Abstract
Objective
We previously tested via randomized controlled trial a novel intervention for adolescents with type 1 diabetes and above-target glycemic control that combined web-delivered incentives for self-monitoring of blood glucose (SMBG) and brief web counseling with working memory training and parental contingency contracting training. Results showed improved SMBG and decreased glycosylated hemoglobin (HbA1c) at 6- and 12-month follow-ups. However, it has not been elucidated if improvements in SMBG mediated the immediate benefits of this treatment on HbA1c nor if this intensive intervention uniquely benefited a subgroup of adolescents with higher problems in emotional control.
Methods
Adolescents with type 1 diabetes and above-target glycemic control (n = 61) were randomized to receive the 6-month intervention (n = 30) or usual care (n = 31). Adolescents completed the Behavior Rating Inventory of Executive Function-Self-Report, problems with emotional control subscale at baseline, and provided meter downloads to assess frequency of SMBG and completed an HbA1c blood draw at baseline and 6 months later.
Results
At 6-month follow-up, improvements in SMBG mediated the effects of receiving the treatment on having lower average HbA1c. Further, problems in emotional control moderated the benefits of the intervention on improvements in SMBG and in turn HbA1c. Only adolescents with above average problems in emotional control evidenced improvements in SMBG in response to treatment, which then explained lower HbA1c levels at 6-month follow-up.
Conclusions
This multicomponent, web-delivered intervention provided unique benefits for improving SMBG and lowering HbA1c in teens with higher problems in emotional control.
Keywords: adherence, adolescence, emotion regulation, intervention, type 1 diabetes
Over 75% of adolescents with type 1 diabetes fail to meet the American Diabetes Association clinical guidelines for glycemic control (glycosylated hemoglobin percentage, HbA1c ≤ 7.5%; Wood et al., 2013). A primary reason for adolescents experiencing above-target glycemic control is the challenge associated with maintaining optimal adherence to the complex medical regimen required to manage type 1 diabetes, including multiple daily self-monitoring of blood glucose (SMBG), accurate carbohydrate counting and insulin dosing, and responding effectively to hyper-and hypoglycemia (Silverstein et al., 2005). Adolescent self-regulation, the ability to modulate cognitions, emotions, and behaviors toward long-term goals, is a key individual difference that has been associated with adherence behaviors, including SMBG, and glycemic control (Lansing, Crochiere, Cueto, Wiebe, & Berg, 2017; McNally, Rohan, Pendley, Delamater, & Drotar, 2010; Perez et al., 2016). A multicomponent, web-delivered intervention designed to target biopsychosocial processes in the self-regulation of SMBG in adolescents with above-target glycemic control was developed and tested, showing improvements in SMBG and HbA1c at 6- and 12-month follow-ups (Stanger, Lansing, Scherer, Budney, Christiano, & Casella, 2018). To target psychological, biological, and social processes associated with self-regulation for chronic illness self-management (Lansing & Berg, 2014), this intervention combined incentives for SMBG and brief web counseling (psychological) with working memory training (biological) for adolescents as well as parental contingency contracting training (focused on adolescent SMBG; social).
First, incentives with brief counseling are a behavioral intervention known to improve self-regulation of health behaviors (Sutherland, Christianson, & Leatherman, 2008). Such protocols have been shown to increase medical adherence and engagement in healthy behaviors and reduce substance use (Kurti & Dallery, 2013; Petry et al., 2015; Lansing, Stanger, Budney, Christiano, & Casella, 2016; Stevens, 2014). In the context of type 1 diabetes management in adolescence, multiple studies have found benefits for incentives for SMBG (Petry et al., 2015; Raiff & Dallery, 2010; Stanger et al., 2013; Lansing, Stanger, et al., 2016; Wong et al., 2017). Second, to further enhance the benefits of the intervention on improvements in self-regulation and SMBG, working memory training and parental contingency contracting training, which focused on parental monitoring of adolescent SMBG, were also included. Among children and adolescents with type 1 diabetes, problems in working memory have been associated with poorer adherence and glycemic control (McNally et al., 2010; Ohmann et al., 2010; Perez et al., 2016). In pilot studies of working memory training in adolescents with type 1 diabetes, evidence has suggested that working memory training may be beneficial for improving executive functioning and SMBG (Lansing, Stanger, et al., 2016; Stanger et al., 2018). Third, increased parental monitoring has also been identified as an important intervention target to improve adherence in adolescents with type 1 diabetes (Ellis et al., 2007; Horton, Berg, Butner, & Wiebe, 2009). Each component of this previously tested intervention had a primary target of increasing SMBG with the goal of in turn decreasing HbA1c. Yet, despite the importance of understanding the mechanisms of treatment to facilitate the process of revising and enhancing this pilot intervention, it has not yet been examined if improvements in SMBG did in fact mediate the initial benefits of this multicomponent web-delivered treatment on HbA1c at 6-month follow-up.
