PET Scanning
Each PDRisk study participant underwent head 18F-DOPA positron emission tomographic (PET) scanning, as described previously by our group [1]. Briefly, 10 mCi of 18F-DOPA was injected intravenously without carbidopa pre-treatment. The putamen/occipital cortex (PUT/OCC) ratio of 18F-DOPA-derived radioactivity was calculated for the static 15-minute image ending at 120 minutes after tracer injection. The putamen posterior/anterior (P/A) ratio of radioactivity was also measured, since in early PD the posterior putamen is more affected than the anterior putamen [8].
Study Size, Data Analysis, and Statistics
To estimate the required numbers of subjects, we used a log-rank test for the interval between biomarkers testing and diagnosis of PD and predicted that among individuals at risk of PD and who had abnormal catecholaminergic biomarkers, 80% would develop PD by 7.5 years of follow-up; and that among at-risk participants without abnormal biomarkers, 20% would develop PD during follow-up. Considering the possibility of dropouts, at an alpha value of 0.05 and beta value of 0.20, follow-up from a group of 26 subjects with complete baseline biomarkers data would be sufficient. An analysis was done after ≥ 3 years of follow-up, for calculation of the positive predictive value of the biomarkers. Calculation of the negative predictive value of absent biomarkers will be done at the end of the study (7.5 years of follow-up).
The individual carrying out the neurochemical assays (C.H.) was blinded as to the clinical risk factors and other biomarkers.
For each of the dependent measures, normality in the distribution of residuals was assessed by the Shapiro-Wilk test. The data for CSF DOPA had to be log-transformed to achieve normality. The data were then subjected to two-sample t-tests, to examine the differences between the PDRisk subjects with pre-clinical PD (Pre-Clinical PD group) and the PDRisk subjects without symptomatic PD at 3 years of follow-up (No-PD group). We also analyzed the data by the non-parametric Wilcoxon rank sum test.
Receiver operating characteristic curve analysis and a method based on the minimum distance between the point (0,1) and points on the curve were used to select the optimal cutoff thresholds for binary predictions. The dichotomized biomarkers data based on the cutoff values were then analyzed by 2-tailed Fisher’s exact test.
A p value less than 0.05 defined statistical significance.
RESULTS
A total of 31 accrued participants had at least 3 of the 4 risk factors confirmed upon on-site screening at the NIH Clinical Center. Of these, 3 reported having been diagnosed with PD between the time of registration at the protocol-specific website and the time of on-site screening and were excluded from further participation. Therefore, 28 at-risk subjects had inpatient biomarkers testing. One subject was withdrawn because of being on an anti-coagulant, which was exclusionary.
A total of 27 subjects underwent inpatient biomarkers testing and were followed for at least 3 years. One had multiple sclerosis, and it was impossible to determine if the participant had reached the end-point. Her data were excluded from the analysis. Of the 26 remaining subjects followed for at least 3 years, 4 (15%) developed clinical PD (3 men and 1 woman, median age 59 years old). The remaining 22 subjects who did not reach the end-point by 3 years of follow-up were in the No-PD group (6 men and 16 women, median age 55 years old). The mean ± SD of the follow-up time in the No PD group was 4.2 ± 1.4 years (median 3.7 years).
None of the PDRisk subjects had an identified mutation of the alpha-synuclein, leucine-rich repeat kinase 2, or glucocerebrosidase genes.
Among the 4 subjects in the Pre-Clinical PD group, 2 developed cognitive dysfunction, visual hallucinations, and fluctuating mentation and were diagnosed with probable dementia with Lewy bodies (DLB). Of the 2, 1 died and was autopsied and had DLB confirmed by the identification of Lewy bodies in the brainstem and sympathetic ganglia.
CSF Neurochemical Biomarkers in the Pre-Clinical vs. No-PD Groups
Two-sample t-testing indicated that the difference in CSF data between the Pre-Clinical PD and the No-PD group was significant, for both DOPA (p=0.0303) and DOPAC (p=0.0354). The median value for DOPA was lower in the Pre-Clinical PD group (2.44 pmol/mL) than in the No-PD group (3.59 pmol/mL). The mean value for CSF DOPAC was also lower in the Pre-Clinical PD group than in the No-PD group (Table 1). By the Wilcoxon test the Pre-Clinical PD and No-PD groups differed in CSF levels of both DOPA (p=0.0128) and DOPAC (p=0.0360).
