Abstract
Objectives
To describe allocation of treatment responsibility (ATR) in adolescents with epilepsy, investigate associations between cognitive skills and ATR, and examine whether ATR for antiepileptic drugs (AEDs) predicted electronically monitored adherence.
Method
Sixty adolescents with epilepsy and their caregivers completed the Allocation of Treatment Responsibility Scale and a battery of self-report measures. Medical chart review data and electronically monitored AED adherence were collected for 1 year. Descriptive data assessed ATR for caregivers and adolescents; multivariate hierarchical regressions tested associations between variables.
Results
ATR for labs and clinic appointments was greatest for caregivers, while ATR for AEDs was more likely to be shared between caregiver and adolescent. Poorer attention was associated with greater caregiver responsibility for AEDs. Greater caregiver responsibility for AEDs was associated with higher electronically monitored adherence over 12 months.
Conclusions
In adolescents with epilepsy, caregivers are responsible for most treatment tasks, although responsibility for taking medication was shared with the adolescent. Greater caregiver responsibility for medication results in better long-term AED adherence. ATR is an important construct that warrants further attention in research and clinical practice, especially in the context of transition and health outcomes in pediatric epilepsy.
Keywords: adolescents, epilepsy, medical adherence, self-management
Epilepsy, a condition characterized by recurrent unprovoked seizures, is the most common neurological condition in adolescents (Macleod & Appleton, 2007), affecting approximately 472,000 youth in the United States (Zack & Kobau, 2017). The primary treatment for epilepsy is the use of antiepileptic drugs (AEDs). Unfortunately, nonadherence is a significant problem for youth with epilepsy, with rates around 25% for adolescents (Smith, Mara, & Modi, 2018). Nonadherence can lead to increased seizures (Modi, Wu, Rausch, Peugh, & Glauser, 2014), higher health-care utilization and costs (Faught, Weiner, Guerin, Cunnington, & Duh, 2009), and even death (Faught, Duh, Weiner, Guerin, & Cunnington, 2008). Adolescents with epilepsy may be particularly at risk for AED nonadherence because of a combination of normative adolescent characteristics (e.g., desire for independence, reduced caregiver responsibility for and supervision of medical tasks, normative rebelliousness; Reed-Knight, Blount, & Gilleland, 2014) and epilepsy-specific characteristics (e.g., cognitive and executive functioning difficulties; Hoie et al., 2008).
Several nonmodifiable predictors of adherence have been identified in adolescents with epilepsy, including age (Buck, Jacoby, Baker, & Chadwick, 1997; Samsonsen, Reimers, Brathen, Helde, & Brodtkorb, 2014), time since diagnosis (Aylward, Rausch, & Modi, 2015; Getnet et al., 2016), and socioeconomic status (SES) (Modi, Rausch, & Glauser, 2011; Paschal, Rush, & Sadler, 2014). Modifiable predictors of adherence, such as epilepsy knowledge (Loiselle, Rausch, & Modi, 2015; Carbone, Zebrack, Plegue, Joshi, & Shellhaas, 2013), family functioning (Loiselle, Rausch, & Modi, 2015), and adherence barriers (Modi, Monahan, Daniels, & Glauser, 2010; Jennum, Gyllenborg, & Kjellberg, 2011), have also been found in this population. While epilepsy knowledge, adherence barriers, and family functioning have been targets for adherence interventions to date (Modi, Guilfoyle, Mann, & Rausch, 2016a; Modi, Mann, Urso, & Peugh, 2016b), allocation of treatment responsibility (ATR) has received less attention in pediatric epilepsy. Notably, adherence interventions for other pediatric conditions (e.g., transplant, asthma) frequently target the transfer of treatment responsibility as a mechanism of change (Annunziato et al., 2008; Duncan et al., 2013; Fredericks et al., 2015). Thus, treatment responsibility warrants further examination in pediatric epilepsy given its potential contribution to adherence.
ATR, the degree to which the patient and caregiver(s) share involvement in or accountability for medical management tasks (Pai et al., 2010), is a central component of adherence (Modi et al., 2012; Reed-Knight et al., 2014). Pai and colleagues (2010) described two conceptualizations of ATR. First, ATR can be conceptualized as discrepancies between caregivers’ and children’s reports of treatment responsibility. Studies have found that greater discrepancies in ATR have been linked with lower adherence in pediatric diabetes and kidney transplant (Cameron et al., 2008; Pai et al., 2010). Second, ATR can be examined as the determination of who is taking responsibility for a given task (e.g., predominantly caregiver, shared, or predominantly child). In the context of this second conceptualization, ATR often changes as children and adolescents mature, with caregivers assuming more or less responsibility for given tasks over time. Although the transfer of responsibility from caregiver(s) to adolescent is often considered to be a linear process (e.g., caregiver gradually assumes less responsibility), ATR can fluctuate over time (Modi, Marciel, Slater, Drotar, & Quittner, 2008). For example, an adolescent may initially assume more independence for taking medication, but after encountering challenges (e.g., forgetting, inaccurate doses), caregivers then increase their responsibility for this task (e.g., monitoring medication administration, providing reminders). Further, adolescents may demonstrate varying levels of responsibility depending on the task. As such, an adolescent may be relatively independent taking AEDs but need more help with scheduling medical appointments. Overall, greater caregiver involvement in medical tasks is associated with greater adherence in adolescent populations, including asthma and transplant (Duncan et al., 2013; Gutiérrez-Colina et al., 2016).
ATR has only recently been examined in youth with epilepsy and their families. The only published investigation of ATR in epilepsy (Ryan, Arnett, Pai, & Modi, 2014) validated the use of the ATR Scale caregiver–child discrepancy scores (Pai et al., 2010) in a sample of adolescents with epilepsy. Like many other health populations (e.g., diabetes, HIV; King, Berg, Butner, Butler, & Wiebe, 2014; Naar-King et al., 2009), older adolescents with epilepsy tended to take on more responsibility for AEDs (Ryan et al., 2014). In addition, caregivers and adolescents generally agreed on ATR, as discrepancies between caregiver and adolescent reports were relatively small. Still, there is much to learn about ATR in adolescents with epilepsy. An important next step is to examine where adolescents and their families fall on the continuum of treatment responsibilities to inform interventions for adolescents with epilepsy.
Epilepsy treatment reflects a range of behaviors, including taking AEDs, scheduling medical appointments, obtaining prescription refills, undergoing blood work, and effectively communicating with providers about seizures and side effects. To independently complete these tasks, adolescents must have both tangible resources (e.g., reliable transportation, access to health insurance information) and individual characteristics (e.g., motivation, cognitive skills). In fact, medical responsibilities require adequate cognitive and executive skills (Reed-Knight et al., 2014), which are often impaired in adolescents with epilepsy; approximately 31% have executive functioning deficits (Hoie et al., 2008), 56% have learning disabilities, and 23% have attention-deficit/hyperactivity disorder (ADHD; Russ, Larson, & Halfon, 2012). To be successful in managing medical tasks, an adolescent must be able to concentrate on the task, act in a planful manner, remember important details (e.g., refill dates, scheduling needs), use language effectively, and engage in effective problem-solving (Gutiérrez-Colina et al., 2016; Reed-Knight et al., 2014). For adolescents with epilepsy, it may be particularly critical to examine the role of cognitive skills in disease management.