Moreover, despite each of the components of this intervention being selected to improve adolescent self-regulation around SMBG, adolescents were not selected into this intervention based on specific types of problems with self-regulation. Thus, it also remains to be seen if there were subgroups of adolescents who particularly benefited from this intervention approach. Specifically, problems with emotional control, that is, regulation of emotion, may be particularly important for understanding and predicting adherence and glycemic control in adolescence (Housiaux, Luminet, Van Broeck, & Dorchy, 2010; Hughes, Berg, & Wiebe, 2012; Lansing, Berg, Butner, & Wiebe, 2016). Beyond the importance of emotional control to diabetes management, two key components of the tested intervention, working memory training and parental contingency contracting, may also be particularly beneficial for adolescents with problems in emotional control. Among individuals without type 1 diabetes, greater working memory capacity has been associated with better ability to regulate negative and positive emotions, including during appraisal of highly emotional stimuli (Brose, Lövdén, & Schmiedek, 2014; Schmeichel, Volokhov, & Demaree, 2008). There is also evidence that one can improve emotional control via working memory training (Schweizer, Grahn, Hampshire, Mobbs, & Dalgleish, 2015). Accordingly, adolescents with above-target glycemic control as well as problems in emotional control may particularly benefit from additional participation in working memory training toward increasing SMBG. In addition, there is also evidence that increased parental monitoring of diabetes management behaviors decreases the impact of problems with emotional control (indexed via delay discounting) on glycemic control (Lansing, Stanger, Crochiere, Carracher, & Budney, 2017). Parental contingency contracting training focused on parental monitoring of SMBG may then also particularly benefit adolescents with higher problems in emotional control. To better understand which adolescents might benefit most from this intervention and support tailoring of the pilot intervention for future research, it was also examined if adolescent problems with emotional control moderated the benefits of this multicomponent intervention on improvements in SMBG and in turn HbA1c at 6-month follow-up.
The goal of this study was to conduct secondary analyses on a previously tested novel intervention combining web-delivered incentives with web counseling, working memory training, and parental contingency contracting training to examine if changes in SMBG-mediated treatment effects on HbA1c at the 6-month follow-up (mediation model) and if that treatment effect was moderated by adolescent problems with emotional control (a moderated mediation model). In the first mediation model, it was hypothesized that when controlling for baseline levels of SMBG and HbA1c, greater improvements in SMBG would mediate the association between receiving the treatment and experiencing lower average HbA1c levels at 6-month follow-up. In the second moderated mediation model, it was theorized that this intervention was particularly well suited to improve SMBG among adolescents experiencing problems with emotional control. Thus, it was also hypothesized that when controlling for baseline levels of SMBG and HbA1c, adolescents with higher problems in emotional control would evidence greater benefits from the treatment in increasing SMBG and, in turn, lower HbA1c at 6-month follow-up compared with adolescents with fewer problems in emotional control.
Methods
Participants
Researchers recruited participants from endocrinology clinics affiliated with a local academic medical center children’s hospital serving both rural and suburban/urban regions in the Northeastern United States. To be included in the study, participants needed to be between 13 and 17 years old and needed to have had type 1 diabetes for over 18 months. The adolescents were only allowed to participate if they had above-target glycemic control: an average HbA1c of ≥8% for the past 6 months, along with an HbA1c of ≥8% as measured at their last clinic visit. Additional inclusion criteria were having a parent or guardian that lived with the participant and could participate in the intervention, as well as having high-speed, broadband Internet at home to make video conferencing possible. Exclusion criteria were pregnancy, severe medical or psychiatric illness, any plans to leave the area within 12 months, or participation in diabetes-related counseling unrelated to the study program.
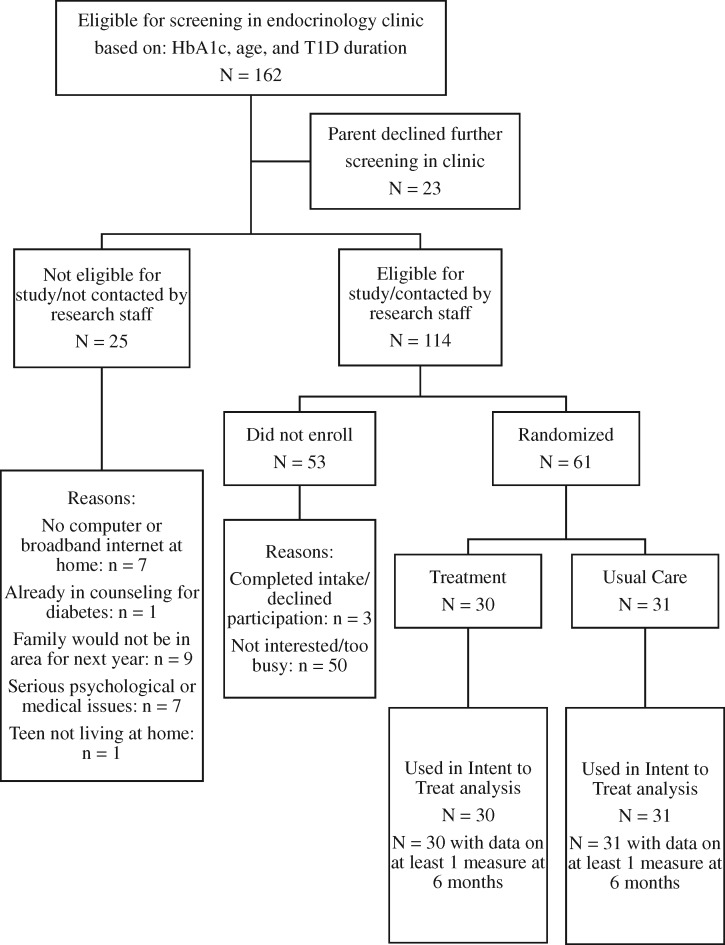
Screening and recruitment are described in detail in Stanger et al. (2018) (all eligibility was assessed by physicians referring patients and confirmed by research staff before enrollment), and the CONSORT table for the trial is included herein (see Figure 1). Sixty-one participants and their parents completed the study’s intake assessment. There were not significant differences in clinical indicators (e.g., HbA1c and type of insurance) between those who participated and declined to participate (see Stanger et al., 2018). This sample size was selected for 80% power to detect a mean difference of 1.5 average daily glucose checks at 6-month follow-up, the primary outcome of the trial. These participants were 43% females with an average age of 15 years. Over half of the participants, 66%, reported using an insulin pump, the average duration of type 1 diabetes diagnosis was 6 years, and their mean HbA1c at intake was 9.1% with an average of four to five blood glucose checks per day. This sample was 97% White, and average SES was 5.4 of 9 on the Hollingshead scale for parental occupation, signifying middle-class status.
