Abstract
Objective
To assess developmental trajectories of decision-making involvement (DMI), defined as the ways in which parents and children engage each other in decision-making about illness management, in youth with type 1 diabetes (T1D) and examine the effects of DMI on levels of and changes in adherence with age.
Methods
Participants included 117 youth with T1D, enrolled at ages 8–16 years and assessed five times over 2 years. The cohort sequential design allowed for the approximation of the longitudinal curve from age 8 to 19 from overlapping cohort segments. Children and parents completed the Decision-Making Involvement Scale, which yields subscales for different aspects of DMI, and a self-report adherence questionnaire. Mixed-effects growth curve modeling was used for analysis, with longitudinal measures nested within participant and participants nested within cohort.
Results
Most aspects of DMI (Parent Express, Parent Seek, Child Express, and Joint) increased with child age; scores on some child report subscales (Parent Express, Child Seek, and Joint) decreased after age 12–14 years. After accounting for age, Child Seek, Child Express, and Joint were associated with overall higher levels of adherence in both child (estimates = 0.08–0.13, p < .001) and parent (estimates = 0.07– 0.13, p < .01) report models, but they did not predict changes in adherence with age.
Conclusion
These data suggest that helping children to be more proactive in T1D discussions, by encouraging them to express their opinions, share information, and solicit guidance from parents, is a potential target for interventions to enhance effective self-management.
Keywords: adherence, chronic illness, diabetes, longitudinal research
The management of type 1 diabetes (T1D) involves critical decision-making and complex tasks that must be carried out daily by children and parents. Maintaining optimal blood glucose levels requires a balance between factors that increase blood glucose levels, such as eating, and those that decrease them, such as exercise and insulin. To achieve this balance, children and families must monitor symptoms of hypo- and hyperglycemia, test blood glucose multiple times per day, and adjust diet, physical activity, and insulin doses (Seiffge-Krenke, 2002). In addition to managing blood glucose levels, children and families must address the logistics of regular clinic visits, supplies and prescriptions, and potentially, new technologies, such as insulin pumps and continuous glucose monitoring (CGM) devices. While parents often retain some responsibility for monitoring, decision-making, and assuring adherence to the regimen, these tasks may become challenging, as children begin to strive for more decision-making independence. The child’s transition to greater autonomy can be difficult for families, because of parental anxiety about disease complications, as well as the child’s resistance to lifestyle changes imposed by the regimen or the perception that parental attempts to monitor adherence are intrusive or limit independence (Berg et al., 2013; Buckloh et al., 2008; Wiebe et al., 2005). Prior studies of youth with T1D suggest that the transfer of responsibility is associated with poor outcomes (Anderson, Ho, Brackett, Finkelstein, & Laffel, 1997; Wysocki et al., 1992; Wysocki et al., 1996). Increased independence, without the skills for making decisions effectively, may contribute to the decreases in adherence that are seen when children with a chronic illness reach adolescence (Bender, Milgrom, Rand, & Ackerson, 1998; Kovacs, Obrosky, Goldston, & Drash, 1997; Miller-Johnson et al., 1994; Miller & Drotar, 2007; Ricker, Delamater, & Hsu, 1998). The way in which families manage this transition may influence effective self-management.
Children’s involvement in decision-making about illness management is a potentially important component of the transition to greater independence (Miller, 2009; White, 1996). Broadly speaking, decision-making is the process of choosing between different courses of action, in light of one’s values and goals; decision-making involvement (DMI) refers to the ways in which parents and children engage each other in decision-making about illness management issues (Miller, 2009). It is a multidimensional construct that includes both active participation by the child (e.g., asking parent for advice, expressing an opinion, giving information) and adult attempts to facilitate the child’s involvement (e.g., asking for the child’s opinion, soliciting questions, sharing information with the child). DMI captures a continuum of potential behaviors that may change as children mature. For example, when children are younger they may be less verbally engaged in decision-making; however, parents’ attempts to solicit their involvement (e.g., by asking for their concerns or opinions) may set the stage for increased active participation over time. As such, the different dimensions of involvement are likely to change in frequency as children mature, with children becoming more actively engaged with increasing age. DMI is differentiated from the constructs of parent–child communication, parent social support, and parent involvement because of its focus on decision-making and both parent and child behaviors.