Cognitive factors have been linked to ATR in diseases other than epilepsy and indicate that caregivers adjust their level of responsibility for their adolescent’s medical tasks based on the adolescent’s cognitive skills. For example, youth with spina bifida who scored lower on tests of intellectual ability and executive function were more likely to have caregivers assume more responsibility for self-management tasks (O’Hara & Holmbeck, 2013; Psihogios, Kolbuck, & Holmbeck, 2015). Further, greater executive function difficulties in pediatric organ transplant recipients were associated with more caregiver reminders for self-care tasks (Gutiérrez-Colina et al., 2016) and less adolescent responsibility (Gutiérrez‐Colina et al., 2017). However, the association between cognitive skills and ATR in pediatric epilepsy is unknown. Determining whether the link between cognitive skills and ATR is present for adolescents with epilepsy will aid in identifying which families struggle to navigate the gradual transfer of responsibility in adolescence, as well as inform the development and refinement of adherence interventions.
The current study had three aims with corresponding a priori hypotheses. First, we examined the level of shared versus independent treatment responsibilities for adolescents with epilepsy using the ATR Scale (Pai et al., 2010). We hypothesized that caregivers would have greater responsibility for managing labs and medical appointments, whereas adolescents would be more likely to share medication responsibility with their caregivers. The second aim was to determine whether cognitive skills predict ATR in adolescents with epilepsy. It was hypothesized that after controlling for key demographic and medical variables, adolescents with greater attention and social problem-solving skills, less hyperactivity, and fewer memory and language concerns would take greater responsibility for treatment compared with adolescents with poorer cognitive skills. Third, this study investigated the association between ATR for AEDs and electronically monitored adherence. It was expected that greater caregiver involvement would be associated with higher adherence across 1 year.
Method
Participants and Procedures
Participants included adolescents aged 13–17 years taking one AED. Adolescents taking multiple AEDs were excluded because of greater epilepsy and regimen complexity, which could negatively affect adherence. All participants had the ability to speak and read English and did not have significant developmental disorders (e.g., autism spectrum disorder, intellectual disability). Patients with ADHD and other common psychological comorbidities (e.g., learning disorders) were not excluded from the sample. Each participant was recruited during a routine epilepsy clinic by trained research assistants who described the overall study timeline and objectives. Adolescents and caregivers provided informed consent/assent and completed questionnaires in clinic or via mail. Both adolescents and caregivers received gift cards for completion of study visits. The current study is a secondary analyses of baseline data derived from a larger longitudinal study (Ryan et al., 2014; Smith, Mara, & Modi, 2018; Smith, Mara, Ollier, Combs, & Modi, 2018; Wagner et al., 2016) that consisted of four time points across 1 year, as well as adherence data across the entire year. The study was approved by the hospital's institutional review board.
A total of 70 families were approached and 10 declined because of being busy or not being interested in research, yielding a participation rate of 86%. Adherence data were available for 48 participants, as data were missing because of study withdrawal (n = 3) or families moving/being lost to follow-up (n = 9). Of the caregivers who participated in the study, 76% were mothers/stepmothers, 22% were fathers, and 2% were aunts. Demographic information for the sample is presented in Table I.
Table I.
Baseline Characteristics (N = 60)
| Variable | M (SD) or % |
|---|---|
| Adolescent age (years) | 15.32 (1.47) |
| Adolescent sex (female) | 65 |
| Adolescent race | |
| White (non-Hispanic) | 78.3 |
| African-American | 15 |
| Other | 6.7 |
| Epilepsy etiology, diagnosis, and syndromes | |
| Localization-related epilepsy | 28.4 |
| Generalized epilepsy | 48.4 |
| Unclassified epilepsy | 23.3 |
| Time since diagnosis (years) | 1.48 (1.88) |
| Had seizure(s) in past year | 83.3 |
| Family Duncan score* | 56.13 (21.18) |
| QOLICE—Memory Subscale | 82.98 (14.64) |
| QOLICE—Language Subscale | 88.64 (13.39) |
| BASC-2-PRS-Attention Problems T-score | 49.21 (8.28) |
| BASC-2-PRS—Hyperactivity T-score | 46.91 (7.90) |
| Social Problem-Solving Inventory Total | 3.05 (0.58) |
| Electronically monitored adherence | 75.00 (25.58) |
Family Duncan scores of 56.13 represent occupations including dieticians, legal assistants, and transportation/ticket agents. BASC-2 = Behavior Assessment Schedule for Children-2; QOLICE = Quality of Life in Childhood Epilepsy Questionnaire.
Measures
Background and Medical Information
Caregivers provided background information, including adolescent age, sex, race, and years since epilepsy diagnosis. Each family’s SES was calculated using the Revised Duncan score (Hauser, 1994; Nakao & Treas, 1992; Stevens & Featherman, 1981), which is an occupation-based score ranging from 15 to 97, with higher scores indicating higher SES.
Seizure occurrence was assessed by medical chart review, which included seizures and suspected seizures recorded by epilepsy providers during clinic visits. To allow for comparison between patients with different seizure types (e.g., absence versus tonic-clonic), seizure occurrence was dichotomized to represent the presence or absence of seizure(s) in the past year.
Allocation of Treatment Responsibility Scale
The ATR Scale (Pai et al., 2010) is a 16-item self-report measure designed to assess who is responsible for various treatment regimen-related tasks for caregivers and patients aged 7–18 years. It includes both patient and caregiver reports. It assesses ATR using a four-point Likert scale (1 = none of the time to 4 = all of the time) for three subscales: oral AEDs (eight items), clinic appointments (five items), and laboratory visits (three items). In addition, an ATR Total score is obtained. Respondents are instructed to first rate themselves on their own level of treatment responsibility for each task and then to rate the caregiver’s or adolescent’s responsibility for each task. The ATR Scale was originally developed for use with pediatric transplant patients and has been adapted for use with pediatric epilepsy patients (Ryan et al., 2014). Prior use of the ATR Scale used separate reports of caregiver responsibility and child responsibility as well as caregiver–child discrepancy scores, such that child-reported responsibility on a given task was subtracted from caregiver-reported responsibility on the task (Pai et al., 2010; Ryan et al., 2014). Support for convergent validity of the ATR has previously been demonstrated by significant correlations in the expected direction between ATR scales and age, electronically monitored medication adherence, and family communication (Pai et al., 2010; Ryan et al., 2014).
