Abstract
Background
Severe infections such as endocarditis and osteomyelitis require long-term treatment with parenteral antibiotics and hence prolonged hospitalisation. Continuous infusion of ceftaroline through elastomeric devices can facilitate early hospital discharge by managing parenteral antibiotics in patient’s home. Therefore, the purpose of this study was to investigate the stability of ceftaroline in a commonly used elastomeric device.
Method
A total of 24 elastomeric devices were prepared, and six elastomeric devices containing 6mg/mL of ceftaroline (three in each type of diluents) were stored at one of the following conditions: 4°C for 6 days, 25°C for 24hours, 30°C for 24hours or 35°C for 24hours. An aliquot was withdrawn before storage and at different time points. Chemical stability was measured using a stability indicating high-performance liquid chromatography, and physical stability was assessed as change in pH, colour and particle content.
Results
Ceftaroline, when admixed with both diluents, was stable for 144, 24 and 12hours at 4°C, 25°C and 30°C, respectively. At 35°C, ceftaroline admixed with normal saline (NS) and glucose 5% was stable for 12hours and for 6hours, respectively. No evidence of particle formation, colour change or pH change was observed throughout the study period.
Conclusions
Our findings support 12 or 24hours continuous elastomeric infusion of ceftaroline-NS admixture, and bulk preparation of elastomeric pumps containing ceftaroline solution in advance. This would facilitate early hospital discharge of patients eligible for the elastomeric-based home therapy and avoid the need for patient’s caregivers travelling to the hospital on a daily basis.
Keywords: ceftaroline, antibiotics, elastomeric devices, continuous infusion, stability, multiple bacterial drug resistance
Introduction
Increasing antibiotic resistance has become an escalating threat worldwide.1 It is estimated that more than two million individuals are infected with multidrug-resistant (MDR) bacteria in the United States (US) each year.2 MDR bacteria are also responsible for more than 50 000 deaths per annum in the US and Europe.3 Additionally, the cost of infections caused by MDR bacteria to the US health system exceeds US$20 billion as per the 2008 estimates.4 Though Pseudomonas aeruginosa or Acinetobacter species are dominant pathogens globally, in some European countries, up to 50% of MDR Gram-positive infections are caused by methicillin-resistant Staphylococcus aureus (MRSA).5
Gram-positive bacteria such as the MDR Streptococcus pneumoniae and MRSA are largely implicated in endocarditis, osteomyelitis, bacteraemia and infective pulmonary exacerbations in cystic fibrosis (CF).6 Clinical treatment options for MRSA infections are limited. Vancomycin and linezolid are recommended as the first-line agents for the treatment of such infections. However, poor lung penetration of vancomycin and the emergence of both vancomycin and linezolid resistant Staphylococcus aureus (S. aureus) have raised considerable debate on the continuous use of these agents as the first-line treatment against MRSA.7 8
Ceftaroline is a fifth-generation parenteral cephalosporin with an extended bactericidal activity against Gram-positive bacteria including MRSA, MDR Streptococcus pneumoniae, vancomycin-resistant S. aureus and linezolid-resistant S. aureus.9 Ceftaroline is currently approved for the treatment of acute bacterial skin infections and community-acquired pneumonia.10 However, recent studies have shown the effectiveness of ceftaroline in the treatment of endocarditis, bacteraemia, infective pulmonary exacerbations in CF and osteomyelitis caused by the MDR bacteria including MRSA.11–13 For example, Lin et al. retrospectively reviewed the outcomes of ceftaroline treatment in 10 in-patients with either endocarditis, pneumonia, septic arthritis or osteomyelitis caused by MRSA. The study concluded that 70% of patients achieved microbiological cure and were discharged from the hospital. In another study, ceftaroline was used as a salvage monotherapy in six in-patients who were suffering from MRSA bacteraemia or endocarditis due to persistent or recurrent endocarditis while on vancomycin or daptomycin. The authors reported the rapid clearance of bacteraemia in all patients after starting ceftaroline and sterilisation of excised heart valve within 13 days of ceftaroline treatment in a patient with endocarditis. These infections often require prolonged treatment with parenteral antibiotics therapy for at least 3–6 weeks due to the deep-seated nature of MRSA.14 Many hospitalised patients with infections such as endocarditis become clinically stable within a week or two after an appropriate antimicrobial treatment, and therefore well enough to be discharged from the hospital.15 Elastomerics are patient-managed, non-battery operated, infusion devices that deliver continuous infusion of drugs at a relatively constant flow rate. Elastomeric-based delivery of ceftaroline can facilitate early hospital discharge that can potentially decrease healthcare costs, reduce nosocomial infections, improve patient’s quality of life and, in some cases, reduce mortality.16 As the stability of ceftaroline in elastomeric devices is unknown beyond 24 and 6 hours at 4°C and 25°C, respectively, it is important to study its stability at various temperatures for an extended period of time to facilitate early hospital discharge of patients who have been optimised on ceftaroline predischarge. Therefore, the purpose of this study was to investigate the physicochemical stability of ceftaroline in one of the commonly used elastomeric infusion devices at four different temperatures at various time points.