Table 1.
Individual data from the PD Risk study. Summary statistics are in bold.
| N | GROUP | Accrual Age | Race | Sex | FH? | Smell? | OH? | RBD? | Risks | F/U Years | DOPA | DOPAC | PUT/OCC | P/A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| years | 1=White | 1=Male | 1=Yes | 1=Yes | 1=Yes | 1=Yes | years | nmol/L | nmol/L | |||||
| CUTOFF | 2.63 | 1.22 | 2.70 | 0.75 | ||||||||||
| 1 | Pre-Clinical PD | 69.0 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 2.0 | 2.63 | 1.21 | 3.24 | 0.25 |
| 2 | Pre-Clinical PD | 59.9 | 1 | 1 | 1 | 1 | 0 | 1 | 3 | 0.5 | 2.50 | 0.69 | 2.36 | 0.58 |
| 3 | Pre-Clinical PD | 58.7 | 1 | 0 | 1 | 0 | 1 | 1 | 3 | 1.5 | 2.38 | 1.63 | 2.30 | 0.61 |
| 4 | Pre-Clinical PD | 52.9 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 1.5 | 2.08 | 0.91 | 2.20 | 0.54 |
| MEAN | 60.8 | 4 | 3 | 4 | 3 | 3 | 4 | 3.5 | 1.4 | 2.40 | 1.11 | 2.53 | 0.50 | |
| SEM | 3.9 | 0 | 1 | 0 | 1 | 1 | 0 | 0.3 | 0.3 | 0.12 | 0.20 | 0.24 | 0.08 | |
| N | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | |
| 5 | No-PD | 50.1 | 1 | 0 | 1 | 1 | 1 | 1 | 4 | 3.2 | 2.65 | 1.07 | 3.14 | 0.89 |
| 6 | No-PD | 50.8 | 1 | 0 | 1 | 0 | 1 | 1 | 3 | 3.0 | 2.05 | 1.73 | 3.17 | 0.88 |
| 7 | No-PD | 55.0 | 1 | 1 | 1 | 0 | 1 | 1 | 3 | 6.5 | 2.50 | 1.23 | 4.28 | 1.03 |
| 8 | No-PD | 58.5 | 1 | 0 | 1 | 1 | 1 | 1 | 4 | 6.2 | 2.81 | 2.29 | 3.68 | 0.86 |
| 9 | No-PD | 50.0 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 3.0 | 3.83 | 1.82 | 4.70 | 1.08 |
| 10 | No-PD | 47.1 | 1 | 0 | 1 | 0 | 1 | 1 | 3 | 6.2 | 3.60 | 0.95 | 3.54 | 0.98 |
| 11 | No-PD | 68.2 | 1 | 1 | 1 | 1 | 0 | 1 | 3 | 6.1 | 4.86 | 0.97 | 4.19 | 0.80 |
| 12 | No-PD | 63.8 | 1 | 0 | 1 | 1 | 1 | 0 | 3 | 6.3 | 6.24 | 2.15 | 4.50 | 0.93 |
| 13 | No-PD | 39.5 | 1 | 0 | 1 | 0 | 1 | 1 | 3 | 3.0 | 4.21 | 1.98 | 3.68 | 1.00 |
| 14 | No-PD | 58.4 | 1 | 0 | 1 | 1 | 0 | 1 | 3 | 6.4 | 3.96 | 2.10 | 2.94 | 0.78 |
| 15 | No-PD | 54.1 | 1 | 0 | 1 | 1 | 0 | 1 | 3 | 3.0 | 3.42 | 1.14 | 3.15 | 0.79 |
| 16 | No-PD | 55.6 | 1 | 0 | 1 | 1 | 1 | 1 | 4 | 3.0 | 2.95 | 2.72 | 2.74 | 0.53 |