Involving children in decision-making may enhance adherence and decision-making skills by increasing knowledge of illness management, providing the opportunity to practice making decisions with the support of a parent, and teaching children what factors to consider when making decisions (White, 1996). In addition, when parents attempt to involve children in decisions, by soliciting their perspectives and sharing information, children may learn that they have an important role to play and that their opinions are important. These hypotheses are informed by social cognitive theory, which asserts that health behaviors are determined in part by knowledge and self-efficacy beliefs gained through both observational learning and repeated practice opportunities (Bandura, 2004). Indeed, prior cross-sectional research using a new measure of DMI, called the Decision Making Involvement Scale (DMIS), found that more youth expression of opinions and information during decision-making interactions with parents was associated with better adherence in youth with T1D after controlling for age (Miller & Jawad, 2014). Prior research using the DMIS also found that when children perceived that adults engaged them more in decision-making about research participation, they reported higher decision self-efficacy (Miller, Feudtner, & Jawad, 2017).
Longitudinal research is now needed to understand how different aspects of DMI change with age and whether these changes are associated with adherence. Data regarding how specific parent and child decision-making behaviors can facilitate or impede the transition to independent illness management can be used to provide anticipatory guidance to families during clinic visits or supplement other interventions to improve adherence and/or facilitate the transition to greater independence, before problems develop. For example, if parental facilitation of children’s involvement at younger ages predicts better adherence or adherence trajectories, then one potential strategy would be to coach parents of younger children to solicit children’s opinions, concerns, and questions regarding illness management decisions. Alternatively, if active engagement of children predicts better adherence or adherence trajectories, or if these behaviors become increasingly important for effective illness management as youth mature, then interventions might focus on teaching children that their opinions and concerns are critical and identifying barriers to active engagement. These strategies may offer improvements beyond those that focus on allocation of responsibility, which refers to “who does what” and does not address how children learn to make complex illness management decisions on their own.
In this longitudinal study, the aims were to describe developmental trajectories of DMI in youth with T1D and examine effects of DMI on both levels of and developmental changes in adherence. Consistent with cross-sectional findings (Miller & Harris, 2012), the first hypothesis was that children’s expression of opinions and sharing of information with parents would increase with age and that parents’ provision of information/guidance to children would initially increase and then decrease with age. The second hypothesis was that when specific aspects of DMI were higher (i.e., children’s seeking of information/guidance from parents; children’s expression of opinions and sharing of information with parents; parents’ provision of information/guidance to children; and joint decision-making behaviors such as brainstorming and negotiating), overall levels of adherence would be higher and adherence would decline more slowly over time. Exploratory analyses also examined if aspects of DMI (i.e., children’s seeking of information/guidance from parents; children’s expression of opinions and sharing of information with parents; parents’ provision of information/guidance to children; and joint decision-making behaviors such as brainstorming and negotiating) predicted overall glycemic control and changes in glycemic control over time.
Methods
This study used a cohort-sequential design, which samples multiple age cohorts at baseline and collects longitudinal data on members of each cohort (Miyazaki & Raudenbush, 2000). In this study, 8–16 year-olds were assessed at Visit 1 and followed-up for 2 years, with assessments occurring every 6 months. For example, 11 year-olds were assessed at ages 11, 11.5, 12, 12.5, and 13, while 12 year-olds were assessed at ages 12, 12.5, 13, 13.5, and 14. This example shows that for each cohort there were three ages at which one of the other cohorts was also assessed. This overlap allowed for the approximation of the full longitudinal curve for the variables of interest from the different cohort segments using growth curve modeling. This approach minimizes the disadvantages of the traditional longitudinal design, which include time constraints, subject attrition, and the cost of repeated assessments while allowing for assessments across a broad range of development (i.e., 8–19 years-old).