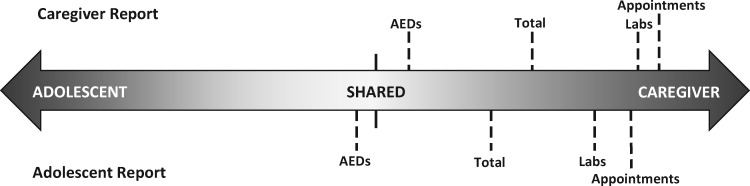
To determine whether caregivers or adolescents were more responsible for a given epilepsy-related task, we modified the scoring method for the ATR. For each reporter and scale, scores were obtained by subtracting ratings of adolescent responsibility from that same reporter’s ratings of caregiver responsibility. Negative values indicate that the adolescent is primarily responsible for the task, positive values indicate that the caregiver is primarily responsible, and values close to 0 indicate equal (i.e., shared) responsibility for the task (see Figure 1). Total possible ranges of scores for each scale were: AED (−24.0 to 24.0), Labs (−9.0 to 9.0), Appointments (−15.0 to 15.0), and Total (−48.0 to 48.0). Internal consistency coefficients ranged from .75 to .89 for subscale scores and .87 to .93 for ATR Total scores.
Figure 1.
Visual depiction of the ATR continuum. For interpretation purposes, ATR scores were converted to a common metric to better understand responsibility across treatment components.
Quality of Life in Childhood Epilepsy Questionnaire
The Quality of Life in Childhood Epilepsy Questionnaire (QOLICE) (Sabaz et al., 2003) is a 79-item caregiver report of their adolescent’s health-related quality of life used for children aged from 4 to 18 years. The measure results in a total score, as well as 15 domains of functioning: Physical Restrictions, Energy and Fatigue, Attention and Concentration, Memory, Language, Other Cognitive, Depression, Anxiety, Control and Helplessness, Self-Esteem, Social Interactions, Social Activities, Stigma, Behavior, and General Health. For the current study, only the Memory and Language scales were used because they represent constructs of cognitive functioning, which were variables of interest. Cronbach’s alphas for the current study were .85 (Memory) and .90 (Language).
Behavior Assessment Schedule for Children-2
The Behavior Assessment Schedule for Children-2 (BASC-2)-PRS (Reynolds & Kamphaus, 2004) is a caregiver-report measure of their adolescent’s behavioral difficulties. It includes multiple scales, including Hyperactivity, Aggression, Conduct Problems, Anxiety, Depression, Somatization, Atypicality, Withdrawal, and Attention Problems. This study used the Attention Problems (α = .82) and Hyperactivity t-scores (α = .71; M of 50 and SD of 10). Higher t-scores indicate greater concerns.
Social Problem-Solving Inventory-Adolescent
The Social Problem-Solving Inventory-Adolescent (SPSI-A) (Frauenknecht & Black, 1995) is a 30-item instrument used to assess problem-solving in adolescents. The adolescent-reported measure uses a five-point Likert scale, ranging from 0 (not at all true of me) to 4 (extremely true of me) across three scales: Automatic Process, Problem Orientation, and Problem-Solving Skills. The total score (α = .93) was used for the present study. Higher scores indicate greater reported problem solving skills and abilities.
Medication Event Monitoring Systems 6 TrackCap
The Medication Event Monitoring Systems (MEMS© 6 Trackcap; AARDEX Corporation, Union City, CA) is an electronic monitoring device that measures daily AED adherence. It includes a bottle and cap that records times and dates of openings. Adherence was defined as the number of doses taken/number of expected doses × 100%. Mean adherence was calculated using daily adherence data over the course of 12 months.
Statistical Analyses
Descriptive data for variables of interest (e.g., ATR subscales and Total Scale) are shown in Table II, and bivariate correlations are presented in Table III. To address Aim 1, Ms, SDs, and ranges were computed for all three ATR subscales and the Total Scale. Paired t-tests were conducted to test for significant differences in caregiver and adolescent report on like measures.
Table II.
Descriptive Data for ATR Subscales and Total Scale
| Caregiver report |
Adolescent report |
t | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ATR Scale | α | Range | Raw M | Raw SD | α | Range | Raw M | Raw SD | |
| AEDs | .79 | −11.0 to 16.0 | 2.29 | 6.14 | .79 | −17.0 to 11.0 | −1.09 | 6.21 | 3.81* |
| Labs | .92 | −9.0 to 9.0 | 7.04 | 4.13 | .94 | −4.0 to 9.0 | 5.80 | 3.81 | 1.48 |
| Appointments | .93 | −14.0 to 15.0 | 12.53 | 6.04 | .95 | −7.0 to 15.0 | 11.33 | 4.64 | 1.06 |
| Total | .86 | −6.0 to 40.0 | 22.57 | 10.01 | .90 | −24.0 to 34.5 | 16.04 | 11.27 | 3.38* |
Note. *p < .01. AED = antiepileptic drug; ATR = allocation of treatment responsibility.
Table III.
Bivariate Correlations Between Study Variables
| Study Variables | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. ATR-AEDC | – | |||||||||||||
| 2. ATR-AEDA | .53** (45) | – | ||||||||||||
| 3. ATR-LabsC | .15 (45) | .04 (45) | – | |||||||||||
| 4. ATR-LabsA | −.06 (45) | .24 (47) | −.01 (45) | – | ||||||||||
| 5. ATR-ApptsC | .12 (45) | .03 (45) | .89** (45) | −.05 (45) | – | |||||||||
| 6. ATR-ApptsA | .13 (45) | .28 (47) | .13 (45) | .64** (47) | .01 (45) | – | ||||||||
| 7. Age | −.28 (45) | −.44** (47) | −.09 (45) | −.10 (47) | −.11 (45) | −.25 (47) | – | |||||||
| 8. Time since diagnosis | .12 (45) | −.11 (47) | .11 (45) | −.35* (47) | .17 (45) | −.24 (47) | .04 (60) | – | ||||||
| 9. Seizures | .01 (45) | .06 (47) | .03 (45) | −.08 (47) | .11 (45) | −.15 (47) | .28* (60) | .38** (60) | – | |||||
| 10. SES | .13 (45) | .18 (47) | −.00 (45) | −.10 (47) | .03 (45) | −.01 (47) | −.25 (54) | .04 (54) | −.07 (54) | – | ||||
| 11. Attention Problems | .42** (44) | .11 (46) | .31* (44) | −.01 (46) | .30* (44) | .02 (46) | −.07 (53) | .01 (53) | .10 (53) | −.34* (51) | – | |||
| 12. Hyperactivity | .144 (44) | −.08 (46) | .15 (44) | −.00 (46) | .17 (44) | −.05 (46) | .30* (53) | −.03 (53) | .15 (53) | −.33* (51) | .51** (53) | – | ||
| 13. Memory | −.30* (44) | −.28 (46) | −.23 (44) | −.35* (46) | −.29 (44) | −.29* (46) | .24 (49) | .06 (49) | .09 (49) | .31* (48) | −.30* (49) | −.15 (49) | – | |
| 14. Language | −.30 (44) | −.15 (46) | −.09 (44) | −.10 (46) | −.22 (44) | −.12 (46) | .15 (49) | .02 (49) | −.04 (49) | .30* (48) | −.39** (49) | −.23 (49) | .77** (49) | – |
| 15. Social problem-solving | −.33* (43) | −.10 (45) | .17 (43) | .00 (45) | −.14 (43) | −.09 (45) | .32* (46) | .00 (46) | .03 (46) | .22 (46) | −.41** (46) | −.21 (46) | .37* (46) | .33* (46) |
Note. *p < .05; **p < .01; C Caregiver report; A Adolescent report; AED = antiepileptic drug; Appts = appointments; ATR = allocation of treatment responsibility; SES = socioeconomic status. Numbers in parentheses indicate n for each correlation.