Methods
Sample preparation
Samples were prepared using aseptic techniques in a class II biological safety cabinet. Ceftaroline fosamil powder for injection (600 mg, AstraZeneca; New South Wales, Australia, Lot 0004D4) was reconstituted with 20 mL Milli-Q Water (Merck MilliPore Purification System, Melbourne, Australia) to achieve the stock solution containing 30 mg/mL of ceftaroline. The stock solution (10 mL) was then mixed with 40 mL of sterile 0.9% normal saline (NS) (Baxter Healthcare; New South Wales, Australia, lot W08L6) or glucose 5% British Pharmacopoeia (Baxter Healthcare, lot W01S6) to obtain a final concentration of 6 mg/mL. This concentration of ceftaroline is recommended for the treatment of endocarditis, osteomyelitis and CF-associated pulmonary infections caused by MDR bacteria.11 The solution was then transferred into the elastomeric pump (Infusor LV10, Baxter, New South Wales, Australia) as per manufacturer’s instructions. Briefly, the solution (6 mg/mL ceftaroline) was withdrawn using a Luer lock syringe, and the syringe was gently tapped to shift air bubbles to the top. Then, the plunger was pushed up to expel air bubbles from the syringe. Protective tape was removed from an elastomeric pump, and its fill port cap was then detached before slowly connecting the tip of the syringe into the fill port. The syringe was twisted clockwise, and its plunger was positioned on the bench while maintaining the elastomeric pump in an upside-down position. Then, the fluid was infused into the elastomeric pump by gradually pushing the syringe plunger all the way in the upward direction.
A total of 24 elastomeric pumps were prepared. Six pumps (three pumps containing ceftaroline in glucose 5% and three pumps containing ceftaroline in NS) were kept at each one of the following four conditions: 4°C in a refrigerator for 6 days, 25°C in an incubator for 24 hours, 30°C in a water bath for 24 hours and 35°C in the water bath for 24 hours. An aliquot (n=3) was then removed aseptically at 0, 2, 6, 24, 48, 72, 96, 120 and 144 hours from the samples stored at 4°C, and at 0, 0.5, 1, 2, 6, 12 and 24 hours from the samples stored at the other three temperatures.
Each sample was analysed for ceftaroline concentration using a stability indicating high-performance liquid chromatography (HPLC) within 15–30 min of the preparation. All the samples were also evaluated for change in pH (using a pH meter), change in colour change (using a UV spectrophotometer) and particle content (microscopically).
HPLC assay
Chromatography was performed using an UHPLC system (Thermo Fisher Scientific, Sunnyvale, CA, US). Ceftaroline was separated using a Kinetex C18 reversed phase column (150 cm × 4.6 mm; 100 Å pore size; 2.6 µm internal diameter), consisted of core-shell silica (Advanced Chromatography Technologies, Sydney, Australia). Mobile phase A was composed of 0.1% trifluoroacetic acid (TFA) in 100 mL of Milli-Q Water, and mobile phase B was composed of 0.1% TFA in 100 mL of acetonitrile. The flow rate was set at 1.0 mL/minute, and the flow gradient for mobile phase A was set as follow: 90% for an initial 4 min, 85% for another 1 min, 65% for the next 2 min, then 90% for another 3 min. Ceftaroline was detected using a Diode Array Detector (Varian, Melbourne, Australia) at 242 nm. The temperatures of the autosampler and the column compartments were set at 5°C and 45°C, respectively. The injection volume was 20 µL.