| 17 | No-PD | 56.9 | 1 | 1 | 1 | 0 | 1 | 1 | 3 | 4.3 | 3.39 | 1.32 | 4.74 | 0.96 |
| 18 | No-PD | 63.3 | 0 | 1 | 1 | 1 | 0 | 1 | 3 | 3.0 | 3.56 | 1.58 | 2.02 | 0.56 |
| 19 | No-PD | 66.9 | 1 | 1 | 1 | 1 | 0 | 1 | 3 | 4.5 | 7.01 | 2.05 | 3.17 | 0.93 |
| 20 | No-PD | 62.5 | 1 | 0 | 1 | 1 | 1 | 0 | 3 | 4.5 | 4.51 | 2.79 | 3.22 | 0.78 |
| 21 | No-PD | 53.2 | 1 | 0 | 1 | 1 | 1 | 1 | 4 | 4.4 | 2.95 | 1.07 | 3.87 | 0.82 |
| 22 | No-PD | 62.2 | 1 | 0 | 1 | 1 | 1 | 0 | 3 | 3.1 | 2.66 | 2.03 | 3.02 | 0.84 |
| 23 | No-PD | 45.6 | 1 | 0 | 1 | 1 | 0 | 1 | 3 | 3.9 | 3.45 | 2.16 | 3.91 | 0.67 |
| 24 | No-PD | 47.5 | 1 | 0 | 1 | 0 | 1 | 1 | 3 | 3.0 | 2.98 | 1.57 | 2.49 | 0.98 |
| 25 | No-PD | 56.7 | 1 | 0 | 0 | 1 | 1 | 1 | 3 | 3.4 | 2.15 | 1.69 | 3.01 | 0.76 |
| 26 | No-PD | 42.0 | 1 | 0 | 0 | 1 | 1 | 1 | 3 | 3.0 | 3.26 | 2.01 | 3.10 | 0.80 |
| MEAN | 54.9 | 21 | 6 | 20 | 16 | 16 | 19 | 3.2 | 4.2 | 3.59 | 1.75 | 1.75 | 1.75 | |
| SEM | 1.7 | 22 | 22 | 22 | 22 | 22 | 22 | 0.1 | 0.3 | 0.26 | 0.12 | 0.12 | 0.12 | |
| N | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 |
Dichotomized Positive Biomarkers by Cutoff Values
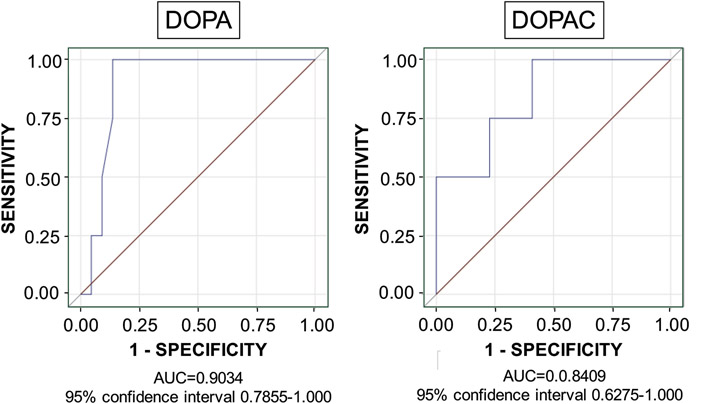
For CSF DOPA the area under the receiver operating characteristic curve was 0.903 (95% confidence interval 0.786-1.000), and for CSF DOPAC the area was 0.841 (95% confidence interval 0.628-1.000; Figure 1). The optimal cutoff value for defining low DOPAC was 1.22 pmol/mL and DOPA 2.63 pmol/mL. Based on the dichotomized biomarkers, all 4 subjects in the Pre-Clinical PD group (100%) had low CSF DOPA, compared to 3 of the 22 subjects in the No-PD group (14%; p=0.0023 by Fisher’s exact test), and 3 of the 4 subjects in the Pre-Clinical PD group (75%) had low CSF DOPAC, compared to 5 of the 22 subjects in the No-PD group (23%; p=0.0721). Three of the 4 subjects in the Pre-Clinical PD group had both low CSF DOPA and CSF DOPAC compared to none of the 22 subjects in the No-PD group (p=0.0015 by Fisher’s exact test).