Missing data ranged between 10 and 33.3% across all analyses variables and were handled via maximum likelihood estimation with auxiliary covariate inclusion because research (Hayes & McArdle, 2017; Shin, Davison, & Long, 2017; Yuan, Yang-Wallentin, & Bentler, 2012) indicates multiple imputation is not recommended at smaller sample sizes. Further, auxiliary correlate variables were included in an analysis only if they (1) were correlated with both the response variable and a binary indicator of response variable missing data (0 = response data observed, 1 = response data missing), at r ≥ .40 (Collins, Schafer, & Kam, 2001; Enders, 2010) and (2) if its inclusion resulted in smaller standard errors versus a model that contained no auxiliary correlate variable information (Enders, 2010; Mazza, Enders, & Ruehlman, 2015). One variable (depressive symptoms) met the first criteria, and was included in one regression analysis (i.e., examining the association between adolescent-reported AED responsibility and 12-month AED adherence) based on the second criteria. All analyses were performed on 5,000 bootstrap replications to address nonnormality and to obtain observed rather than estimated standard errors. Additionally, all analyses were performed using 100 random start values to ensure the estimation converged on the true maximum of the likelihood function and not a localized likelihood function error (Hipp & Bauer, 2006).
To test Aims 2 and 3, multivariate hierarchical regressions were conducted in MPlus (Muthén & Muthén, 1998-2017). Specifically, the three ATR subscales were allowed to correlate and were analyzed simultaneously as dependent variables as follows. A “base model” of demographic and epilepsy-related predictors (i.e., adolescent age, years since diagnosis, seizures in the past year, and SES) served as the comparison model against which a second model with all five cognitive predictors (attention problems, hyperactivity, memory, language, social problem-solving) was compared. Changes in R2 between the two models were assessed for significance using F-tests to determine whether the second model produced a nonzero increase in R2 (indicated as R2Δ). Associations between individual predictors and dependent variables were examined for significance. This process was repeated to test the ATR Total Scale as well as the associations between ATR-AED scores and 12-month adherence, resulting in seven multivariate hierarchical regressions (i.e., three different base models, two models with cognitive predictors, and two models with ATR-AED scores). For N = 60 and assuming α = .05, analyses were powered (.80) to find medium-to-large effects defined as f2 = .24 (or, R2 = .19).
Results
Aim 1: Descriptive Data for the ATR Scales
All caregiver-reported ATR scales yielded positive values, indicating that caregivers reported that they were more responsible for AEDs, appointments, and labs compared with their adolescents. While adolescents’ scores for labs and appointments also suggested greater caregiver responsibility, adolescents reported more personal responsibility for AEDs. Paired t-tests demonstrated that adolescents reported significantly more personal responsibility for AEDs (M = −1.09; t = 3.81, p < .01) and total epilepsy self-management responsibilities (M = 16.04; t = 3.38, p < .01) compared with caregiver report (M = 2.29 and M = 22.57, respectively). Caregiver and adolescent reports of ATR regarding labs and appointments were not statistically different, suggesting agreement on these responsibilities.
Aim 2: Associations Between Cognitive Variables and ATR Scales
Owing to the limited variability in the ATR Labs and Appointments scales (i.e., caregivers were predominantly responsible for these tasks), they were eliminated from subsequent regression analyses.
Demographic and Medical Predictors
In the multiple regression analyses, demographic and medical variables were unrelated to caregiver report of responsibility for AEDs or total responsibilities. In contrast, occurrence of seizures within the past year (B = 4.85, β = .30, p = .029) and lower adolescent age (B = −1.97, β = −.47, p = .011) were related to greater adolescent-reported caregiver responsibility for AEDs. In addition, both younger age (B = −3.05, β = −.41, p = .039) and more recent epilepsy diagnosis (B = −1.73, β = −.30, p = .035) were related to greater adolescent-reported caregiver responsibility for total epilepsy treatment tasks. See Table IV for all regressions related to Aim 2.
Table IV.
Hierarchical Linear Regression Analyses With Demographic and Cognitive Variables Predicting ATR Scales
| Predictors | ATR-AEDs |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
B |
β |
95% CI |
R2 |
95% CI |
B |
β |
95% CI |
R2 |
95% CI |
|
| Caregiver |
Adolescent |
|||||||||
| Demographic predictors | ||||||||||
| Age | −1.25 | −.30 | −.65, .05 | −1.97 | −.47* | −.82, −.12 | ||||
| Time since diagnosis | .44 | .13 | −.18, .44 | −.51 | −.15 | −.43, .13 | ||||
| Seizures | .93 | .06 | −.21, .33 | 4.85 | .30* | .03, .57 | ||||
| SES | .02 | .07 | −.31, .45 | .12 | −.02, .26 | .02 | .07 | −.29, .43 | .25 | .08, .42 |
| Cognitive predictors | ||||||||||
| Attention problems | .31 | .40** | .14, .66 | .10 | .13 | −.25, .51 | ||||
| Hyperactivity | .06 | .07 | −.27, .41 | .04 | .04 | −.33, .42 | ||||
| Memory | −.11 | −.26 | −.73, .21 | −.20 | −.49* | −.93, −.04 | ||||
| Language | −.01 | −.03 | −.43, .37 | .10 | .21 | −.23, .65 | ||||
| Social problem-solving | −2.01 | −.19 | −.56, .18 | .46 | .31, .61 | 1.04 | .10 | −.29, .49 | .36 | .20, .52 |
| Cohen’s f2 | Cohen’s f2 | |||||||||
| ΔR2 | .34* | .63 | .11 | .17 | ||||||
| ATR—Total | ||||||||||
| Demographic predictors | ||||||||||
| Age | 2.17 | −.26 | −.68, .16 | −3.05 | −.41* | −.77, −.06 | ||||
| Time since diagnosis | 1.11 | .17 | −.05, .39 | −1.73 | −.30* | −.63, .04 | ||||
| Seizures | 2.54 | .08 | −.16, .32 | 6.06 | .21 | −.13, .54 | ||||
| SES | .01 | .02 | −.26, .30 | .10 | −.03, .23 | −.02 | −.04 | −.43, .36 | .21 | .04, .38 |
| Cognitive predictors | ||||||||||
| Attention problems | .72 | .47* | .10, .83 | .06 | .05 | −.24, .34 | ||||
| Hyperactivity | .25 | .16 | −.14, .45 | .00 | .00 | −.36, .36 | ||||
| Memory | −.41 | −.49* | −.86, −.12 | −.49 | −.67** | −1.07, −.28 | ||||
| Language | .19 | .21 | −.14, .55 | .28 | .35 | −.01, .71 | ||||
| Social problem-solving | −1.70 | −.08 | −.47, .31 | .50 | .35, .65 | .24 | .12 | −.23, .47 | .61 | .48, .74 |
| Cohen’s f2 | Cohen’s f2 | |||||||||
| ΔR2 | .40** | .81 | .40** | 1.04 | ||||||
Note. *p < .05; **p < .01. Cohen’s f2 small, medium, and large effects are interpreted as .02, .15, and .35, respectively (Cohen, 1992). AED = antiepileptic drug; ATR = allocation of treatment responsibility; CI = confidence interval; SES = socioeconomic status.