HPLC assay performance
Linearity
The stock solution (1 mg/mL) was first prepared by dissolving 10 mg of ceftaroline powder into 10 mL of Milli-Q water. The stock solution was then further diluted to obtain five different concentrations (300, 200, 100, 50 and 25 µg/mL) of ceftaroline. Determination coefficient (r2) over a period of 5 days was calculated by plotting the peak areas versus ceftaroline concentrations.
Accuracy, precision and reproducibility
Three different concentrations (25, 100 and 300 µg/mL) of ceftaroline were prepared and used for precision, accuracy and repeatability analysis. Accuracy was determined by investigating mean intraday and interday (>7 consecutive days) values for the ceftaroline peak (n=3) by using the following regression equation: (observed concentration − expected concentration)/expected concentration × 100. Intraday and interday (>7 consecutive days) precision were calculated using peak areas with repeat analysis (n=3) of the ceftaroline solutions. Repeatability was performed by investigating mean interday and intraday (>7 consecutive days) retention time of ceftaroline (n=3).
Forced degradation
The developed HPLC method was validated for its stability indicating capability by stressing ceftaroline under acidic, basic and thermal conditions. Under acidic stress condition, ceftaroline (500 µL, 200 µg/mL) was mixed with an equal volume of 2 M hydrochloric acid and then kept at room temperature for 24 hours. Under basic stress condition, ceftaroline (500 µL, 200 µg/mL) was mixed with an equal volume of 0.006 M sodium hydroxide and then kept at room temperature for 10 min. Under thermal stress condition, ceftaroline (1 mL, 200 µg/mL) was kept at 50°C in capped glass vials for 4 hours. Control samples (n=3) were also prepared the same way except ceftaroline was omitted. Each sample was prepared in triplicate and analysed in duplicate.
Sample preparation for HPLC analysis
Ceftaroline powder was reconstituted with 20 mL Milli-Q water to achieve the stock solution containing 30 mg/mL of ceftaroline. The solution was then further diluted to prepare the standard solutions (25, 50, 100, 200 and 300 µg/mL). An aliquot withdrawn from each elastomeric pump was diluted with Milli-Q water to obtain a concentration of 100 µg/mL. The diluted solution was subjected to HPLC analysis within 15–30 min of sampling. Standard curve (n=2) was generated on each day of sample analysis by plotting the peak areas of ceftaroline in standard solutions versus its corresponding concentration. Each analysis was analysed in triplicate.
Microscopic analysis
An aliquot (50 µL) withdrawn from each sample was carefully transferred on the surface of microscopic slide (76.2×25.4 mm) (Dako Limited, Sydney, Australia) and was covered with a coverslip (24×50 mm). The slide was then examined for particle content under a microscope (Nikon microscope ECLIPSE 50i, Adelaide, Australia) using 4× and 10× magnifications. Positive control samples (n=3) were prepared by dissolving ceftaroline (2 mg) in 1 mL glucose 5% solution. Then, 1 mL of solution containing 70 mg sodium phosphate dibasic was mixed with an equal volume of the ceftaroline in glucose 5% solution. The sample was then kept on the bench at room temperature until ceftaroline crystals were formed. Particles were counted using the Image J software (NIH, Baltimore, MD, US). Negative control samples (n=3) were prepared the same way except that ceftaroline was omitted. Each sample was analysed in duplicate.
UV spectrophotometric analysis
A standard solution (n=6) containing 100 µg/mL of ceftaroline was prepared by dissolving 1 mg of ceftaroline powder with 10 mL of Milli-Q water. Three samples were stored at 4°C, and three samples were kept at 70°C for 16 hours. An aliquot was withdrawn after 0 (baseline sample), 2, 4, 8 and 16 hours of storage at 4°C and 70°C. The UV-visible absorbance of each sample was determined in duplicate at 242 nm using a spectrophotometer (UV mini 1240, Shimadzu, Japan).