Participants and Recruitment
Participants were recruited from a tertiary children’s hospital between October 2011 and June 2013. Inclusion criteria included that parents and children were English-speaking, the child was diagnosed with T1D for at least 1 year, the parent participant was the biological or adoptive parent, and the child lived with the parent participant for at least 50% of the week. Exclusion criteria included developmental delay or pervasive developmental disorder in the child, psychiatric hospitalization of the child in the past year, and the child participant had another life-threatening medical condition, not related to T1D, which required daily treatment for >6 months of the past year (e.g., cancer). Research staff identified potential participants from outpatient clinic lists and schedules sent their parents a letter describing the study, contacted them by telephone or in person (at clinic), and screened them for eligibility.
Research staff identified and screened 167 families by telephone, and of these, 151 (90%) were eligible for the study. Of these, 148 (98%) agreed to participate, but 10 (7%) could not be reached again or scheduled for a study visit, and 14 (9%) did not show up for the study visit. One (1%) additional participant declined in person at the study visit. Of the 123 (81%) dyads who consented and enrolled in the study, four (3%) did not complete Visit 1, and two (1%) were withdrawn by study personnel because they no longer met eligibility criteria. The final Visit 1 sample was composed of 117 participant dyads. Chi-square and independent samples t-tests indicated that there were no significant differences found between the participants and those who were eligible but declined, could not be scheduled, or did not complete Visit 1 (n = 34) with respect to age, duration of diagnosis, child sex, child race, or child ethnicity (all p > .20).
Procedures
The study was approved by the institutional review board, and procedures were in accordance with U.S. guidelines for the ethical conduct of human subject research. Eligible families were met for their Visit 1 assessment at a routine clinic appointment or on another convenient day of their choice. Research personnel gave a thorough explanation of the study to parents and children and gave them an informed consent document to review and sign.
Study procedures included questionnaires and medical chart review. Research personnel read questionnaires to children of age 8–10 years to promote comprehension. In general, Visit 1, 3, and 5 assessments were conducted in person, while Visit 2 and 4 assessments could be done either in person or over the phone. Child and parent participants each received $20 for completing each visit and an additional $20 each at Visit 5 if they completed all five visits.
Measures
Demographics
At Visit 1, parents completed a demographic questionnaire. Child data included sex, age, race and ethnicity, and date of diagnosis. Parent/family data included sex, age, race and ethnicity, highest educational grade, income, employment status, and family structure.
Decision-Making Involvement
At all visits, children and parents completed the DMIS (Miller & Harris, 2012). When administering the DMIS, the interviewer first asks the parent–child dyad to identify a discussion related to a decision or problem they had about illness management in the past 2 weeks. The interviewer then instructs the parent and child to respond independently to the 20 DMIS items, which assess different aspects of the child’s involvement in the discussion and yield five subscales: (1) Child Express assesses child behaviors such as expressing an opinion or giving information to the parent, (2) Child Seek assesses child behaviors such as asking for advice or information from the parent, (3) Parent Express assesses parent behaviors such as expressing advice or an opinion or giving information to the child, (4) Parent Seek assesses parent behaviors such as asking for an opinion or information from the child, and (5) Joint assesses negotiation and brainstorming between parent and child, as well as parental provision of options to the child. There are four response options: not at all, a little bit, quite a bit, and a lot. Psychometrics and validity were supported in prior research (Miller & Harris, 2012; Miller & Jawad, 2014). Items are averaged to create a composite score for each subscale, which can range from 1 to 4. Higher scores indicate more of the behavior assessed by the subscale. Cronbach’s alphas across all visits for all subscales ranged from 0.70 to 0.92 for parent report and 0.66 to 0.85 for youth report. An additional item at the end of the DMIS asks, “Think about how you and your mom/dad [child] acted during this talk. Was this similar to how you both acted in other talks about the illness in the past 2 weeks?” The response options are the same as for the other items.