Antiepileptic Drugs
Regarding the AED subscale, greater attention problems were related to greater caregiver-reported caregiver responsibility (B = .31, β = .40, p = .002). Further, poorer memory skills were associated with greater caregiver responsibility by adolescent report (B = −.20, β = −.49, p = .029). No other cognitive variables were associated with caregiver- or adolescent-reported responsibility for AEDs. The cognitive variables as a whole accounted for significantly greater variance in caregiver-reported AED responsibility (R2Δ = .34, F(5, 54) = 3.24, p = .013), above and beyond demographic and medical predictors. Cognitive variables did not explain significantly more variability for adolescent report of AED responsibility.
Total Responsibilities
When the ATR Total Scale was examined, more attention problems (B = .72, β = .47, p = .036) and poorer memory abilities (B = −.41, β = −.49, p = .017) were associated with greater caregiver-reported caregiver responsibilities. Likewise, poorer memory abilities were also related to greater adolescent-reported caregiver responsibility for epilepsy treatment (B = −.49, β = −.67, p = .017). Altogether, the cognitive variables explained significant variance in the ATR-Total score beyond demographic and medical predictors (caregiver report: R2Δ = .40, F(5, 54) = 4.13, p = .003; adolescent report: R2Δ = .40, F(5, 54) = 5.31, p < .001).
Aim 3: Predictors of AED Adherence
SES was the only significant demographic and medical predictor of 12-month electronically monitored adherence. Specifically, higher SES was associated with higher adherence, (B = .42, β = .35, p = .015). Greater caregiver responsibility for AEDs reported by adolescents at baseline was associated with higher 12-month adherence (B = 1.98, β = .46, p = .004).
Discussion
Overall, caregivers of adolescents with epilepsy assume a significant role in treatment tasks, including clinic appointments, obtaining laboratory tests, and taking AEDs. Our data suggest that attention and memory issues may be particularly important for determining the balance of responsibility for AEDs and overall treatment responsibility in adolescents with epilepsy. More caregiver involvement in treatment-related tasks by adolescent report was related to higher 1-year AED adherence in this sample, indicating that caregivers may adapt their level of involvement according to their child’s cognitive skills. Results mirror those from pediatric transplant, diabetes, and spina bifida (Gutiérrez-Colina et al., 2017; King et al., 2014; O’Hara & Holmbeck, 2013).
Unlike previous usage of the ATR Scale that featured caregiver–child discrepancy scores (Pai et al., 2010; Ryan et al., 2014), the current study presents an alternative scoring approach for examining ATR in adolescents with epilepsy. Compared with forced choice response formats on other measures, including the Diabetes Family Responsibility Questionnaire (Anderson, Auslander, Jung, Miller, & Santiago, 1990) and Allocation of Responsibility Survey (Bilhartz et al., 2015), our approach allowed for scores that did not elicit social desirability for respondents (i.e., reporters rated caregiver and adolescent responsibility separately without having to choose the most responsible person). In fact, our scoring method conceptualizes ATR on a continuum, which is similar to the Perspectives on Adolescent Decision-Making Questionnaire (PADM; Bosma et al., 1996), a measure of adolescent autonomy in the context of everyday tasks that uses a five-point Likert-type scale. In place of caregiver–child discrepancies, use of a continuum of ATR (ranging from sole responsibility by caregiver to adolescent, with shared responsibility as a midpoint) allowed for identification of primary responsibility.
The present scoring system also simplified the ability to examine predictors and outcomes by collapsing four scores (i.e., adolescent-reported adolescent and caregiver responsibility, caregiver-reported adolescent and caregiver responsibility) into two scores. In doing so, we were able to more easily determine who was responsible for a given responsibility; by subtracting adolescent responsibility from caregiver responsibility, there is a greater context for ATR that is not easily apparent when examining caregiver and adolescent responsibility scores individually. However, one limitation of our scoring method is the inability to demonstrate convergent validity with other measures of adolescent responsibility. In the future, we recommend that the scoring method described here is compared with like measures of ATR or autonomy, such as the PADM. Altogether, our alternative scoring method yielded valuable insight to examining treatment responsibilities for adolescents with epilepsy and their caregivers; other researchers may find utility in adopting a similar scoring method in studies of youth with chronic health conditions.
Both respondents indicated that caregivers are more responsible for labs and appointments, which is expected given that caregivers are typically responsible for managing transportation, family schedules, and navigating health insurance. Further, health-care providers typically contact caregivers for scheduling purposes, which removes the opportunity for adolescents to participate and develop skills in these aspects of treatment. Compared with lab and clinic appointments, caregivers and adolescents were more likely to report that AED responsibilities were shared (see Figure 1). In fact, adolescents reported that they were significantly more responsible for AEDs compared with their caregivers. Taking AEDs does not require external resources (e.g., transportation), allowing adolescents more opportunities to engage in and exercise autonomy for this particular treatment task. In addition, because AEDs are taken daily, adolescents can practice this behavior in the context of their daily lives. For example, adolescents may spend the night at a friend’s house and are thus required to manage their AED more independently; in contrast, adolescents rarely attend clinic appointments without caregivers. Our data also support that as adolescents grow older and have more increased independence, they assume more responsibility for their AEDs and overall epilepsy management responsibilities, which is consistent with the larger pediatric literature (King et al., 2014; Modi et al., 2008; Naar-King et al., 2009).
Consistent with our hypothesis, attention and memory were significant predictors of ATR scores. Specifically, attention problems were related to greater caregiver responsibility for AEDs and overall epilepsy management (by caregiver report); poorer memory was associated with greater caregiver responsibility for medications (by adolescent report) and overall epilepsy management (across both reporters). Attention is a necessary skill to manage AEDs, as adolescents need to shift attention from competing activities (e.g., homework, sports, video games) to take their AEDs, follow through on taking the AED, and monitor the times they take AEDs. Memory skills are also important for sufficient self-management. In fact, forgetfulness is often identified as the primary barrier to treatment adherence in youth (Asato et al., 2009). Studies in pediatric diabetes, spina bifida, and transplant (Gutiérrez-Colina et al., 2016; Gutiérrez-Colina et al., 2017; O’Hara & Holmbeck, 2013; Turner, Berg, Butner, & Wiebe, 2018) have suggested that executive functioning difficulties, including memory and attention, negatively affect self-management and adherence behaviors. Of note, the association between attention problems and greater caregiver responsibility for AEDs was not present for adolescent report of ATR. It is possible that adolescents with more attention problems may be less aware of the help provided by their caregivers in managing medications. Alternatively, caregiver-reported attention problems may not be accurate reflections of attention problems for all adolescents with epilepsy. Further, several cognitive characteristics (i.e., hyperactivity, language, social problem-solving) were not significantly related to ATR for AEDs or overall epilepsy management. These cognitive characteristics may be less salient when families are deciding who is responsible for treatment tasks.