An aliquot withdrawn from each elastomeric pump was diluted with Milli-Q water to obtain the final concentration of 100 µg/mL. Then the aliquot was subjected to spectrophotometric analysis in duplicate within 30 min of sampling. The change in colour of ceftaroline–NS or ceftaroline–glucose 5% admixture stored at different temperatures for various time points was determined by comparing the UV-visible absorbance of baseline samples with the absorbance values obtained after spectrophotometric analysis of ceftaroline samples. A negative control was prepared using either NS (n=3) or glucose 5% (n=3) solution only.
The absorbance values obtained after spectrophotometric analysis of ceftaroline standard solution before and after storage at 4°C and 70°C for various time points or ceftaroline sample stored at four different temperatures for various time points or negative control solution before and after storage at 35°C for 12 hours were compared statistically by paired t-test. p value of <0.05 was considered statistically significant.
Results
Stability indicating capability of the newly developed HPLC method
Chromatographic profiles of stressed and unstressed samples (100 µg/mL) are shown in figure 1. The peak of ceftaroline was eluted at 6.3 min. After exposure to 0.003 M sodium hydroxide for 10 min, the remaining concentration of ceftaroline left was found to be 80% compared with unstressed samples, with two degradation peaks were eluted at 6.6 and 6.8 min. Under thermal stress, ceftaroline lost 22% of its initial concentration after 5 hours of incubation, with appearance of two degradation peaks at 3.0 and 6.8 min, respectively. After exposure to 1 M hydrochloric acid for 24 hours, the concentration of ceftaroline was decreased by 70% and four degradation peaks were eluted at 2.4, 3.0, 6.6 and 6.8 min. Importantly, no interference was observed between the peaks from degradation products and the peak of ceftaroline, suggesting the developed HPLC method is capable of investigating the chemical stability of ceftaroline.
Figure 1.
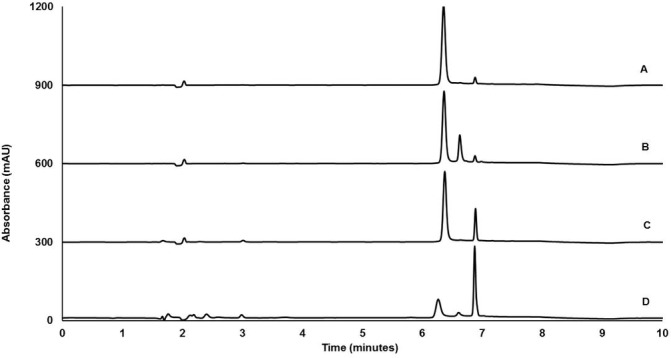
Chromatograms of ceftaroline before (A) and after stressing under basic (B), thermal (C) and acidic (D) conditions.
HPLC assay performance
Determination coefficient (r2), used to assess linearity, at five different concentrations (25, 50, 100, 200 and 300 µg/mL) over 5-day period was found to be greater than 0.999. The intraday and interday precision relative standard deviation (RSD) at three different concentrations (25, 100 and 300 µg/mL) were found to be 0.3% and 0.2%, respectively. The intraday and interday accuracy RSD values at the level of 25, 100 and 300 µg/mL were less than 0.4% and 0.2%, respectively. The intraday and interday retention time RSD were less than 0.15% and 0.4%, respectively.
Chemical stability of ceftaroline
At time 0, the average concentration (±SD) of ceftaroline admixed with NS and glucose 5% was found to be 5.70±0.02 mg/mL and 5.63±0.01 mg/mL, respectively. These concentrations were considered as 100%. The percentage of ceftaroline concentration remaining at different temperatures at various time points are shown in table 1. The remaining concentration of ceftaroline in NS after 6 days (144 hours) of storage at 4°C was found to be more than 98%. Ceftaroline when prepared in glucose 5% retained more than 99% of its initial concentration at 4°C for 6 days. At 25°C, ceftaroline retained more than 90% of its initial concentration for at least 24 hours when admixed with NS or glucose 5%. Ceftaroline–NS admixture retained more than 94% of ceftaroline initial concentration for 12 hours at either 30°C or 35°C. However, it lost more than 12% of its initial concentration after 24 hours of storage at either 30°C or 35°C. When admixed with glucose 5%, ceftaroline lost less than 10% and more than 19% of its initial concentration after 12 hours of storage at 30°C or 35°C, respectively.