Figure 1.
Consort table.
Procedures
At intake, researchers explained the study to adolescents and their parent/guardian. They obtained assent from adolescents and consent from parents. Adolescents and parents completed tasks and questionnaires and provided SMBG data from all meters. A blood draw HbA1c test was also completed. Families were randomized after intake completion. Researchers used computerized minimum likelihood allocation to randomize participants into two groups—usual care (continued care with current pediatric endocrinologist consistent with standards of medical care in diabetes) and intervention. No research staff conducting intakes or participants were aware of allocation until at least 1 day after their intake session, and those individuals were never provided with the allocation rules entered by the principal investigator into the computerized system used by research staff (not therapists) to determine allocations. Further details on randomization are available in Stanger et al., 2018. Adolescents and parents were paid $50 each for the intake assessment. The intervention lasted 25 weeks, and the first follow-up assessment was completed at the end of treatment or at the equivalent time for the usual care group, 6 months later. During this 6-month follow-up, adolescents and parents completed the same tasks and questionnaires and again provided SMBG data from all meters and completed a blood draw HbA1c test. Each teen and each parent was paid an additional $50 for completing the 6-month follow-up. Both intake and follow-up were completed at either the pediatric endocrinology clinic or in an office centrally located in the region. Participants were enrolled between January 2014 and September 2015, and follow-up assessments were completed by September 2016. Adverse events were assessed for at every therapy session and follow-up assessment, and no study-related adverse events occurred. The institution IRB reviewed and approved this study (Clinical Trials # is NCT01722643).
Intervention
All intervention sessions were conducted via HIPAA compliant video conferencing software. The intervention contained the following Internet-based components: incentives with brief web counseling sessions, working memory training, and parent contingency contracting training sessions. Further details about the intervention model and providers’ specialized training and supervision, beyond what is provided below, are available in Stanger et al., 2018. Incentives with brief web counseling and parental contingency contracted were delivered by two master’s level therapists (participants allocated to balance work load and fit with schedules for family) and working memory training by certified working memory training coaches. Fidelity to the treatment model was monitored and coded, and good fidelity was found across the intervention components.
Incentives With Brief Web Counseling
The incentive schedule was composed of three different sections over the 25 weeks. Weeks 1 and 2 were a baseline phase: adolescents received $10 weekly for uploading data from their glucometers. In Weeks 3 through 7, adolescents earned incentives for meeting the self-monitoring goal for SMBG. The goal was five or more checks daily, with checks over 2 hr apart. In these weeks, the goal was 1 more day than the previous week, for a maximum of 5 days per week. Weekly incentives increased by $5 every week, starting from $10 and up to $30 weekly. Finally, in Weeks 8 through 25, the target goal remained consistent at meeting the daily checking goal 5 days per week. In Weeks 1 through 11, participants received the incentive payments weekly. In following weeks, every payment was delayed by 1 more week. So, participants were paid on Weeks 13, 16, 20, and 25. This fading strategy was intended increase resistance to extinction once incentives ended. Adolescents could earn a maximum of $845 for incentives. All incentives were loaded onto prepaid debit cards.
Those incentives overlapped with brief web counseling sessions. In Weeks 1–11, adolescents received 20-min sessions with therapists over video chat. In Weeks 1–5, therapists used motivational exercises to help adolescents in understanding the costs and benefits of changing their behavior, identifying and evaluating alternative actions, and setting goals (Channon et al., 2007). In Weeks 6–12, therapists helped adolescents complete functional analysis to identify antecedents and consequences of completing and not completing self-care behaviors and taught problem-solving and mood management skills. Web counseling sessions were also held at Weeks 13, 16, 20, and 25 to review SMBG and help families problem-solve.
Working Memory Training
Adolescents could earn an additional maximum of $245 for completing 25 working memory training sessions. Working memory training began in Week 3 of the intervention and continued for 5 weeks or until 25 sessions were completed, with five training sessions planned per week. Working memory training was administered through the Cogmed-RM v.2 program. Incentives for completion could total up to $10 per session, with $5 awarded if an adolescent completed all the session’s tasks in 1 day and an additional $5 awarded for either maintaining or improving performance for three of the eight tasks within a session. Research staff provided feedback on adolescents’ task performance and helped motivate them during weekly coaching calls (separate from web counseling sessions).
Parent Contingency Contracting Training
Coordinated with the adolescent incentives and brief web counseling sessions, parents also completed 20-min sessions where they developed and deployed a contingency contract for SMBG. Beginning at Week 11, therapists also asked them to complete a weekly family meeting where they reviewed the SMBG logs and would problem-solve around a specific concern. Along with the parent contingency contracting training sessions, parents also received incentives for reporting adolescents’ daily SMBG frequency and contracting progress. The goal for parents was to report 5 days each week, though otherwise their incentive schedule was the same as adolescents’: $10 in Weeks 1 and 2, from $10 to $30 in following weeks, with delayed incentives after Week 11. Researchers encouraged families to review adolescents’ SMBG weekly by paying both adolescents and parents an extra $5 weekly for this review in Weeks 12–25.