Similar to other studies of AED adherence in youth with epilepsy (Modi, Rausch, & Glauser, 2011), SES was a significant predictor of AED adherence. In addition, adolescent report of ATR was associated with AED adherence over the course of 1 year, such that adolescents who had greater caregiver oversight of AEDs were more likely to be adherent to their AED. This finding reinforces the importance of caregiver involvement for adherence during adolescence that has been demonstrated across pediatric populations (Duncan et al., 2013; Gutiérrez-Colina et al., 2016; Psihogios et al., 2015; Turner et al., 2018). Parent scaffolding (e.g., building independence while providing support and supervision) of adherence behaviors during adolescence is critical to adolescents establishing good adherence habits, which can affect health outcomes during the transition from pediatric to adult health care. Further, the association between ATR scores and adherence data provides support for convergent validity of the alternative scoring method described here.
Results from this study have clear implications for clinical practice, especially as adolescents with epilepsy plan to transition from pediatric to adult health care. In general, patients with epilepsy are not well prepared for the transition, resulting in discontinuity of care (Geerlings et al., 2015) and continued caregiver involvement (Schultz, 2013). Consistent with recent guidelines for the transition to adult care in epilepsy (Camfield et al., 2012), and evidence that transition clinics result in improved AED adherence (Geerlings, Aldenkamp, Gottmer-Welschen, van Staa, & De Louw, 2016), our results provide continued support for medical providers (e.g., physicians, nurses, behavioral medicine clinicians) to encourage families to determine how to appropriately share responsibility for epilepsy tasks; responsibilities can be gradually transferred as the adolescent shows increasing mastery for a given task.
Moreover, adolescence is the ideal time to practice independent adherence behaviors with caregiver support. For instance, adolescents can make clinic appointments with the aid of their caregivers, allowing for failure without the negative consequences, as caregivers provide a safety net to ensure follow-through. Behavioral interventions that specifically target ATR may be particularly helpful for adolescents and families struggling to adhere to AED regimens; several promising examples (Jantzen et al., 2009; Modi et al., 2016b) have been described. Although often considered a stable factor (Modi et al., 2012), cognitive skills have increasingly been shown to improve through targeted interventions and strategies (Kurowski et al., 2014; Wade, Michaud, & Brown, 2006), including in youth with epilepsy (Fuentes & Kerr, 2017). Interventions that aim to improve executive function skills, such as attention and working memory, may also be valuable in supporting families navigating medical adherence and the sharing of epilepsy responsibilities (Modi et al., 2017).
While this study contributes to the adolescent epilepsy adherence literature, there were several limitations. First, the sample was homogenous (e.g., no adolescents with development disorders) and small with missing data. Additional studies are needed to better understand ATR in more diverse samples of pediatric patients with epilepsy (e.g., youth with more severe epilepsy and associated complex medical regimens, preadolescents, young adults). To minimize missingness, future researchers should ensure completeness of measures at the time of recruitment (via extensive consent/assent procedures) and data collection (via visual review). Second, data were cross-sectional in nature, which precluded our ability to examine ATR over time and the transfer of responsibility between caregivers and adolescents with epilepsy. Third, shared method variance must be acknowledged, although data from multiple reporters were analyzed in an effort to minimize this issue. While the measurement of cognitive variables was limited to caregiver or self-report, which lacks robustness compared with neuropsychological testing, researchers have questioned the real-world validity of neuropsychological testing in daily tasks (e.g., remembering to take medications, being organized, and completing tasks; Toplak, West, & Stanovich, 2013). However, we acknowledge that a more comprehensive assessment of cognitive skills is needed to further research this topic. Future studies may explore whether cognitive skills, particularly executive functioning skills, serve as moderators for the association between ATR for AEDs and adherence. We expect that youth with poor cognitive skills who are given greater responsibility for AEDs would be most at risk for lower adherence. Overall, our data suggest that ATR is an important construct that warrants further research and clinical attention, especially in the context of transition and health outcomes in pediatric epilepsy.
Funding
This work was supported by the Charlotte M. Schmidlapp Women's Scholar Award through Cincinnati Children's Hospital Medical Center (A.M.) and Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (T32HD068233; C.H.).
Conflicts of interest: None declared.
References
- Anderson B., Auslander W. F., Jung K. C., Miller J. P., Santiago J. V. (1990). Assessing family sharing of diabetes responsibilities. Journal of Pediatric Psychology, 15, 477–492. [DOI] [PubMed] [Google Scholar]
- Annunziato R. A., Emre S., Shneider B. L., Dugan C. A., Aytaman Y., McKay M. M., Shemesh E. (2008). Transitioning health care responsibility from caregivers to patient: A pilot study aiming to facilitate medication adherence during this process. Pediatric Transplantation, 12, 309–315. [DOI] [PubMed] [Google Scholar]
- Asato M. R., Manjunath R., Sheth R. D., Phelps S. J., Wheless J. W., Hovinga C. A., Pina-Garza J. E., Haskins L. S., Zingaro W. M. (2009). Adolescent and caregiver experiences with epilepsy. Journal of Child Neurology, 24, 562–571. [DOI] [PubMed] [Google Scholar]
- Aylward B. S., Rausch J. R., Modi A. C. (2015). An examination of 1-year adherence and persistence rates to antiepileptic medication in children with newly diagnosed epilepsy. Journal of pediatric psychology, 401, 66–74. 10.1016/S1059-1311(97)80060-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilhartz J. L., Lopez M. J., Magee J. C., Shieck V. L., Eder S. J., Fredericks E. M. (2015). Assessing allocation of responsibility for health management in pediatric liver transplant recipients. Pediatric Transplantation, 19, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma H. A., Jackson S. E., Zijsling D. H., Zani B., Cicognani E., Xerri M. L., Honess T. M., Charman L. (1996). Who has the final say? Decisions on adolescent behaviour within the family. Journal of Adolescence, 19, 277–291. [DOI] [PubMed] [Google Scholar]
- Buck D., Jacoby A., Baker G. A., Chadwick D. W. (1997). Factors influencing compliance with antiepileptic drug regimes. Seizure, 62, 87–93. 10.1016/S1059-1311(97)80060-X [DOI] [PubMed] [Google Scholar]
- Cameron F. J., Skinner T. C., de Beaufort C. E., Hoey H., Swift P. G., Aanstoot H., Aman J., Martul P., Chiarelli F., Daneman D., Danne T., Dorchy H., Kaprio E. A., Kaufman F., Kocova M., Mortensen H. B., Njølstad P. R., Phillip M., Robertson K. J., Schoenle E. J., Urakami T., Vanelli M., Ackermann R. W., Skovlund S. E.. Hvidoere Study Group on Childhood Diabetes. (2008). Are family factors universally related to metabolic outcomes in adolescents with type 1 diabetes? Diabetic Medicine, 25, 463–468. [DOI] [PubMed] [Google Scholar]
- Camfield P., Camfield C., Pohlmann-Eden B. (2012). Transition from pediatric to adult epilepsy care: A difficult process marked by medical and social crisis. Epilepsy Currents, 12, 13–21. 10.5698/1535-7511-12.4s.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone L., Zebrack B., Plegue M., Joshi S., Shellhaas R. (2013). Treatment adherence among adolescents with epilepsy: What really matters? Epilepsy & Behavior, 271, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1992). A power primer. Psychological Bulletin, 112, 155–159. 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Collins L. M., Schafer J. L., Kam C. M. (2001). A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychological Methods, 6, 330–351. 10.1037/1082-989X.6.4.330 [DOI] [PubMed] [Google Scholar]