Table 1.
The percentage of ceftaroline concentration remaining in the elastomeric pumps stored at various temperatures for different time intervals. Results are presented as mean±SD
| Time (hours) | % Initial drug concentration remaining (mean±SD) | |||||||
| 4°C | 25°C | 30°C | 35°C | |||||
| NS | Glucose 5% | NS | Glucose 5% | NS | Glucose 5% | NS | Glucose 5% | |
| 0.5 | - | - | 97.7±0.4 | 98.9±0.6 | 98.9±0.5 | 99.9±0.5 | 99.7±0.5 | 98.9±0.2 |
| 1 | - | - | 99.6±0.6 | 98.5±0.7 | 98.5±0.4 | 99.7±0.5 | 99.3±1.1 | 97.7±0.6 |
| 2 | 99.7±0.5 | 99.8±0.9 | 99.6±0.7 | 97.5±0.5 | 98.4±0.2 | 98.1±0.4 | 99.3±0.2 | 96.6±0.3 |
| 6 | 99.6±0.4 | 101±0.3 | 98.1±0.8 | 96.4±0.4 | 96.9±0.5 | 94.4±0.8 | 96.1±0.4 | 92.4±0.2 |
| 12 | - | - | 95.2±0.2 | 93.0±0.2 | 94.8±0.5 | 90.9±0.2 | 94.3±0.8 | 80.2±0.3 |
| 24 | 98.9±0.2 | 100±0.8 | 90.1±0.1 | 90.2±0.2 | 85.0±0.2 | 80.9±0.5 | 87.3±0.4 | 70.2±0.3 |
| 48 | 98.8±0.3 | 100±0.5 | - | - | - | - | - | - |
| 72 | 98.3±0.5 | 99.8±0.4 | - | - | - | - | - | - |
| 96 | 98.5±0.5 | 100±0.5 | - | - | - | - | - | - |
| 120 | 98.6±0.3 | 100±0.5 | - | - | - | - | - | - |
| 144 | 98.4±0.5 | 101±0.9 | - | - | - | - | - | - |
NS, Normal saline.
Physical stability of ceftaroline
The pH of ceftaroline when admixed with either NS or glucose 5% was found to be 5.27±0.1 (mean±SD) or 5.42±0.2. The pH of the admixtures remained essentially unchanged at four different storage temperatures for the duration of study. Microscopic images of positive and negative controls are shown in figure 2. The estimated number of particles in the positive control was found to be 1500 particles/mL. Microscopic analysis did not show the presence of particles in samples withdrawn from elastomeric pumps containing ceftaroline stored at the four tested temperatures for various time points.
Figure 2.
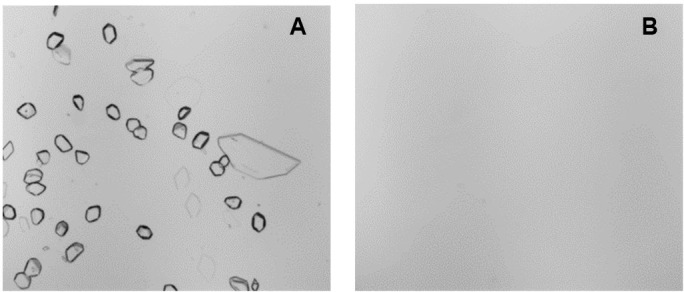
Microscopic images of positive control (A) and negative control (B) samples.
There was no significant change in the absorbance of ceftaroline standards when stored at 4°C for 16 hours. However, when stored at 70°C, a significant change in the absorbance was observed suggesting the used spectrophotometric method is suitable for the analysis of colour change in samples containing ceftaroline. The spectrophotometric analysis showed no significant change in UV-visible absorbance when the ceftaroline samples were stored at 4°C and 25°C. However, significant change in the absorbance was observed when the ceftaroline–NS samples were stored at 30°C and 35°C for 24 hours. Similarly, a significant change in absorbance was noted when the ceftaroline–glucose 5% samples were stored at 30°C and 35°C for 24 and 12 hours, respectively. Glucose solution is known to undergo discolouration at high temperatures. However, no significant change in the absorbance of negative controls was observed when stored at 35°C for 12 hours indicating the change in the absorbance of ceftaroline solution at 35°C for 12 hours was not due to the discolouration of glucose.