Treatment Adherence
Children and parents completed the Self-Care Inventory (SCI) (Greco et al., 1990) at all visits. The SCI contains 14 items that assess adherence to multiple aspects of the diabetes treatment regimen. The SCI has been classified as a “well established” measure of adherence (Quittner, Modi, Lemanek, Ievers-Landis, & Rapoff, 2008), and research suggests that it is reliable and valid for youth on both conventional and intensive insulin regimens (Lewin et al., 2009). Items are averaged to create a composite score, which can range from 1 to 5. Higher scores indicate better adherence. Cronbach’s alphas across all visits ranged from 0.70 to 0.81 for parent report (parent report on SCI, PSCI) and 0.69 to 0.83 for youth report (child report on SCI, CSCI).
Chart Review
Research staff completed a chart review after Visit 1, 3, and 5 to obtain information regarding insulin regimen and hemoglobin A1C (HbA1C, a measure of glycemic control).
Data Analytic Plan
SAS PROC Mixed (9.4) was used for estimating growth curve parameters based on the cohort sequential design. Models to examine whether age-related changes in Child Seek, Child Express, Parent Express, and Joint would be associated with both levels of and changes in adherence were chosen a priori, and the restricted maximum likelihood estimation was used for model estimation. Therefore, participants with partially missing data were included in model estimations under the assumption that missingness occurred at random (MAR)(Little & Rubin, 2002). Assumptions included that the intercepts and slopes for each participant were random effects and that the averages of the intercepts and slopes for all participants were fixed effects. The associated variance components, which represent how much the individual trajectories vary from the average trajectory (i.e., variations in the individuals’ slopes), were defined as unstructured, allowing for every term of the variance–covariance matrix to be different. A series of two-level models with linear (age) and quadratic (age2) effects in the models predicting adherence were estimated. Level 1 represents the within-participant repeated measures over time (measurement nested within individual), and Level 2 represents the between-participants measurements (measurement nested within age cohort 8, 9,…, 16). The specification of Level 1 and Level 2 random intercept and random slope model is presented in the online Supplementary Material.
Parent and child report models were tested separately. Before modeling the relationship between age and adherence, subject’s age (age_cnt) was centered by subjects’ mean age. In all the mixed-effect models, a linear and quadratic fixed-effect model and the inclusion of random intercepts and slopes were consecutively examined and tested. The quadratic term (age_cnt2) and its fixed-effect term were removed from the model if not significant. Such models were rerun using a linear (age) term. For models with significant quadratic age_cnt2 but nonsignificant linear age_cnt term, the linear and quadratic terms were included as predictors. The final models were chosen based on examining model fit using linear and quadratic terms of age and the mixed and random effect components of the model. The improvement in the log likelihood values and their chi-square differences guided us in choosing the final models. Significance was assessed at p ≤ .05. Main effects of DMIS subscales were used to test whether DMI predicted overall levels of adherence, while interactions of DMIS subscales with age were used to test whether DMI impacted the slope of adherence (i.e., Did higher levels of DMI slow the rate of decline in adherence with age?). Different models were defined and fitted based on the aims of the study.
Sample size estimation was determined using the statistical power analysis for growth curve models using SAS macros developed by Zhang and Wang (2009) and a power calculation table for comparing means between outcomes measured repeatedly over time (Vonesh & Chinchilli, 1997). Using Zhang and Wang macros and based on prior data, assuming a slope of age of 0.30 (effect size), SD of 1, correlation between repeated measure of 0 (conservative assumption), and power of 0.80, a total of 100 dyads were needed. In this scenario, with type 1 error of .05 and five repeated measurements, we would be able to find a standard difference between means of 0.5 SD as statistically significant. The standardized effect size is defined as the minimum difference between mean values measured repeatedly over the five time points divided by the common SD. Therefore, the standardized difference = Δmin/σ. Sample size estimation accounted for a potential of up to 15% of the dyads to be excluded from the analysis because of dropout or incomplete data, so a total of 117 T1D dyads were enrolled to provide at least 100 T1D evaluable dyads.
