Abstract
Objective
This study is aimed at assessing the stability of dabigatran etexilate (Pradaxa) capsules repackaged into a dose administration aid (DAA), in order to inform appropriate storage conditions that ensure quality. Although Pradaxa is used chronically by patients, and DAAs are known to improve adherence, removal of the capsules from their original packaging is not recommended by the manufacturer due to sensitivity to moisture.
Methods
Pradaxa capsules containing dabigatran etexilate 110 mg were repackaged into a commercially available DAA and stored under ambient conditions (30°C±2°C and 75%±5% relative humidity) for periods of 14 and 28 days and in a domestic refrigerator for 28 days. The capsules were evaluated for changes in their physical appearance and weight. Content uniformity and the drug concentration during dissolution were determined using a validated high-performance liquid chromatography method.
Results
Storage at ambient conditions for 14 and 28 days resulted in a percentage drug remaining of 92.5% and 71.6%, respectively, indicating a lack of compendial compliance (88.4%–111.8%) for the 28-day ambient sample. There was a statistically significant difference (p=0.015) in the dissolution behaviour of the 14-day samples, when compared with control capsules. In contrast, repackaged capsules stored in the refrigerator for 28 days had a drug content of 98.2% and dissolution was not significantly affected (p=0.132).
Conclusion
This study has clearly demonstrated that if repackaging of Pradaxa capsules is required, storage under refrigerated conditions ensures quality for 28 days.
Keywords: Dabigatran etexilate, physicochemical stability, moisturesensitive, humidity, repackaged, multicompartment compliance aid, refrigeration
Introduction
Dabigatran etexilate, an anticoagulant drug that inhibits both free and clot-bound thrombin, is formulated as multilayered pellets, with the drug coated onto a tartaric acid core and enclosed within a hard-shelled capsule. The purpose of this formulation is to ensure that absorption is independent of gastrointestinal activity and unaffected by proton pump inhibitors.1 The stability of the drug product is however impacted by this formulation, due to its susceptibility to moisture2 and the potential for dabigatran etexilate to undergo acid hydrolysis, the rate of which may be accelerated by an increase in temperature.3 Figure 1 presents the chemical structure of dabigatran etexilate showing the ester linkage, which is the preferred site for acid hydrolysis. Recently, regulatory authorities such as the U.S. Food and Drug Administration (FDA) and Australia’s Therapeutic Goods Administration (TGA) have issued safety alerts regarding the risk of moisture exposure and the effect on efficacy.4 5 These agencies, and the manufacturer of dabigatran etexilate capsules (Boehringer Ingelheim),6 have all documented that dabigatran etexilate capsules should not be removed from the original blister pack and repackaged into dose administration aids (DAAs), such as dosette boxes, tablet organisers or weekly medication packs.
Figure 1.
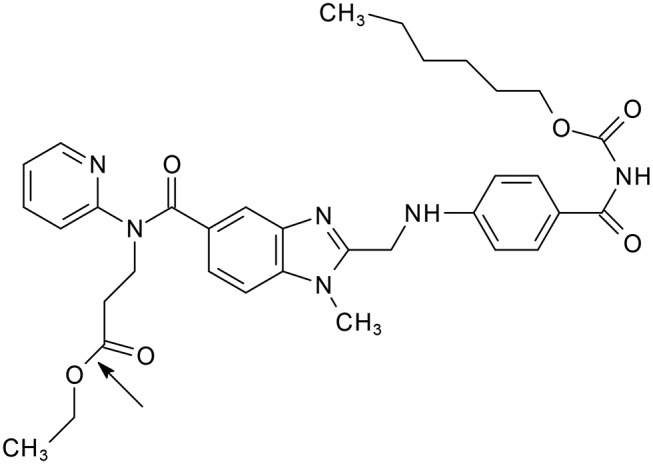
Chemical structure of dabigatran etexilate, showing the site of acid hydrolysis.3
An emerging challenge as a consequence of an ageing population is the increased incidence of chronic disease, the management of which often requires the taking of multiple medications. Compliance aids, also referred to as DAAs or multicompartment compliance aids, are widely employed to manage complex medicine regimes and improve adherence.7 While debate about the effectiveness of such devices continues,8 9 medicine adherence remains difficult for the elderly and the use of compliance aids has increased10 as health authorities attempt to manage this significant public health issue.