Discussion
Patients with endocarditis, osteomyelitis, bacteraemia and infective pulmonary exacerbations in CF caused by MDR bacteria need an extended treatment with parenteral antibiotics and therefore prolonged hospitalisations. This will increase healthcare expenditures and the risk for acquiring nosocomial infections. It is estimated that 1 in 25 patients will develop a nosocomial infection during their hospital stay,17 and the estimated cost for treating a single nosocomial infection is reported to be approximately US$2100.16 Studies conducted in Australia and USA estimated that the annual cost savings associated with the use of elastomeric intravenous pumps can be up to US$500 000.18 19 Furthermore, a prospective study found that patients had improved physical, mental and emotional experience when they were transferred from the hospital-based treatment to home-based treatment.20 Ceftaroline is effective in the treatment of infections caused by MDR Gram-positive bacteria.11 In this study, the physical and chemical stability of ceftaroline was investigated in Baxter LV 10 elastomeric pump because of two reasons. One, if ceftaroline–NS or glucose 5% intravenous admixture is found to be stable for 24 hours, then the pump would allow once daily dosing of ceftaroline. Second, this type of elastomeric device is used in many countries around the world including Australia, UK and France. The results of our study indicated that ceftaroline 6 mg/mL is stable when stored in Baxter LV10 elastomeric pump at various temperatures for an extended period of time.
While in use, elastomeric pumps are recommended to be kept inside a belt bag close to the patient’s body for easy mobilisation and better accuracy. Vella-Brincat et al. studied the stability of benzyl penicillin in elastomeric pumps and found that the temperature in elastomeric containing bag can raise to as high as 34.9°C.21 In view of this finding, we conducted the study at 25, 30 and 35°C to replicate a typical hot day scenario where the temperature can elevate beyond 25°C.
Ceftaroline, when admixed with NS, was found to be stable for 24 hours at 25°C, and for 12 hours at 30°C and 35°C. Therefore, ceftaroline can be administered as a 24 hours continuous infusion using one pump per day when the ambient temperatures do not exceed 25°C (eg, during winter season). Alternatively, ceftaroline can be infused over a 12-hour period when the ambient temperature is beyond 25°C. Infusion of ceftaroline over a period of 12 or 24 hours can provide two important advantages. First, extended infusion would minimise the risk of intravenous line-associated microbial contamination. Second, continuous infusion can result in a better bacterial killing rate and hence improved clinical efficacy because bactericidal activity will continue as long as the plasma concentration is greater than the minimum inhibitory concentration.22
The current study found that ceftaroline in elastomeric pumps is stable for at least 6 days under refrigerated conditions when admixed with either NS or glucose 5%. This crucial information would allow healthcare professionals to prepare and supply elastomeric pumps containing ceftaroline in advance. This would avoid the need for patient’s caregivers or nurses travelling to the hospital or patient’s home on a daily basis to collect or deliver the required elastomeric pumps. Also, this approach would save nursing time and avoid delays in the timely administration of antibiotics allowing the allocation of valuable human resources to other competing priorities such as managing complex patients with acute health issues requiring close clinical follow-up by medical staff. Not to mention the cost savings from early discharge and reallocating stretched nursing staff will result in better utilisation of available resources in the healthcare system. In addition, bulk preparation of elastomeric devices containing the required dose of an antibiotic has the potential for reducing drug wastage and the risk of medication misadventure.23
To the best of our knowledge, only one study has investigated the stability of ceftaroline in elastomeric pumps. However, the study did not investigate the stability of ceftaroline beyond 24 hours at 4°C, and beyond 6 hours at 25°C.24 The lack of extended stability data can cause unnecessary inconvenience because either patients/or their caregivers need to travel to the hospital on a daily basis to collect the required elastomerics. Moreover, the authors examined the stability of ceftaroline at 25°C for 6 hours in elastomeric devices that deliver the entire solution (50 mL) over a period of 1 hour. As such, the study findings mean a thrice daily administration of ceftaroline, making it an inconvenient and costly approach. Ceftaroline is a cephalosporin and its bactericidal activity can be maximised by prolonging the time its concentration is above the minimum inhibitory concentration of bacteria in the blood. Therefore, a prolonged 12 or 24 hours daily infusion of ceftaroline will, at least theoretically, result in better bactericidal killing while minimising the cost and maximising patient comfort. Another limitation of the previous study is the lack of data beyond the room temperature of 25°C, thus limiting the scope of results as the temperature of the admixture in elastomeric pumps can reach close to 35°C during drug administration.