Measures
Emotional Control
To measure problems with emotional control at baseline, adolescents completed the Behavior Rating Inventory of Executive Function-Self-Report (BRIEF-SR), an 80-item measure (Guy, Isquith, & Kenworthy, 2004). This scale has been validated by Gioia et al. (2002) and is an adolescent self-report. The BRIEF-SR contains multiple clinical subscales and the Emotional Control subscale, which indexes executive function problems that affect the modulation and control of emotional responses across 10 items, was used for this study. Factor analysis in clinical adolescent samples supports the Emotional Control subscale as an index of emotion regulation (Gioia et al., 2002). Example items include “I overreact to small problems,” “I become tearful easily,” “I get upset easily,” and “I have angry outbursts.” Higher ratings on the BRIEF-SR emotional control subscale indicate more problems with emotional control. Gender- and age-adjusted t-scores of the BRIEF-SR emotional control subscale were used in analyses. This measure has been validated in other samples of adolescent with type 1 diabetes (Berg et al., 2014; Duke & Harris, 2014) and showed strong internal consistency (α =.91) in this sample.
Self-Monitoring of Blood Glucose
SMBG was the primary target for the intervention’s incentive program. To measure SMBG, participants were asked to bring their blood glucose meters to each assessment. If they forgot these meters, research assistants would obtain their data either through information downloaded through the meter website, or through the parent reading their information over the phone to a staff member. Researchers obtained the number of times adolescents self-monitored their blood glucose over the past 14 days and calculated the average daily frequency of SMBG at baseline and 6-months follow-up.
Glycosylated Hemoglobin
HbA1c was used to measure teens’ glycemic control. Blood samples for HbA1c testing were drawn at the hospital laboratory by hospital lab technicians at intake and at 6-month follow up. The HbA1c level was determined via a Roche immunoassay.
Analyses
The primary aims of this study were to examine if improvements in SMBG mediated the benefits of treatment on lower HbA1c and if those effects were moderated by adolescent problems with emotional control. These aims were tested via baseline corrected structural equation models in Mplus v8.1.5. For all models, fit indices for the overall model included chi-square, comparative fit index (CFI), and root mean square error of approximation (RMSEA). Chi-square p > .05, CFI > .9, and RMSEA < .05 indicated good model fit. Correlated error terms were not modeled for any variables. Bias-corrected bootstrapping with 95% confidence intervals (CIs) was used to test direct, indirect, and total effects, as well as conditional indirect effects. Unstandardized coefficients, standard errors, and confidence intervals are also provided.
Regarding missing data, there was no missing data on problems with emotional control. There was one participant missing HbA1c at 6-month follow-up. This participant was included in all analyses, and full information maximum likelihood estimation was used to account for missing data. There was one participant with outlier values on SMBG (testing nearly 16 times per day at baseline and 17 times per day at follow-up). Given that this checking behavior was nonnormative and well above medical recommendations, with a clear ceiling for improvement, this participant was not included, resulting in N = 60 (n = 29 treatment; n = 31 usual care).
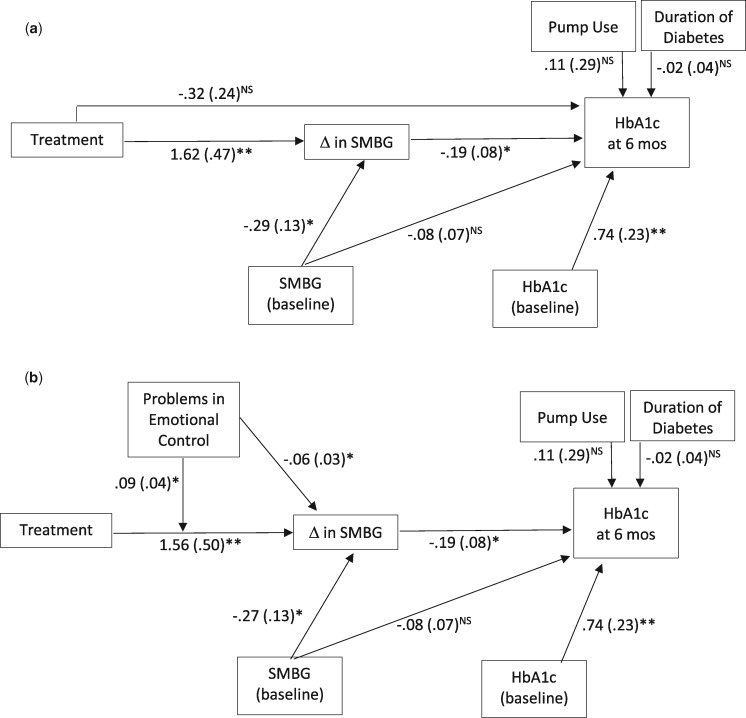
The first mediation model (see Figure 2a) tested if changes in SMBG mediated the association of treatment with level of HbA1c at the 6-month follow-up. Residualized (baseline score as covariate) change scores were used for SMBG, as the primary target of this intervention was to change (increase) frequency of SMBG. Residualized (baseline score as covariate) post-scores for HbA1c at 6-month follow-up (not change scores) were used. Residualized change and post-scores result in the same estimated treatment effects; however, the interpretation of the post-scores results is different, that is, how the two groups differed on average HbA1c at end of treatment. The primary interest of this study was how changes in SMBG in turn explain lower average HbA1c in the treatment group posttreatment, so residualized HbA1c post-scores were used. Both baseline covariates, SMBG and HbA1c at intake, were centered at the grand mean. The only additional predictor of changes in SMBG in the first mode was treatment (0 = usual care, 1= treatment). For Hba1c at 6-month follow-up, the additional predictors included treatment, changes in SMBG, SMBG at baseline (centered at grand mean), and covariates: pump status (0 = no pump; 1= uses a pump) and duration of diabetes in years (centered at grand mean).