Results
Participants
The sample included 117 children of age 8–16 years with T1D and a parent (84% mothers). Demographic characteristics are in Table I, and descriptive statistics for adherence and HbA1C at each visit are in the Online Supplementary Material. Overall, six dyads withdrew from the study; 78 completed all 4 follow-up visits; 33 completed between 1 and 3 follow-up visits; and 6 did not complete any follow-up visits. Chi-square and analysis of variance tests indicated that there were no significant differences between participants who completed no follow-ups, one to three follow-ups, and all four follow-ups with respect to demographics (age, duration of diagnosis, child sex, child race, child ethnicity, parent sex, parent education, family structure) or any of the primary variables that were analyzed for the present study (DMIS subscales, PSCI, CSCI, HbA1C; all p > .05). On the DMIS for Visit 1, 62% of youth and 81% of parents indicated that their behaviors during the identified discussion were “a lot” or “quite a bit” similar to other talks they had about the illness in the past 2 weeks.
Table I.
Participants’ Characteristics (Visit 1)
| M (SD), range or n (%) | |
|---|---|
| Child age | 12.87 (2.53), 8.14–16.97 |
| Parent age | 43.04 (7.29), 26–60 |
| Illness duration (years) | 5.63 (3.53), 1.10–14.74 |
| Child sex: Female | 66 (56) |
| Parent sex: Female | 98 (84) |
| Child race | |
| White | 70 (60) |
| African-American | 29 (25) |
| Asian | 1 (1) |
| Other | 14 (12) |
| Child Hispanic/Latino: No | 102 (87) |
| Income | |
| <19,999 | 19 (16) |
| 20,000–39,999 | 13 (11) |
| 40,000–59,999 | 16 (14) |
| 60,000–79,999 | 14 (12) |
| 80,000–99,999 | 13 (11) |
| >100,000 | 33 (28) |
| Parent education | |
| Some high school | 1 (1) |
| Completed high school | 15 (13) |
| Some college or technical school | 40 (34) |
| College graduate | 34 (29) |
| Some postcollege graduate education | 11 (9) |
| Masters, PhD, MD, law degree, etc. | 11 (9) |
| Employment status | |
| Not currently employed | 28 (24) |
| Working part-time | 28 (24) |
| Working full-time | 58 (50) |
| Family structure | |
| Two parent | 76 (65) |
| Two parents—step family | 14 (12) |
| Single parent | 23 (20) |
| Insulin regimen | |
| Basal bolus | 47 (40) |
| Pump | 46 (39) |
| Premixed (70/30 insulin) | 24 (21) |
Note: Numbers may not add up to 100% because of missing data (i.e., parent refused the item).