Although manufacturers’ packaging is designed to offer protection from exposure to environmental factors, such as light, air (oxygen) and moisture, the repackaging of medicines into these aids invalidates the manufacturer’s stability guarantee11 and the lack of short-term stability data for drugs repackaged into compliance aids is an ongoing challenge for healthcare professionals.12 Drugs that exhibit sensitivity to light are perhaps the simplest to manage as pharmacists can advise patients to store the compliance aid ‘protected from light,12 13 while management of moisture-sensitive medications is more complex. The effect of repackaging on the stability of some moisture-sensitive and hygroscopic medicines, including dabigatran etexilate, has been investigated.14–16 However, the outcomes of repackaging, especially in relation to exposure to moisture, are often difficult to predict as they are complicated both by patients living in different climatic zones, where temperature and relative humidity (RH) can differ significantly, and the fact that the permeability of these aids to moisture is often unknown.17
Studies by Llewelyn et al 15 and Redmayne et al 16 have shown that the physical stability of both immediate-release and enteric-coated sodium valproate, a hygroscopic drug, was compromised when repacked and stored under various ambient conditions. In both cases, however, their chemical stability was confirmed due to a lack of susceptibility of sodium valproate to hydrolysis. When the repackaged enteric-coated tablets were stored in the refrigerator, both the physical and chemical stability was assured for 28 days.16
Wang et al 14, on the other hand, reported that dabigatran etexilate was stable for up to 120 days when stored at room temperature (25°C) in both unit-dose packaging (individual plastic bag) and community pharmacy blister packs despite the susceptibility of dabigatran etexilate to moisture and hydrolysis. The lack of humidity data for this study is noted and may provide an explanation for these positive results.
While moisture may impact the chemical stability of drugs susceptible to hydrolysis, it is also the physical stability in terms of weight gain, and changes in hardness, friability, disintegration and dissolution, which may compromise the physical integrity of the tablets and the efficacy due to altered dissolution behaviour and potentially bioavailability. Findings by Redmayne et al 16 reported the success of storage under refrigerated conditions, highlighting the impact of both temperature and humidity on the stability of a moisture-sensitive drug.
The aim of this study was thus to investigate the physicochemical stability of dabigatran etexilate (Pradaxa) repackaged into a DAA and stored under ambient conditions, specifically at 30°C±2°C and 75%±5% RH and refrigeration (2°C–8°C). These are the long-term stability ambient conditions recommended for climatic zone IVB,18 which includes Indonesia, Singapore and Thailand. While Australia encompasses zones II and IVA, it might be argued that regions in northern Australia are more realistically represented by the hot and very humid conditions of zone IVB. Given the sensitivity of the dabigatran etexilate formulation to moisture, the findings will allow the accurate prediction of stability to any region that experiences hot and very humid weather conditions.
Methods
Materials
Pradaxa capsules (dabigatran etexilate 110 mg; Boehringer Ingelheim) were purchased from a local pharmacy, removed from their blister and repackaged separately into DAAs (Webster-pak Multi Dose Aid; Webstercare). Dabigatran etexilate mesylate powder was kindly donated by Boehringer Ingelheim, Sydney, Australia. High-performance liquid chromatography (HPLC)-grade methanol was purchased from Fischer Scientific, triethylamine (TEA) from Merck and phosphoric acid from Ajax Finechem.