Figure 2.
Changes in SMBG mediate the association between the treatment and Hba1c at 6-month follow-up (a) and problems in emotional control moderate those associations (b).
Note. Unstandardized coefficients are presented followed by their standard error in parentheses. In Model (2b), for clarity, the direct effect of treatment on HbA1c at 6 months is not shown, and the coefficient, standard error, and significance for that effect are the same in both models. *p < .05, **p < .01, nsp > .05
The second moderated mediation model (Figure 2b) tested if problems in emotional control moderated the effect of treatment on changes in SMBG, and if that moderated mediation effect in turn explained HbA1c at 6-month follow-up (Preacher, Rucker & Hayes, 2007). The same model as the first was tested with additional predictors. Problems with emotional control (centered at the grand mean) and the treatment by problems with emotional control interaction were also entered as predictors of changes in SMBG. Conditional indirect effects (moderated mediation effects) were examined at 1 SD above and below and at the mean for problems in emotional control.
Results
Primary analyses conducted in the preliminary trial evaluation found no differences in any key study variables at intake between the treatment and control groups (e.g., SMBG at baseline in Treatment was 4.8 times per day, SD = 2.8, and in Usual Care was 4.5 times per day, SD = 2.1; HbA1c in Treatment was 9.1%, SD = 1.0% and in Usual Care was 9.2%, SD = .9%). There were also no baseline differences in problems with emotional control between the treatment and control groups, t(59) = 1.25, p = .22; Usual Care M = 52.84, SD = 13.07 and Treatment M = 48.97, SD = 11.02.
The hypothesis that changes in SMBG would mediate the association of treatment with level of HbA1c at the 6-month follow-up was tested in the first model (Figure 2a). The full model had moderately good fit, with a nonsignificant chi-square test, χ2(3) = 3.60, p = .31, and a CFI above .9 (CFI = .99) but an RMSEA above .05 (RMSEA = .06, 90% CI [0, .23]). This model explained 24% of the variance in changes in SMBG and 50% of the variance in HbA1c at 6-month follow-up. Both lower baseline SMBG and receiving the treatment predicted greater increases in SMBG (baseline SMBG: b = −.29, p = .03, 95% CI [−.53, −.02]); treatment: (b = 1.62, p = .001, 95% CI [.66, 2.54]). The only significant predictors of lower average HbA1c at 6-month follow-up were lower baseline Hba1c and greater increases in SMBG (baseline HbA1c: b = .74, p = .002, 95% CI [.25, 1.13]); changes in SMBG: b = −.19, p = .02, 95% CI [−.39, −.05]). Nonsignificant predictors of HbA1c at 6-month follow-up included treatment, baseline SMBG, pump use, and duration of diagnosis (see figure for parameters). There was a significant total effect of receiving the treatment on HbA1c at 6-month follow-up (total = −.62, SE = .25, 95% CI [−1.16, −.16]). The total effect comprised a significant indirect effect of treatment on HbA1c through changes in SMBG (indirect effect = −.31, SE = .17, 95% CI [−.77, −.06]) and a nonsignificant direct effect of treatment (direct effect = −.32, SE = .24, 95% CI [−.77, .18]). With nonsignificant covariates removed, this pattern of effects remained the same, and there were not changes in the interpretation of findings. These findings suggest that a participant with average baseline SMBG who received the treatment experienced an increase in SMBG frequency of 1.6 times per day, and that improvement explained lower average HbA1c at 6-month follow-up.
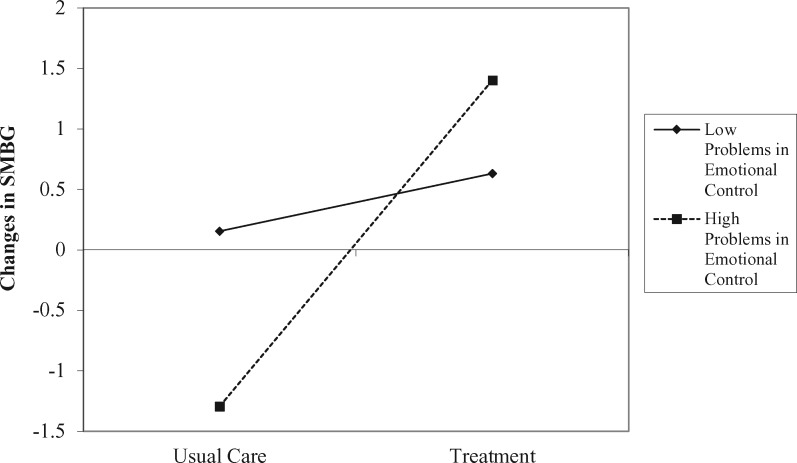
Next, the hypothesis that problems in emotional control would moderate the effect of treatment on changes in SMBG and in turn explain HbA1c at 6-month follow-up was tested (Figure 2b). The full model had good fit, with a nonsignificant chi-square test, χ2(5) = 2.30, p = .81, a CFI above .9 (CFI > .99), and an RMSEA below .05 (RMSEA < .001, 90% CI [0, .11]). This model explained 33% of the variance in changes in SMBG and 51% of the variance in HbA1c at 6-month follow-up. No parameters differed from the first model in predicting HbA1c at 6-month follow-up (i.e., changes in SMBG and baseline HbA1c were the only significant predictors, and all parameter estimates were equivalent between models and removing nonsignificant covariates did not change the pattern of effects or the interpretation of findings). All predictors of changes in SMBG were significant: baseline SMBG (b = −.27, p = .05, 95% CI [−.53, −.02]), treatment (b = 1.59, p = .001, 95% CI [.66, 2.54]), problems in emotional control (b = −.06, p = .02, 95% CI [.66, 2.54]), and treatment by problems with emotional control interaction (b = .09, p = .03, 95% CI [.66, 2.54]). The interaction predicting changes in SMBG is visualized at 1 SD above and below the mean in Figure 3, showing that adolescents with higher problems in emotional control benefited more from treatment on SMBG, simple slope = 2.70, t(5) = 3.70, p =.014, than those with fewer problems in emotional control who showed no benefit, simple slope = .48, t(5) = .69, p = .52.