Conclusion
This study supports 12 or 24 hours continuous elastomeric infusion of ceftaroline–NS admixture. This will facilitate early hospital discharge using elastomeric-based administration in clinically stable patients who have been optimised on the antibiotics predischarge, decreasing the risk of nosocomial infections and improving patient’s quality of life.
What this paper adds.
What is already known on this subject
Severe infections such as endocarditis, osteomyelitis and infective pulmonary exacerbations in cystic fibrosis require long-term treatment with parenteral antibiotics and hence prolonged hospitalisation.
Continuous infusion of ceftaroline through elastomeric devices can facilitate early hospital discharge by managing parenteral antibiotics in patient’s home.
Physicochemical stability of ceftaroline in elastomeric devices for extended period of time is currently unknown, and therefore such devices cannot be used as an alternative to the hospital-based administration of intravenous ceftaroline.
What this study adds
Ceftaroline admixed with 0.9% sodium chloride is chemically and physically stable for at least 6 days at 4°C, 24 hours at 25°C and 12 hours at both 30°C and 35°C in one of the commonly used elastomeric devices.
These findings will facilitate early hospital discharge of patients requiring prolonged treatment with ceftaroline.
This would reduce the risk of nosocomial infections and improve patients’ quality of life.
Acknowledgments
We would like to thank Israa Khaleel (BPharm, MPharmSc, Division of Pharmacy, School of Medicine, University of Tasmania) for her input with microscopic analysis.
Footnotes
Contributors: Conceived and designed the experiments: RP, STRZ, TW and LCM. Performed the experiments: FAM and RP. Analysed the data: FAM and RP. Wrote the manuscript: FAM, STRZ, RP, TW and LCM.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Draenert R, Seybold U, Grützner E, et al. . Novel antibiotics: are we still in the pre-post-antibiotic era? Infection 2015;43:145–51. 10.1007/s15010-015-0749-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. US department of health and human service. Antibiotic resistance threats in the United States. Med Benefits 2014;31:12. [Google Scholar]
- 3. O`Neill J. Review on antimicrobial resistance Antimicrobial resistance: tackling a crisis for the health and wealth of nations. UK: UK government, 2014. [Google Scholar]
- 4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. 2013. http://www.cdc.gov/drugresistance/threat-report (accessed 22 Oct 2016).
- 5. European Centre for Disease Prevention and Control Antimicrobial Resistance. Antimicrobial resistance surveillance in Europe: annual report of the European antimicrobial resistance surveillance network (EARS-Net). 2012. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/antimicrobial-resistance-surveillance-europe-2012.pdf (accessed 22 Oct 2016).
- 6. Crossley KB, Archer G, Jefferson K, et al. . The staphylococci in human disease. New Jersey, USA: Wiley Online Library, 1997. [Google Scholar]
- 7. Kollef MH. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin Infect Dis 2007;45 (Suppl 3):S191–S195. 10.1086/519470 [DOI] [PubMed] [Google Scholar]
- 8. Endimiani A, Blackford M, Dasenbrook EC, et al. . Emergence of linezolid-resistant Staphylococcus aureus after prolonged treatment of cystic fibrosis patients in Cleveland, Ohio. Antimicrob Agents Chemother 2011;55:1684–92. 10.1128/AAC.01308-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saravolatz L, Pawlak J, Johnson L. In vitro activity of ceftaroline against community-associated methicillin-resistant, vancomycin-intermediate, vancomycin-resistant, and daptomycin-nonsusceptible Staphylococcus aureus isolates. Antimicrob Agents Chemother 2010;54:3027–30. 10.1128/AAC.01516-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. AstraZeneca A. Zinforo 600 mg powder for concentrate for solution for infusion: EU summary of product characteristics. 2012.