Analytical method
A Varian ProStar system consisting of a 240 solvent delivery module, 410 autosampler and 330 diode array detector was used to quantify the dabigatran etexilate. A Varian Pursuit XRs C8 5 µm (150×4.6 mm) column was maintained at 30°C. An isocratic mobile phase was prepared by mixing water with the required volume of TEA and adjusting the pH to 2.4 with sufficient phosphoric acid. This was added to methanol producing a final composition of 65% methanol/10 mM TEA/35% acidified aqueous solution. A flow rate of 1.3 mL/min was employed, the detection wavelength was set at 340 nm and a 20 µL injection volume was used. All solutions were centrifuged prior to analysis.
Standard solutions were prepared from dabigatran etexilate mesylate powder (>99.5%) at concentrations ranging from 4 to 45 µg/mL dabigatran etexilate diluted with 40% methanol. The method was validated for linearity, precision and accuracy in accordance with ICH guideline Q2(R1).19 Specificity was evaluated using PolyView 2000 software for comparison of ultraviolet (UV) spectra. An acid-stressed sample was produced by dissolving Pradaxa capsule material in 0.1 M hydrochloric acid. The solution was stored at 23°C while protected from light, and during a 24-hour period samples were analysed to view the degradation profile obtained using the HPLC method developed.
Storage conditions
The DAAs were stored at 30°C±2°C and 75%±5% RH for periods of 14 and 28 days. Temperature and humidity control were achieved using a KBF 720 Climatic Chamber (Binder). Capsules were evaluated for changes in weight and physical appearance, content uniformity and dissolution per the respective British Pharmacopoeia (BP) general monographs.20 Additional DAAs were placed in a domestic refrigerator for 28 days and TinytagPlus data loggers recorded the temperature and RH at 5 min intervals. To investigate the effect of refrigerator performance on capsule stability, two studies were performed. In the first study, the door was left ajar for 19 hours on day 2 of the experiment while for the second, refrigerator operation was normal. The above listed tests were performed on capsules from the first study and only weight data were collected from the latter.
Weight uniformity and physical appearance
Twenty capsules selected at random were individually weighed on an AND HM-200 analytical balance. Changes in the physical appearance of the capsules were observed by opening the shell and comparing the contents against a control capsule recently removed from the manufacturer’s blister.
Content uniformity
Six capsules were individually analysed by opening the shell and quantitatively transferring the contents, plus shell, to 150 mL of methanol. After sonication for 20 min, dissolution was complete and the solution was made to a final volume of 200 mL with water. Samples were diluted 1 in 50 with aqueous methanol, centrifuged and the dabigatran etexilate concentration measured by HPLC.
Dissolution
In the absence of a pharmacopoeial dissolution method specific for dabigatran etexilate, a BP Apparatus I (basket apparatus) was used with 900 mL of reverse osmosis water (pH 5.2) as the medium and a final time of 45 min. The tests were performed using a VanKel VK7000 dissolution apparatus with a basket rotation rate of 100 rpm and vessel temperature of 37°C±0.5°C. At timed intervals, 2 mL samples were withdrawn, centrifuged and diluted. The dabigatran etexilate concentration was measured by HPLC. For each test condition, dissolution profiles for n=6 capsules were constructed.
Data analysis
The dissolution results were analysed using a two-tailed Mann-Whitney U test (p=0.05). Calculations were performed with IBM SPSS Statistics V.23.
Results
Validation of analytical method
Dabigatran etexilate eluted at 4.0 min and the identity was confirmed by comparison of both retention time and UV spectra of standard and sample solutions. Peak purity was demonstrated by analysing the UV data across the width of the peak. The principle acid degradation product was found to be more polar, eluting at 2.5 min. There were no overlapping peaks at the wavelength of detection (340 nm). Linearity, precision and accuracy were confirmed and the results are available in online supplementary table S1.
ejhpharm-2017-001224supp001.pdf (74.8KB, pdf)
Weight uniformity
Repackaged capsules increased in weight under all storage conditions (figure 2). The change was greatest for DAAs exposed to 30°C and 75% RH, with the mean capsule weight increasing by 7.2%±0.7% and 10.9%±0.6% at 14 days and 28 days, respectively. For the refrigerator studies, a slight difference was noted between the results at 28 days. A weight increase of 2.6%±0.6% was measured for the study in which the door had been left ajar, while the gain was 1.6%±0.4% when the refrigerator was operated normally. Logger data showed that during the period of impaired performance, 8.8°C±0.2°C and 82%±1% RH were experienced and that under the normal operating conditions 3.08°C±0.02°C and 58%±1% RH were recorded.