Figure 3.
The interaction of treatment and problems with emotional control predicting changes in SMBG at 6-month follow-up.
The conditional indirect effects of treatment by problems in emotional control predicting HbA1c at 6-month follow-up via changes in SMBG were also tested within this model. There was a significant indirect effect of treatment on HbA1c at 6-months follow-up via changes in SMBG when problems with emotional control were above average (at mean, indirect effect = −.30, SE = .17, 95% CI [−.75, −.06]; at 1 SD above mean, indirect effect = −.51, SE = .25, 95% CI [−1.12, −.09]). These findings suggest that improvements in SMBG explained the benefits of treatment on HbA1c among adolescents with above average problems in emotional control, with the benefits of treatment more pronounced the higher the problems with emotional control. However, there was no significant indirect effect when problems with emotional control were below average (at 1 SD below mean, indirect effect = −.09, SE = .14, 95% CI [−.52, .10]). There did remain a total effect for treatment beyond mediation by changes in SMBG when problems with emotional control were lower (at 1 SD below mean, total effect = −.41, SE = .22, 95% CI [−.92, −.03]). Thus, the treatment still provided benefit to HbA1c for adolescents with lower problems in emotional control, but not via improved SMBG. Last, compared with the first mediation model above, this moderated mediation model evidenced stronger fit indices and explained greater variance in HbA1c, further supporting the importance of considering emotional control as a moderator of the intervention effects on HbA1c.
Discussion
This study conducted secondary analyses of a web-delivered multicomponent intervention targeting adherence and glycemic control in adolescents with type 1 diabetes to examine both a key mediator (changes in SMBG) and moderator (problems with emotional control) of the previously established benefits. First, it was supported that improvements in SMBG, the primary target of the intervention, mediated the benefits of the treatment on HbA1c. Second, it was also supported that problems with emotional control moderated the effects of the treatment on changes in SMBG and in turn on HbA1c at 6-month follow-up. Adolescents with above average problems with emotional control, but not those with lower problems, experienced greater improvements in SMBG in response to treatment and in turn lower average HbA1c. This study suggests that the web-delivered multicomponent treatment designed to enhance self-regulation was most beneficial for adolescents with higher problems in emotional control.
The finding that changes in SMBG mediated the effects of the treatment on HbA1c at 6-month follow-up was consistent with hypotheses and with the focus of the treatment model on SMBG. For example, adolescent incentives and web counseling directly targeted SMBG, while parent contingency contracting training with incentives focused on parental monitoring of adolescent SMBG. In examining the indirect (via SMBG) and direct effects of the treatment on HbA1c, it is important to note that while only the indirect effect was significant, both effects had similar coefficients (effect size). The direct effect of treatment was nonsignificant because of a larger standard error. Given the smaller sample size in this trial and that it was powered to detect changes in SMBG as the primary outcome and not this secondary mediation analysis, it is possible that a larger replication would show both significant direct and indirect effects and only partial mediation by changes in SMBG. Further research is needed in a larger sample to examine the mediating effects for the components of this intervention (e.g., changes in working memory, parental monitoring) to identify the key components to retain in the final intervention package.
In addition, the hypothesis that problems with emotional control would moderate the benefits of this intervention on changes in SMBG and in turn HbA1c at 6-month follow-up was also supported. Adolescents with above average or greater problems in emotional control benefited from the intervention on improvements in SMBG, while those with below average problems in emotional control did not benefit via improvements in SMBG. The intervention in this study was not specifically targeted at or for adolescents with higher problems in emotional control. However, multiple intervention components, working memory training and parental contingency contracting training, were hypothesized to provide greater benefits in the context of poor emotional control. Thus, the finding was reasonably expected. This research suggests that a multicomponent, biopsychosocial approach to targeting self-regulation and improving glycemic control is likely important for adolescents with higher problems in emotional control. Those adolescents with lower problems in emotional control still benefited from treatment with regards to HbA1c, but via mechanisms outside of improvements in SMBG, and this was not because of an association between lower problems in emotional control and SMBG at baseline (r = .19, p =.16). Further research is needed to clarify which intervention components are most beneficial for the lower problems in emotional control subgroup.
The conclusions drawn from this study must also be considered in the context of limitations. Foremost, the sample size of this pilot randomized controlled trial study was powered a priori to detect outcomes in SMBG, the primary outcome of the study. Thus, it is reasonable that there may be significant effects not found in this study because of increased type 2 error in the post hoc models tested. Replication is needed in a larger sample to address this limitation. In addition, the sample in the study, although consistent with the population of the clinics and surrounding locale, was almost entirely white and largely middle class. This limits the generalizability of the conclusions to more diverse and lower socio-economic status populations. Another limitation of this study was measurement of SMBG, as SMBG at baseline was higher than typically found in adolescent samples (4–5 times per day). A “white coat” phenomenon has been studied in type 1 diabetes where adolescents might evidence improvements in adherence just before study visits (Driscoll et al., 2017). For this study, only the 14 days before the study visit were assessed for SMBG, and future research might benefit from extending the assessment period for SMBG to address possible “white coat” effects. Last, measurement of problems in emotional control was by adolescent report only, and future research would also benefit from using more reporters and perhaps an objective indicator.