Developmental Trajectories of Decision-Making Involvement
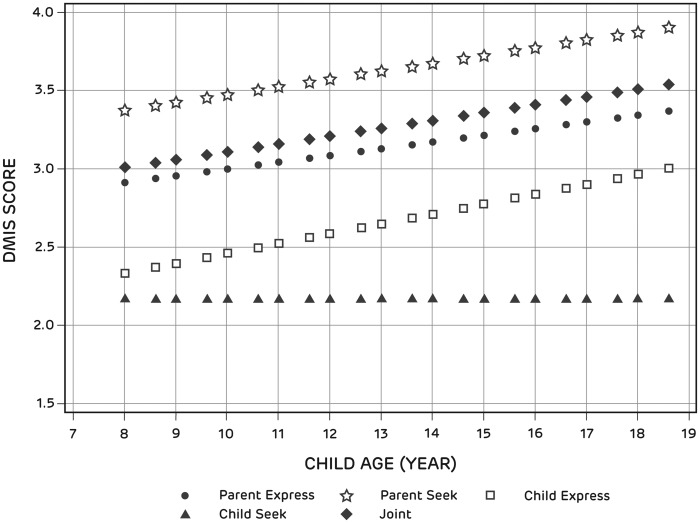
Figure 1 shows the developmental trajectories for all parent report DMIS subscales. As expected, Child Express increased with age. The increase in Child Express because of a 6-month increase in age was estimated to be .06 units (p = .0021). Although the hypothesis was for a nonlinear trajectory, parent report of Parent Express showed a linear increase with age, increasing by .04 units for each 6 month increase in age (p = .0187). Parent report of Parent Seek increased with age. The increase in Parent Seek because of a 6-month increase in age was estimated to be .06 units (p = .007). Parent report of Child Seek did not change with age. Parent report of Joint increased with age in a linear fashion, increasing .05 units for each 6 month increase in age (p < .02).
Figure 1.
Developmental trajectories of DMIS subscale scores (parent report).
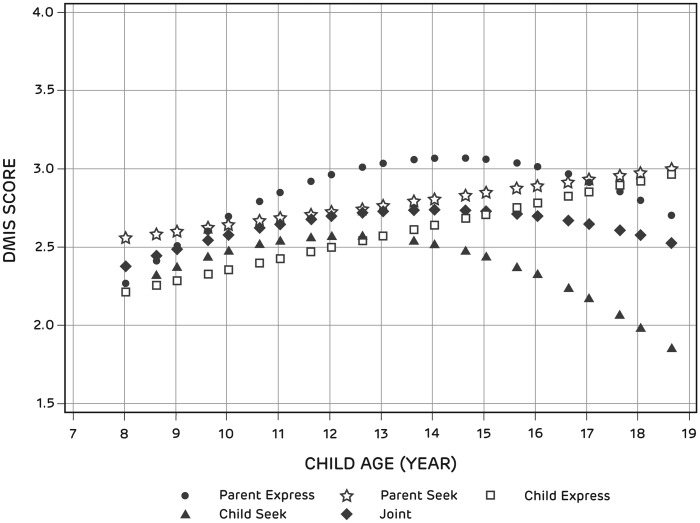
Figure 2 shows the developmental trajectories for all child report DMIS subscales. As expected, Child Express increased with age. The increase in Child Express because of a 6-month increase in age was estimated to be .07 units (p = .0011). As expected, child report of Parent Express had a nonlinear relationship with age, increasing until age 14 years and decreasing thereafter (age2 = −.02, p = .0004). Child report of Parent Seek increased with age. The increase in Parent Seek because of a 6-month increase in age was estimated to be .04 units (p < .05). Furthermore, child report of Child Seek had a nonlinear relationship with age, initially increasing until age 12 years and decreasing thereafter (age = −.04, p < .07; age2 = −.02, p < .02). Joint had a nonlinear relationship with age, initially increasing until age 14 years and decreasing thereafter (age2 = −.01, p < .03).
Figure 2.
Developmental Trajectories of DMIS Subscale Scores (child report).
Decision-Making Involvement as a Predictor of Adherence
For the parent report models, adherence scores decreased with age (Table II). In addition to the negative effects of age on adherence, aspects of youth decision-making involvement predicted higher levels of adherence. Specifically, higher scores for parent report of Child Seek, Child Express, and Joint predicted higher PSCI scores. However, the interactions of these DMIS subscales with age were not significant, indicating that the rate of decline in adherence did not vary based on DMIS scores. For illustrative purposes, Figure 3 shows the trajectory of predicted PSCI scores for the lowest quartile of Child Express and the highest quartile of Child Express, showing that children in the lowest quartile of Child Express had lower adherence across the full developmental trajectory compared with children in the highest quartile. Contrary to expectation, increases in parent report of Parent Express did not predict PSCI scores. When examined together in a single model, only age (−0.09, p < .0001) and Child Express (0.11, p = .0008) were significant predictors of adherence. Child Seek and Joint were no longer significant.