Figure 2.
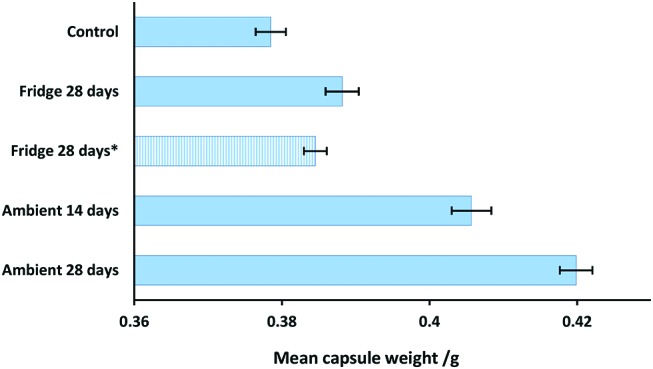
Weight uniformity for capsules stored under various conditions. ‘Control’ refers to capsules recently removed from the manufacturer’s blister and ‘Ambient’ to 30°C and 75% RH.18 Fridge 28 days* indicates duplicate study where refrigerator was allowed to operate normally. Error bars represent 95% CI.
Physical appearance
The external appearance of the capsules remained unchanged for all test conditions. However, opening of the hard shell revealed that the pellets were significantly affected when stored at 30°C/75% RH (figure 3). After 14 days, the contents were not free flowing and instead remained clumped inside the shell. By 28 days, a discoloured gel-like mass was observed. In contrast, after storage in the refrigerator for 28 days, the contents appeared unchanged.
Figure 3.
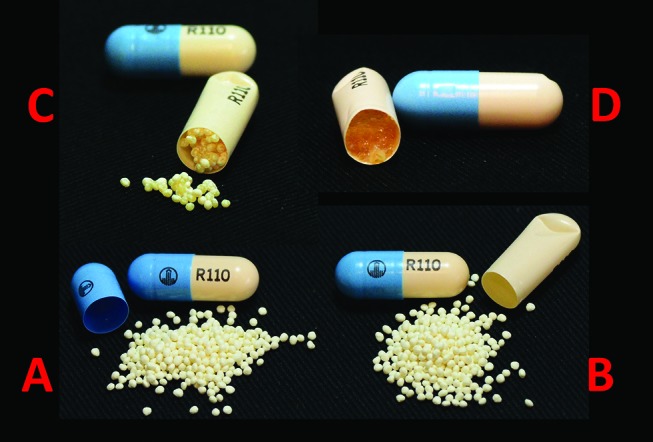
Physical appearance of repackaged capsules stored under various conditions. (A) Control capsule removed from manufacturer’s blister; (B) refrigerated for 28 days; (C) 14 days at 30°C/75% relative humidity (RH); (D) 28 days at 30°C/75% RH.
Content uniformity
Wang et al 14 chose to define stability as the maintenance of at least 90% of the initial drug concentration. In the absence of a BP monograph for dabigatran etexilate, the content requirement limits of 90%–110% were similarly adopted for the current study. Thus, in accordance with the BP general monograph for capsules,21 with 5<n<10 samples the content tolerance was calculated to be 88.4%–111.8%. Table 1 displays the results of the chemical analysis for (n=6) capsules. After 14 days at 30°C and 75% RH, the dabigatran etexilate content had decreased to the lower limit acceptable and after 28 days the capsules clearly are non-compliant with the content requirement.