- Duncan C. L., Hogan M. B., Tien K. J., Graves M. M., Chorney J. M., Zettler M. D., Koven L., Wilson N. W., Dinakar C., Portnoy J. (2013). Efficacy of a parent–youth teamwork intervention to promote adherence in pediatric asthma. Journal of Pediatric Psychology, 38, 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders C. K. (2010). Applied missing data analysis. New York, NY: Guilford Press. [Google Scholar]
- Faught E., Duh M. S., Weiner J. R., Guerin A., Cunnington M. C. (2008). Nonadherence to antiepileptic drugs and increased mortality: Findings from the RANSOM Study. Neurology, 71, 1572–1578. [DOI] [PubMed] [Google Scholar]
- Faught R. E., Weiner J. R., Guerin A., Cunnington M. C., Duh M. S. (2009). Impact of nonadherence to antiepileptic drugs on health care utilization and costs: Findings from the RANSOM study. Epilepsia, 50, 501–509. [DOI] [PubMed] [Google Scholar]
- Frauenknecht M., Black D. R. (1995). Social Problem-Solving Inventory for Adolescents (SPSI-A): Development and preliminary psychometric evaluation. Journal of Personality Assessment, 64, 522–539. 10.1207/s15327752jpa6403_10 [DOI] [PubMed] [Google Scholar]
- Fredericks E. M., Magee J. C., Eder S. J., Sevecke J. R., Dore-Stites D., Shieck V., Lopez M. J. (2015). Quality improvement targeting adherence during the transition from a pediatric to adult liver transplant clinic. Journal of Clinical Psychology in Medical Settings, 22, 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes A., Kerr E. N. (2017). Maintenance effects of working memory intervention (Cogmed) in children with symptomatic epilepsy. Epilepsy and Behavior, 67, 51–59. 10.1016/j.yebeh.2016.12.016 [DOI] [PubMed] [Google Scholar]
- Geerlings R., Aldenkamp A., Gottmer-Welschen L., van Staa A., De Louw A. (2016). Long-term effects of a multidisciplinary transition intervention from paediatric to adult care in patients with epilepsy. Seizure, 38, 46–53. [DOI] [PubMed] [Google Scholar]
- Geerlings R. P., Aldenkamp A. P., de With P. H., Zinger S., Gottmer-Welschen L. M., de Louw A. J. (2015). Transition to adult medical care for adolescents with epilepsy. Epilepsy and Behavior, 44, 127–135. [DOI] [PubMed] [Google Scholar]
- Getnet A., Woldeyohannes S. M., Bekana L., Mekonen T., Fekadu W., Menberu M., Belete H. (2016). Antiepileptic drug nonadherence and its predictors among people with epilepsy. Behavioural Neurology, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Colina A. M., Eaton C. K., Lee J. L., Reed-Knight B., Loiselle K., Mee L. L., LaMotte J., Liverman R., Blount R. L. (2016). Executive functioning, barriers to adherence, and nonadherence in adolescent and young adult transplant recipients. Journal of Pediatric Psychology, 41, 759–767. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Colina A. M., Reed-Knight B., Eaton C., Lee J., Loiselle Rich K., Mee L., Travers C., Blount R. L. (2017). Transition readiness, adolescent responsibility, and executive functioning among pediatric transplant recipients: Caregivers' perspectives. Pediatric Transplantation, 21, e12898. [DOI] [PubMed] [Google Scholar]
- Hauser R. M. (1994). Measuring socioeconomic status in studies of child development. Child Development, 65, 1541–1545. 10.2307/1131279 [DOI] [PubMed] [Google Scholar]
- Hayes T., McArdle J. J. (2017). Should we impute or should we weight? Examining the performance of two CART-based techniques for addressing missing data in small sample research with nonnormal variables. Computational Statistics and Data Analysis, 115, 35–52. [Google Scholar]
- Hipp J. R., Bauer D. J. (2006). Local solutions in the estimation of growth mixture models. Psychological Methods, 11, 36–53. 10.1037/1082-989X.11.1.36 [DOI] [PubMed] [Google Scholar]
- Hoie B., Sommerfelt K., Waaler P. E., Alsaker F. D., Skeidsvoll H., Mykletun A. (2008). The combined burden of cognitive, executive function, and psychosocial problems in children with epilepsy: Apopulation-based study. Developmental Medicine and Child Neurology, 50, 530–536. [DOI] [PubMed] [Google Scholar]
- Jantzen S., Muller-Godeffroy E., Hallfahrt-Krisl T., Aksu F., Pust B., Kohl B., Redlich A., Sperner J., Thyen U. (2009). FLIP&FLAP—A training programme for children and adolescents with epilepsy, and their parents. Seizure, 18, 478–486. [DOI] [PubMed] [Google Scholar]
- Jennum P., Gyllenborg J., Kjellberg J. (2011). The social and economic consequences of epilepsy: A controlled national study. Epilepsia, 525, 949–956. 10.1111/j.1528-1167.2010.02946.x [DOI] [PubMed] [Google Scholar]
- King P. S., Berg C. A., Butner J., Butler J. M., Wiebe D. J. (2014). Longitudinal trajectories of parental involvement in type 1 diabetes and adolescents' adherence. Health Psychology, 33, 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowski B., Wade S. L., Kirkwood M. W., Brown T. M., Stancin T., Taylor H. G. (2014). Long-term benefits of an early online problem-solving intervention for executive dysfunction after traumatic brain injury in children: A randomized controlled trial. JAMA Pediatrics, 168, 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiselle K., Rausch J. R., Modi A. C. (2015). Behavioral predictors of medication adherence trajectories among youth with newly diagnosed epilepsy. Epilepsy & Behavior, 50, 103–107. 10.1016/j.yebeh.2015.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod S., Appleton R. (2007). Neurological disorders presenting mainly in adolescence. Archives of Disease in Childhood, 92, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza G. L., Enders C. K., Ruehlman L. S. (2015). Addressing item-level missing data: A comparison of proration and full information maximum likelihood estimation. Multivariate Behavioral Research, 50, 504–519. 10.1080/00273171.2015.1068157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A. C., Guilfoyle S. M., Mann K. A., Rausch J. R. (2016a). A pilot randomized controlled clinical trial to improve antiepileptic drug adherence in young children with epilepsy. Epilepsia, 57, e69–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A. C., Mann K. A., Urso L., Peugh J. (2016b). Preliminary feasibility and efficacy of a text and application-based adherence intervention in adolescents with epilepsy. Epilepsy and Behavior, 63, 46–49. [DOI] [PubMed] [Google Scholar]
- Modi A. C., Marciel K. K., Slater S. K., Drotar D., Quittner A. L. (2008). The influence of parental supervision on medical adherence in pre and late-adolescents with cystic fibrosis: Developmental shifts from early to late adolescence. Children’s Health Care, 37, 78–92. [Google Scholar]
- Modi A. C., Monahan S., Daniels D., Glauser T. A. (2010). Development and validation of the pediatric epilepsy medication self-management questionnaire. Epilepsy & Behavior, 181, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A. C., Pai A. L., Hommel K. A., Hood K. K., Cortina S., Hilliard M. E., Guilfoyle S. M., Gray W. N., Drotar D. (2012). Pediatric delf-management: A framework for research, practice, and policy. Pediatrics, 129, e473–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A. C., Rausch J. R., Glauser T. A. (2011). Patterns of non-adherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA, 305, 1669–1676. 10.1001/jama.2011.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A. C., Schmidt M., Smith A. W., Turnier L., Glaser N., Wade S. L. (2017). Development of a web-based executive functioning intervention for adolescents with epilepsy: The epilepsy journey. Epilepsy and Behavior, 72, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A. C., Wu Y. P., Rausch J. R., Peugh J. L., Glauser T. A. (2014). Antiepileptic drug nonadherence predicts pediatric epilepsy seizure outcomes. Neurology, 83, 2085–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L., Muthén B. (1998-2017). Mplus user's guide (8th ed). Los Angeles: Muthén & Muthén. [Google Scholar]
- Naar-King S., Montepiedra G., Nichols S., Farley J., Garvie P. A., Kammerer B., Malee K., Sirois P. A., Storm D., PACTG P1042S Team. (2009). Allocation of family responsibility for illness management in pediatric HIV. Journal of Pediatric Psychology, 34, 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K., Treas J. (1992). The 1989 socioeconomic index of occupations: Construction from the 1989 occupational prestige scores. GSS Methodological Report No. 74. Chicago: National Opinion Research Center.