- 11. Lin JC, Aung G, Thomas A, et al. . The use of ceftaroline fosamil in methicillin-resistant Staphylococcus aureus endocarditis and deep-seated MRSA infections: a retrospective case series of 10 patients. J Infect Chemother 2013;19:42–9. 10.1007/s10156-012-0449-9 [DOI] [PubMed] [Google Scholar]
- 12. Ho TT, Cadena J, Childs LM, et al. . Methicillin-resistant Staphylococcus aureus bacteraemia and endocarditis treated with ceftaroline salvage therapy. J Antimicrob Chemother 2012;67:1267–70. 10.1093/jac/dks006 [DOI] [PubMed] [Google Scholar]
- 13. Autry EB, Rybak JM, Leung NR, et al. . Pharmacokinetic and Pharmacodynamic Analyses of Ceftaroline in Adults with Cystic Fibrosis. Pharmacotherapy 2016;36:13–18. 10.1002/phar.1681 [DOI] [PubMed] [Google Scholar]
- 14. Liu C, Bayer A, Cosgrove SE, et al. . Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011;52:e18–e55. 10.1093/cid/ciq146 [DOI] [PubMed] [Google Scholar]
- 15. Siefers R, Massey D. Is hospital the best place for patient with infective endocarditis: a review of the literature. B J Cardiac Nursing 2006;5:232–7. [Google Scholar]
- 16. Paladino JA, Poretz D. Outpatient parenteral antimicrobial therapy today. Clin Infect Dis 2010;51 (Suppl 2):S198–S208. 10.1086/653520 [DOI] [PubMed] [Google Scholar]
- 17. Davey PWM, Irving W, Guy Thwaites G, et al. . OPAT: outpatient parenteral antimicrobial therapy. London: Oxford University Press, 2013. [Google Scholar]
- 18. Ashley S, Hajkowicz K, Halford M, et al. . Outpatient parenteral antibiotic therapy: an economic, clinical, and humanistic analysis at a quaternary hospital. Queensland government http://mm2015shpa.com/wp-content/uploads/2015/11/052-Ashley-Sahra-Outpatient-Parenteral-Antibiotic-Therapy-OPAT-an-economic-clinical-and-humanistic-analysis-at-a-quaternary-hospital.pdf (accessed 22 Oct 2016).
- 19. Nathwani D, Barlow GD, Ajdukiewicz K, et al. . Cost-minimization analysis and audit of antibiotic management of bone and joint infections with ambulatory teicoplanin, in-patient care or outpatient oral linezolid therapy. J Antimicrob Chemother 2003;51:391–6. 10.1093/jac/dkg061 [DOI] [PubMed] [Google Scholar]
- 20. Goodfellow AF, Wai AO, Frighetto L, et al. . Quality-of-life assessment in an outpatient parenteral antibiotic program. Ann Pharmacother 2002;36:1851–5. 10.1345/aph.1C153 [DOI] [PubMed] [Google Scholar]
- 21. Vella-Brincat JW, Begg EJ, Gallagher K, et al. . Stability of benzylpenicillin during continuous home intravenous therapy. J Antimicrob Chemother 2004;53:675–7. 10.1093/jac/dkh146 [DOI] [PubMed] [Google Scholar]
- 22. Craig WA. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 2003;17:479–501. 10.1016/S0891-5520(03)00065-5 [DOI] [PubMed] [Google Scholar]
- 23. Cheragi MA, Manoocheri H, Mohammadnejad E, et al. . Types and causes of medication errors from nurse’s viewpoint. Iran J Nurs Midwifery Res 2013;18:228-31. [PMC free article] [PubMed] [Google Scholar]
- 24. Bhattacharya S, Parekh S, Dedhiya M. In-use Stability of Ceftaroline Fosamil in Elastomeric Home Infusion Systems and MINI-BAG Plus Containers. Int J Pharm Compd 2015;19:432–6. [PubMed] [Google Scholar]