Table 1.
Dabigatran etexilate content of capsules (n=6) expressed as percentage of nominal content. Error represents 95% CI.
| Condition | Duration | % Dabigatran etexilate |
| Control | t=0 | 100.6±1.2 |
| Domestic refrigerator | 28 days | 98.2±1.4 |
| 30°C/75% RH | 14 days | 92.5±1.6 |
| 30°C/75% RH | 28 days | 71.6±0.8 |
RH, relative humidity.
Dissolution
The dissolution profiles for Pradaxa capsules repackaged and stored in the refrigerator for 28 days were similar to those obtained with control capsules, recently removed from the manufacturer’s blister (figure 4). A Mann-Whitney U test performed on the final dissolution values measured at 45 min found that there was no statistical difference between the two test groups (p=0.132).
Figure 4.
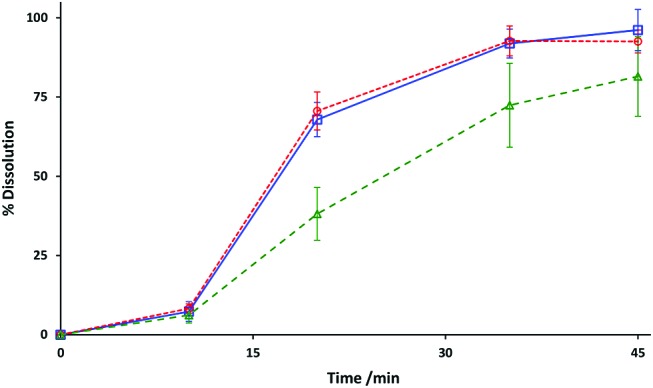
Dissolution profiles after storage under various conditions. Control (blue) and refrigerated (red) results at 28 days expressed as percentage of nominal content. Ambient (green) results at 14 days are displayed as a percentage of measured content. Data points represent mean and 95% CIs for n = 6 tests.
Evaluation of the dissolution results obtained with capsules stored at 30°C/75% RH for 14 days was complicated by the loss of dabigatran etexilate content over this time period (table 1). Data were therefore calculated as a percentage of the measured drug content, rather than the nominal amount. Comparison against the results obtained with control capsules demonstrated that the rate of drug dissolution was slower. Furthermore, it was observed that for the n=6 tests, the dissolution profile for individual capsules was more variable as reflected by the larger CIs in figure 3. The Mann-Whitney U test found that a statistical difference (p=0.015) existed between the 45 min results obtained with control capsules and those repackaged and stored at 30°C/75% RH for 14 days. Dissolution testing was not performed on capsules stored for 28 days due to failure to meet the content requirement.
Discussion
The proposed analytical method for dabigatran etexilate was validated for linearity, precision and accuracy, and proved to be specific, without interference from either tablet excipients or acid degradants. Repackaging and storage at the ambient temperature and humidity conditions selected for the study clearly compromised the physicochemical stability of the dabigatran etexilate 110 mg capsules. After only 14 days, the capsule contents displayed signs of moisture ingress affecting the rate of dissolution and potentially the bioavailability of dabigatran etexilate. These findings are consistent with the warnings, not to remove the capsules from the original packing issued by the FDA, TGA and Boehringer Ingelheim.4–6 It is evident that even brief periods (≤14 days) of exposure to warm and very humid conditions can cause significant deterioration and the lack of physical change in the external appearance of the hard capsule shell can be misleading, especially for patients.
The slower dissolution rate observed after 10 min for capsules stored at 30°C and 75% RH (14 days) corresponded to a period after complete rupture of the hard shell. Thus, the change in physical integrity of the capsule filling, caused by moisture ingress, appears to have adversely affected the release of dabigatran etexilate. This is most likely explained by the reduction in the available surface area for contact with the dissolution medium, due to the agglomeration of the pellets.