Figure 1: Receiver operating characteristic curves for CSF DOPA and DOPAC in PDRisk study participants.
CSF DOPA and DOPAC distinguished the Pre-Clinical PD group from the No-PD group. Abbreviation: AUC=area under the curve.
18F-DOPA PET Scanning
The Pre-Clinical PD group had a lower mean PUT/OCC ratio of 18F-DOPA-derived radioactivity and a lower mean putamen P/A ratio of radioactivity than did the No-PD group (p=0.0188 and p=0.0002 by 2-sample t-tests). CSF DOPA and DOPAC levels were not significantly correlated with PUT/OCC or putamen P/A ratios in the PDRisk group.
DISCUSSION
The data from this small prospective cohort study show that among people with multiple risk factors for PD (family history, olfactory dysfunction, symptoms of rapid eye movement behavior disorder, and orthostatic hypotension), those who subsequently develop clinical PD have neurochemical biomarkers of central dopamine deficiency (low CSF DOPA, low CSF DOPAC, or both abnormalities), whereas among risk-matched individuals with normal DOPA and DOPAC levels, none so far has developed clinical PD (mean 4.2 years of follow-up). Thus, in the setting of multiple PD risk factors, having positive CSF biomarkers of central dopaminergic neurodegeneration substantially increases the likelihood of developing clinical PD. We believe that the reason for this predictive strength is that the catecholaminergic neurochemical biomarkers reflect key underlying pathogenetic processes, so that positive biomarkers identify the disease in a pre-clinical phase.
The PDRisk study involves a relatively small number of highly selected, intensively studied, closely followed subjects. These aspects enabled sufficient statistical power to test the main hypotheses while minimizing risks involved with the research tests. On the other hand, since all the PDRisk subjects had multiple risk factors, generalizability to people with fewer or different risk factors is unclear.
The Pre-Clinical PD group had low PUT/OCC and putamen P/A ratios of 18F-DOPA-derived radioactivity, as one would expect in early PD [8, 9]. Thus, the Pre-Clinical PD group had both CSF neurochemical and dopaminergic neuroimaging evidence of central dopamine deficiency before the onset of parkinsonism.
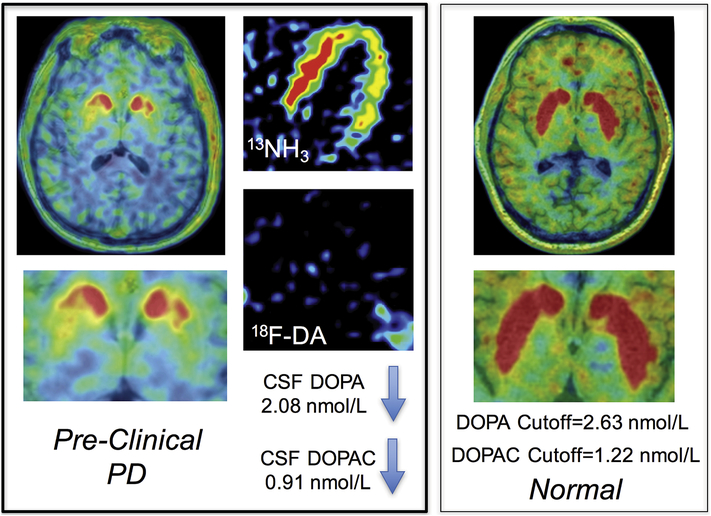
The same subjects with pre-clinical PD also had myocardial noradrenergic deficiency assessed by cardiac sympathetic neuroimaging, as noted in a companion report [10]. Therefore, among at-risk people, the combination of CSF neurochemical evidence of central dopamine deficiency with neuroimaging evidence of cardiac sympathetic denervation seems to provide a powerful means to identify pre-clinical PD. Figure 2 shows neurochemical and neuroimaging results in a PDRisk study participant with pre-clinical PD.
Figure 2: Neurochemical and neuroimaging results in a PDRisk study participant with pre-clinical PD.