There are also multiple recommendations for future research. Foremost, to elaborate on the research questions posed on this article, future research would benefit from the use of a factorial design consistent with the Multiphase Optimization Strategy (Collins, Murphy, Nair, & Strecher, 2005). This type of design allows for highly efficient comparison of effective components in multicomponent interventions and would clarify which components should be maintained in the final intervention package. This design also allows for selection of components based on both improvements in glycemic control and cost-effectiveness, a key issue for complex multicomponent interventions. Future studies should also analyze and collect longer-term outcomes (e.g., 12- and 24-month follow-up) to examine which components of the intervention have the greatest effects on maintenance of improved SMBG and lower HbA1c.
From a clinical implications perspective, the findings of this study suggest that simultaneously targeting biological, psychological, and social processes that affect self-regulation of SMBG, via incentives for adolescents with counseling, cognitive training, and parent training may be effective in improving SMBG and, in turn, glycemic control. This multicompetent incentive-based approach may be especially important to increasing SMBG among adolescents with higher problems in emotional control. It is notable that this subgroup of adolescents still showed greater benefits even though this program did not specifically train emotion regulation skills (e.g., Dialectical Behavior Therapy skills group). This suggests that skills training may not be requisite for enhancing adherence in the context of emotion regulation problems. Also, incentives are a generalizable behavioral approach that can be readily applied to new health behavior skills that these adolescents struggle with as new technologies beyond SMBG continue to emerge in diabetes care (e.g., incentives for continuous glucose monitor adherence). As adolescents with lower problems with emotion control also evidenced benefits from this treatment on glycemic control but not via SMBG, it may be that mechanisms outside of SMBG-focused incentives were more important targets for intervention. For example, parental monitoring has been identified as a target for intervention in adolescents with broad challenges in self-regulation around diabetes management (Wasserman, Hilliard, Schwartz, & Anderson, 2015). Focusing interventions on increasing parental monitoring may be more beneficial in improving glycemic control outside of SMBG for these youths. The use of cognitive training programs to improve self-regulation remains a novel area of research, so although these findings are promising, further work is needed before specific recommendations can be made about integrating cognitive training into clinical practice.
Acknowledgments
Cogmed and Cogmed Working Memory Training are trademarks, in the United States and/or other countries, of Pearson Education, Inc., or its affiliate(s).
Funding
This study was supported by a grant from the National Institute of Child Health and Human Development (DP3 HD076602). Dr. Stanger was also supported by a grant from NIDA- P30 DA029926 (PI: Lisa A. Marsch).
Conflicts of interest: None declared.
References
- Berg C. A., Wiebe D. J., Suchy Y., Hughes A. E., Anderson J. H., Godbey E. I., Butner J., Tucker C., Franchow E. I., Pihlaskari A. K., King P. S., Murray M. A., White P. C. (2014). Individual differences and day-to-day fluctuations in perceived self-regulation associated with daily adherence in late adolescents with type 1 diabetes. Journal of Pediatric Psychology, 39, 1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose A., Lövdén M., Schmiedek F. (2014). Daily fluctuations in positive affect positively co-vary with working memory performance. Emotion, 14, 1–6. [DOI] [PubMed] [Google Scholar]
- Channon S. J., Huws-Thomas M. V., Rollnick S., Hood K., Cannings-John R. L., Rogers C., Gregory J. W. (2007). A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabetes Care, 30, 1390–1395. [DOI] [PubMed] [Google Scholar]
- Collins L. M., Murphy S. A., Nair V. N., Strecher V. J. (2005). A strategy for optimizing and evaluating behavioral interventions. Annals of Behavioral Medicine, 30, 65–73. [DOI] [PubMed] [Google Scholar]
- Driscoll K., Wang Y., Johnson S., Gill E., Wright N., Deeb L. (2017). White coat adherence occurs in adolescents with type 1 diabetes receiving intervention to improve insulin pump adherence dehaviors. Journal of Diabetes Science and Technology, 11, 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke D. C., Harris M. A. (2014). Executive function, adherence, and glycemic control in adolescents with type 1 diabetes. Current Diabetes Reports, 14, 532.. [DOI] [PubMed] [Google Scholar]
- Ellis D. A., Podolski C. L., Frey M., Naar-King S., Wang B., Moltz K. (2007). The role of parental monitoring in adolescent health outcomes: impact on regimen adherence in youth with type 1 diabetes. Journal of Pediatric Psychology, 32, 907–917. [DOI] [PubMed] [Google Scholar]
- Gioia G., Isquith P., Retzlaff P., Esypy K. (2002). Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function in a clinical sample. Child Neuropsychology, 8, 249–257. [DOI] [PubMed] [Google Scholar]
- Guy S. C., Isquith P. K., Kenworthy L. (2004). Behavior rating inventory of executvie function—Self report version. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Horton D., Berg C. A., Butner J., Wiebe D. J. (2009). The role of parental monitoring in metabolic control: Effect on adherence and externalizing behaviors during adolescence. Journal of Pediatric Psychology, 34, 1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housiaux M., Luminet O., Van Broeck N., Dorchy H. (2010). Alexithymia is associated with glycaemic control of children with type 1 diabetes. Diabetes and Metabolism, 36, 455–462. [DOI] [PubMed] [Google Scholar]