- O’hara L. K., Holmbeck G. N. (2013). Executive functions and parenting behaviors in association with medical adherence and autonomy among youth with spina bifida. Journal of Pediatric Psychology, 38, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal A. M., Rush S. E., Sadler T. (2014). Factors associated with medication adherence in patients with epilepsy and recommendations for improvement. Epilepsy & Behavior, 31, 346–350. 10.1016/j.yebeh.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Pai A. L., Gray E., Kurivial K., Ross J., Schoborg D., Goebel J. (2010). The allocation of treatment responsibility scale: A novel tool for assessing patient and caregiver management of pediatric medical treatment regimens. Pediatric Transplantation, 14, 993–999. [DOI] [PubMed] [Google Scholar]
- Psihogios A. M., Kolbuck V., Holmbeck G. N. (2015). Condition self-management in pediatric spina bifida: A longitudinal investigation of medical adherence, responsibility-sharing, and independence skills. Journal of Pediatric Psychology, 40, 790–803. 10.1093/jpepsy/jsv044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Knight B., Blount R. L., Gilleland J. (2014). The transition of health care responsibility from parents to youth diagnosed with chronic illness: A developmental systems perspective. Families, Systems, and Health, 32, 219–234. 10.1037/fsh0000039 [DOI] [PubMed] [Google Scholar]
- Reynolds C. R., Kamphaus R. W. (2004). Behavior assessment system for children (2nd ed.). Circle Pines: American Guidance Service, Inc. [Google Scholar]
- Russ S. A., Larson K., Halfon N. (2012). A national profile of childhood epilepsy and seizure disorder. Pediatrics, 129, 256–264. 10.1542/peds.2010-1371 [DOI] [PubMed] [Google Scholar]
- Ryan J. L., Arnett A. D., Pai A. L., Modi A. C. (2014). An examination of the allocation of treatment responsibility scale in adolescents with epilepsy. Epilepsy and Behavior, 41, 1–5. 10.1016/j.yebeh.2014.08.136 [DOI] [PubMed] [Google Scholar]
- Sabaz M., Lawson J. A., Cairns D. R., Duchowny M. S., Resnick T. J., Dean P. M., Bye A. M. (2003). Validation of the quality of life in childhood epilepsy questionnaire in American epilepsy patients. Epilepsy and Behavior, 4, 680–691. [DOI] [PubMed] [Google Scholar]
- Samsonsen C., Reimers A., Bråthen G., Helde G., Brodtkorb E. (2014). Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia, 5511, e125–e128. [DOI] [PubMed] [Google Scholar]
- Schultz R. J. (2013). Parental experiences transitioning their adolescent with epilepsy and cognitive impairments to adult health care. Journal of Pediatric Health Care, 27, 359–366. 10.1016/j.pedhc.2012.03.004 [DOI] [PubMed] [Google Scholar]
- Shin T., Davison M. L., Long J. D. (2017). Maximum likelihood versus multiple imputation for missing data in small longitudinal samples with nonnormality. Psychological Methods, 22, 426–449. 10.1037/met0000094 [DOI] [PubMed] [Google Scholar]
- Smith A. W., Mara C., Ollier S., Combs A., Modi A. C. (2018). Rebellious behaviors in adolescents with epilepsy. Journal of Pediatric Psychology, 43, 52–60. [DOI] [PubMed] [Google Scholar]
- Smith A. W., Mara C., Modi A. (2018). Adherence to antiepileptic drugs in adolescents with epilepsy. Epilepsy and Behavior, 80, 307–311. [DOI] [PubMed] [Google Scholar]
- Stevens G., Featherman D. L. (1981). A revised socioeconomic index of occupational status. Social Science Research, 10, 364–395. 10.1016/0049-089X(81)90011-9 [DOI] [Google Scholar]
- Toplak M. E., West R. F., Stanovich K. E. (2013). Practitioner review: Do performance‐based measures and ratings of executive function assess the same construct? Journal of Child Psychology and Psychiatry, 54, 131–143. [DOI] [PubMed] [Google Scholar]
- Turner S. L., Berg C. A., Butner J. E., Wiebe D. J. (2018). Attention problems as a predictor of type 1 diabetes adherence and metabolic control across adolescence. Journal of Pediatric Psychology, 43, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade S. L., Michaud L., Brown T. M. (2006). Putting the pieces together: Preliminary efficacy of a family problem-solving intervention for children with traumatic brain injury. Journal of Head Trauma Rehabilitation, 21, 57–67. [DOI] [PubMed] [Google Scholar]
- Wagner J. L., Kellermann T., Mueller M., Smith G., Brooks B., Arnett A., Modi A. C. (2016). Development and validation of the NDDI-E-Y: A screening tool for depressive symptoms in pediatric epilepsy. Epilepsia, 57, 1265–1270. [DOI] [PubMed] [Google Scholar]
- Yuan K. H., Yang-Wallentin F., Bentler P. M. (2012). ML versus MI for missing data with violation of distribution conditions. Sociological Methods and Research, 41, 598–629. 10.1177/0049124112460373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack M. M., Kobau R. (2017). National and state estimates of the numbers of adults and children with active epilepsy—United States, 2015. Morbidity and Mortality Weekly Report, 66, 821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]