The outcome of this study is in contrast with the findings of Wang et al 14 who reported that repackaged Pradaxa capsules were stable for up to 120 days at room temperature (25°C). It seems likely that their experiments were conducted under low humidity conditions, which were not specified. The difference of 5°C between the two studies is not thought to account for the different conclusions as dabigatran etexilate is stable for 6 months at 40°C/75% RH when stored in the original packing.2
Findings from a previous study16 on the repackaging of the hygroscopic drug, sodium valproate (enteric coated), indicating that storage in the refrigerator maintained the physicochemical stability of the drug product in fact informed the design of our study. Storage of the Webster-style DAAs containing Pradaxa capsules in the refrigerator is thus proposed as a satisfactory alternative. Our results have clearly demonstrated that drug content and dissolution behaviour were not compromised even when refrigerator performance was suboptimal and a weight gain of 2.6%±0.6% was measured due to moisture ingress. It is proposed that the rate of dabigatran etexilate hydrolysis was insignificant over the 28-day period due to the low temperature in the refrigerator, slowing down the rate of reaction.
Conclusions
Although it is the recommendation not to repackage dabigatran etexilate by the manufacturer, regulators and in compliance aid guidelines, health professionals are often challenged to provide a solution for patients on multiple medications and/or those that are non-adherent. While the ambient temperature and humidity conditions of climatic zone IVB might not routinely be encountered in many countries, the findings are cautionary for regions that experience short periods of warm and humid conditions, since the physicochemical stability of Pradaxa capsules is compromised within only 14 days. This study therefore identifies the option of storing repacked dabigatran etexilate capsules in the refrigerator, where quality is assured. The findings also make a valuable contribution to the growing body of information on repackaging, namely that although exposure to moisture impacts the stability of dabigatran etexilate, it is the combination of temperature and humidity that compromises the quality of the repackaging process.
What this paper adds.
What is already known on this subject
Medications are increasingly being repackaged into dose administration, especially for patients on chronic medications and the elderly, in order to reduce medication misadventures and improve adherence.
The challenge is that the manufacturer’s stability guarantee does not extend to the repackaged medication and there are limited stability data to support this process.
A previous study on a drug sensitive to moisture has highlighted the option for storage of the dose administration aid (DAA) in the refrigerator.
What this study adds
This study demonstrated that Pradaxa 110 mg capsules maintained their physicochemical stability when repackaged in a DAA and stored in the refrigerator.
These findings add to body of research on the stability of repackaged medications, and in particular for those moisture-sensitive drugs, where repackaging is not recommended, especially under conditions of high humidity which is routinely encountered in the tropics.
Acknowledgments
The authors wish to acknowledge Boehringer Ingelheim (Australian Office) for the donation of the pure dabigatran etexilate mesylate powder used in this study.
Footnotes
Contributors: SGR performed the study and authored the paper. BDG authored the paper.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Hankey GJ, Eikelboom JW. Dabigatran etexilate: a new oral thrombin inhibitor. Circulation 2011;123:1436–50. 10.1161/CIRCULATIONAHA.110.004424 [DOI] [PubMed] [Google Scholar]
- 2. European Medicines Agency. CHMP assessment report for pradaxa EMEA/174363/2008. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Public_assessment_report/human/000829/WC500041062.pdf (accessed 2 Feb 2017).
- 3. Nagadeep J, Kamaraj P, Arthanareeswari M. Gradient RP-HPLC method for the determination of potential impurities in dabigatran etexilate in bulk drug and capsule formulations. Arab J Chem (Published Online First: 9 Oct 2015). 10.1016/j.arabjc.2015.09.006 [DOI] [Google Scholar]
- 4. US Food & Drug Administration. FDA drug safety communication: special storage and handling requirements must be followed for Pradaxa (dabigatran etexilate mesylate) capsules. http://www.fda.gov/Drugs/DrugSafety/ucm248746.htm#sa (accessed 2 Feb 2017).