The subject has low putamen 18F-DOPA-derived radioactivity, low myocardial 18F-DA-derived radioactivity (13N-ammonia perfusion image in the same subject shows the location of the left ventricle), low CSF DOPA, and low CSF DOPAC. Cutoff values shown are the optimum for separating the Pre-Clinical PD from the No-PD groups. PD and DLB were diagnosed at the 1.5-year follow-up visit.
In conclusion, based on data from the PDRisk study, at-risk individuals with CSF neurochemical biomarkers of central dopamine deficiency have a substantially increased likelihood of developing clinical PD subsequently within 3 years. At-risk individuals without these biomarkers remain disease-free in this time period.
HIGHLIGHTS.
Study on whether low CSF DOPAC & DOPA predicts PD
Risk factors: genetic, olfactory, dream enactment, orthostatic hypotension
26 people with ≥ 3 risk factors followed ≥ 3 years after LP
4 with pre-clinical PD all had antecedent low CSF DOPAC, DOPA, or both
Biomarkers of central dopamine deficiency predict PD in at-risk individuals.
ACKNOWLEDGEMENT
The Division of Intramural Research, NINDS, NIH supported the research reported here (Project ZIA NS003034).
We acknowledge the assistance of Sandra Pechnik, RN, LaToya Sewell, CRNP, and Janna Gelsomino, RN, in the conduct of the study.
The funding source was the Division of Intramural Research, National Institute of Neurological Disorders and Stroke.
Footnotes
FINANCIAL DISCLOSURES OF ALL AUTHORS FOR THE PAST YEAR
David S. Goldstein: Stock ownership in medically-related fields; royalty (book).
Courtney Holmes: None.
Grisel Lopez: None.
Tianxia Wu: None.
Yehonatan Sharabi: None.
Disclosures: None of the authors has a conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Goldstein DS, Holmes C, Bentho O, Sato T, Moak J, Sharabi Y, Imrich R, Conant S, Eldadah BA, Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy, Parkinsonism Relat. Disord. 14(8) (2008) 600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andersen AD, Blaabjerg M, Binzer M, Kamal A, Thagesen H, Kjaer TW, Stenager E, Gramsbergen JBP, Cerebrospinal fluid levels of catecholamines and its metabolites in Parkinson’s disease: effect of l-DOPA treatment and changes in levodopa-induced dyskinesia, J. Neurochem. 141(4) (2017) 614–625. [DOI] [PubMed] [Google Scholar]
- [3].Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Kim HM, Leverenz JB, Montine TJ, Ginghina C, Kang UJ, Cain KC, Wang Y, Aasly J, Goldstein D, Zhang J, Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression, Ann. Neurol. 69(3) (2011) 570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW, Idiopathic hyposmia as a preclinical sign of Parkinson’s disease, Ann. Neurol. 56(2) (2004) 173–181. [DOI] [PubMed] [Google Scholar]
- [5].Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D, Montplaisir JY, Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials, Neurology 84(11) (2015) 1104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goldstein DS, Orthostatic hypotension as an early finding in Parkinson disease, Clin. Auton. Res 16 (2006) 46–64. [DOI] [PubMed] [Google Scholar]
- [7].Goldstein DS, Holmes C, Sharabi Y, Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson’s disease and other synucleinopathies, Brain 135(Pt 6) (2012) 1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nurmi E, Ruottinen HM, Bergman J, Haaparanta M, Solin O, Sonninen P, Rinne JO, Rate of progression in Parkinson’s disease: a 6-[18F]fluoro-L-dopa PET study, Mov Disord 16(4) (2001) 608–15. [DOI] [PubMed] [Google Scholar]
- [9].Jokinen P, Helenius H, Rauhala E, Bruck A, Eskola O, Rinne JO, Simple ratio analysis of 18F-fluorodopa uptake in striatal subregions separates patients with early Parkinson disease from healthy controls, J. Nucl. Med 50(6) (2009) 893–9. [DOI] [PubMed] [Google Scholar]
- [10].Goldstein DS, Holmes C, Lopez G, Wu Tianxia, Sharabi Y, Cardiac sympathetic denervation predicts PD in at-risk individuals, Parkinsonism Relat. Disord ((in press)). [DOI] [PMC free article] [PubMed] [Google Scholar]