- Hughes A. E., Berg C. A., Wiebe D. J. (2012). Emotional processing and self-control in adolescents with type 1 diabetes. Journal of Pediatric Psychology, 37, 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurti A. N., Dallery J. (2013). Internet-based contingency management increases walking in sedentary adults. Journal of Applied Behavior Analysis, 46, 568–581. [DOI] [PubMed] [Google Scholar]
- Lansing A. H., Berg C. A. (2014). Adolescent self-regulation as a foundation for chronic illness self-management. Journal of Pediatric Psychology, 39, 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing A., Berg C., Butner J., Wiebe D. (2016). Self-control, daily negative affect and blood glucose control in adolescents with type 1 diabetes. Health Psychology, 35, 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing A. H., Crochiere R., Cueto C., Wiebe D. J., Berg C. A. (2017). Mother, father, and adolescent self-control and adherence in adolescents with type 1 diabetes. Journal of Family Psychology, 31, 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing A. H., Stanger C., Budney A., Christiano A. S., Casella S. J. (2016). Pilot study of a web-delivered multicomponent intervention for rural teens with poorly controlled type 1 diabetes. Journal of diabetes research, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing A. H., Stanger C., Crochiere R., Carracher A., Budney A. (2017). Delay discounting and parental monitoring in adolescents with poorly controlled type 1 diabetes. Journal of Behavioral Medicine, 40, 864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally K., Rohan J., Pendley J. S., Delamater A., Drotar D. (2010). Executive functioning, treatment Adherence, and glycemic control in children with type 1 diabetes. Diabetes Care, 33, 1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmann S., Popow C., Rami B., Konig M., Blaas S., Fliri C., Schober E. (2010). Cognitive functions and glycemic control in children and adolescents with type 1 diabetes. Psychological Medicine, 40, 95–103. [DOI] [PubMed] [Google Scholar]
- Perez K., Patel N. J., Lord J. H., Savin K. L., Monzon A. D., Whittemore R., Jaser S. S. (2016). Executive function in adolescents with type 1 diabetes: relationship to adherence, glycemic control, and psychosocial Outcomes. Journal of Pediatric Psychology, 19, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry N., Cengiz E., Wagner J., Weyman K., Tichy E., Tamborlane W. (2015). Testing for rewards: a pilot study to improve type 1 diabetes management in adolescents. Diabetes Care, 38, 1952–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher K., Rucker D., Hayes A. (2007). Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behavioral Research, 42, 185–227. [DOI] [PubMed] [Google Scholar]
- Raiff B. R., Dallery J. (2010). Internet-based contingency management to improve adherence with blood glucose testing recommendations for teens diagnosed with type 1 diabetes. Journal of Applied Behavior Analysis, 43, 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel B. J., Volokhov R. N., Demaree H. A. (2008). Working memory capacity and the self-regulation of emotional expression and experience. Journal of Personality and Social Psychology, 95, 1526–1540. [DOI] [PubMed] [Google Scholar]
- Schweizer S., Grahn J., Hampshire A., Mobbs D., Dalgleish T. (2015). Emotional working memory training improves affective control. Biological Psychiatry, 77, 292S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein J., Klingensmith G., Copeland K., Plotnick L., Kaufman F., Laffel L., Deeb L., Grey M., Anderson B., Holzmeister L. A., Clark N. (2005). Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care, 28, 186–212. [DOI] [PubMed] [Google Scholar]
- Stanger C., Ryan S. R., Delhey L. M., Thrailkill K., Li Z., Li Z., Budney A. J. (2013). A multicomponent motivational intervention to improve adherence among adolescents with poorly controlled type 1 diabetes: a pilot study. Journal of pediatric psychology, 386, 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. (2014). Topical review: behavioral economics as a promising framework for promoting treatment adherence to pediatric regimens. Journal of Pediatric Psychology, 39, 1097–1103. [DOI] [PubMed] [Google Scholar]
- Stanger C., Lansing A. H., Scherer E., Budney A., Christiano A. S., Casella S. J. (2018). A Web-Delivered Multicomponent Intervention for Adolescents with Poorly Controlled Type 1 Diabetes: A Pilot Randomized Controlled Trial. Annals of Behavioral Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland K., Christianson J. B., Leatherman S. (2008). Impact of targeted financial incentives on personal health behavior. Medical Care Research and Review, 65, 36S–78S. [DOI] [PubMed] [Google Scholar]
- Wasserman R. M., Hilliard M. E., Schwartz D. D., Anderson B. J. (2015). Practical strategies to enhance executive functioning and strengthen diabetes management across the lifespan. Current Diabetes Reports, 15, 52.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. A., Miller V. A., Murphy K., Small D., Ford C. A., Willi S. M., Feingold J., Morris A., Ha Y. P., Zhu J., Wang W., Patel M. S. (2017). Effect of financial incentives on adherence and glycemic control among adolescents and young adults with type 1 diabetes. JAMA Pediatrics, 171, 1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. R., Miller K. M., Maahs D. M., Beck R. W., DiMeglio L. A., Libman I. M., Quinn M., Tamborlane W. V., Woerner S. E.; T1D Exchange Clinic Network. (2013). Most youth with type 1 diabetes in the T1D exchange clinic registry do not meet American diabetes association or international society for pediatric and adolescent diabetes clinical guidelines. Diabetes Care, 36, 2035–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]