- 5. Australian Government Therapeutic Goods Administration. Dabigatran (Pradaxa) safety update: information for consumers. https://www.tga.gov.au/alert/dabigatran-pradaxa-safety-update-information-consumers (accessed 2 Feb 2017).
- 6. Boehringer Ingelheim Canada Ltd. Product monograph Pradaxa, date of revision August 11, 2016. https://www.boehringer-ingelheim.ca/sites/ca/files/documents/pradaxapmen.pdf (accessed 2 Feb 2017).
- 7. Nunney J, Raynor D. How are multi-compartment compliance aids used in primary care? Pharm J 2001;267:784–9. [Google Scholar]
- 8. Watson SJ, Aldus CF, Bond C, et al. Systematic review of the health and societal effects of medication organisation devices. BMC Health Serv Res 2016;16:202 10.1186/s12913-016-1446-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edirisinghe S, Raimi-Abraham BT, Gilmartin JF-M, et al. Multi-compartment compliance aids (MCAs): application to the geriatric community. Eur Geriatr Med 2015;6:65–8. 10.1016/j.eurger.2014.05.004 [DOI] [Google Scholar]
- 10. Church C, Smith J. How stable are medicines moved from original packs into compliance aids? Pharm J 2006;276:75–81. [Google Scholar]
- 11. Royal Pharmaceutical Society. Improving patient outcomes. The better use of multi-compartment compliance aids. http://www.rpharms.com/support-pdfs/rps-mca-july-2013.pdf (accessed 2 Feb 2017).
- 12. Haywood A, Glass BD. Evidence of stability of medicines repackaged in compliance aids: a review. Curr Drug Saf 2016;11:69–77. 10.2174/1574886310666150928104931 [DOI] [PubMed] [Google Scholar]
- 13. Yamazaki N, Taya K, Shimokawa K, et al. The most appropriate storage method in unit-dose package and correlation between color change and decomposition rate of aspirin tablets. Int J Pharm 2010;396:105–10. 10.1016/j.ijpharm.2010.06.031 [DOI] [PubMed] [Google Scholar]
- 14. Wang EH, Bolt JL, Décarie D, et al. Stability of dabigatran etexilate in manufacturer’s Blister Pack, Unit-Dose Packaging, and Community Pharmacy Blister Pack. Can J Hosp Pharm 2015;68:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Llewelyn VK, Mangan MF, Glass BD. Stability of sodium valproate tablets repackaged into dose administration aids. J Pharm Pharmacol 2010;62:838–43. 10.1211/jpp.62.07.0004 [DOI] [PubMed] [Google Scholar]
- 16. Redmayne N, Robertson S, Kockler J, et al. Repackaged sodium valproate tablets—meeting quality and adherence to ensure seizure control. Seizure 2015;31:108–11. 10.1016/j.seizure.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 17. Modamio P, Loscertales HR, Braza AJ, et al. Degree of hermeticity of a multicompartment compliance aid: implications for the quality of professional pharmaceutical services : Muñoz-Torrero D, Vázquez-Carrera M, Estelrich J, Recent advances in pharmaceutical sciences IV. Kerala, India: Research Signpost, 2014:133–42. [Google Scholar]
- 18. World Health Organization. Annex 2, stability testing of active pharmaceutical ingredients and finished pharmaceutical products. http://www.gmp-compliance.org/guidemgr/files/WHO_TRS_953_ANNEX2.PDF (accessed 2 Feb 2017).
- 19. ICH Guideline. Validation of analytical procedures: text and methodology. Q2 (R1). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed 2 Feb 2017).
- 20. British Pharmacopoeia Commission. British pharmacopoeia. Volume V London: TSO, 2015. [Google Scholar]
- 21. British Pharmacopoeia Commission. British pharmacopoeia. Volume III London: TSO, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2017-001224supp001.pdf (74.8KB, pdf)