Table II.
Individual Models Predicting Adherence
| Parent report models |
Child report models |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Standard error | t | p value | Estimate | Standard error | t | p value | ||
| 1 | Intercept | 4.83 | 0.15 | 31.95 | <.0001 | 4.51 | 0.17 | 27.16 | <.0001 |
| Age | −0.07 | 0.01 | −5.61 | <.0001 | −0.04 | 0.01 | −2.95 | .0039 | |
| 2 | Intercept | 4.65 | 0.15 | 30.65 | <.0001 | 4.29 | 0.15 | 27.90 | <.0001 |
| Age | −0.06 | 0.01 | −5.16 | <.0001 | −0.04 | 0.01 | −3.28 | .0014 | |
| Child Seek | 0.07 | 0.02 | 3.00 | .0036 | 0.08 | 0.02 | 3.77 | .0003 | |
| 3 | Intercept | 4.52 | 0.15 | 29.55 | <.0001 | 4.35 | 0.15 | 29.06 | <.0001 |
| Age | −0.07 | 0.01 | −5.29 | <.0001 | −0.05 | 0.01 | −3.94 | .0001 | |
| Child Express | 0.13 | 0.03 | 4.72 | <.0001 | 0.10 | 0.03 | 3.75 | .0003 | |
| 4 | Intercept | 4.66 | 0.17 | 27.24 | <.0001 | 4.30 | 0.18 | 23.81 | <.0001 |
| Age | −0.07 | 0.01 | −4.75 | <.0001 | −0.04 | 0.01 | −3.36 | .0011 | |
| Parent Express | 0.06 | 0.04 | 1.51 | .1338 | 0.08 | 0.03 | 2.60 | .0109 | |
| 5 | Intercept | 4.63 | 0.16 | 28.20 | <.0001 | 4.20 | 0.16 | 26.89 | <.0001 |
| Age | −0.07 | 0.01 | −5.14 | <.0001 | −0.04 | 0.01 | −3.64 | .0004 | |
| Joint | 0.08 | 0.03 | 2.67 | .009 | 0.13 | 0.02 | 5.39 | <.0001 | |
Note. Interactions of DMIS subscales with age were tested but were not significant and dropped from the models.
Figure 3.
Predicted PSCI scores based on child age and parent report of Child Express.
For the child report models, adherence scores decreased with age (Table II). In addition to the negative effects of age on adherence, aspects of youth decision-making involvement predicted overall levels of adherence. Specifically, scores for child report of Child Seek, Child Express, Parent Express, and Joint predicted higher CSCI scores. However, the interactions of these DMIS subscales with age were not significant, indicating that the rate of decline in adherence did not vary based on DMIS scores. For illustrative purposes, Figure 4 shows the trajectory of predicted CSCI scores for the lowest quartile of Child Express and the highest quartile of Child Express, showing that children in the lowest quartile of Child Express had lower adherence across the full developmental trajectory compared with children in the highest quartile. When examined together in a single model, only age (−0.04, p = .0006) and Joint (0.12, p = .0007) were significant predictors of adherence. Child Seek, Child Express, and Parent Express were no longer significant.
Figure 4.
Predicted CSCI scores based on child age and child report of Child Express.
Exploratory Analyses Predicting HbA1C
HbA1C increased from age 8 to 19 years (Table III). In exploratory analyses to examine whether aspects of DMI predicted overall levels of HbA1C and slower increases in HbA1C over time, there were significant interactions of Child Seek, Child Express, and Joint with age. These interactions indicated that when parents reported higher levels of Child Seek, Child Express, and Joint, the increases in HbA1C were less steep than when parents reported lower levels of these variables. Parent report of Parent Express was not associated with HbA1C. Child reports of Child Seek, Child Express, Parent Express, and Joint were not associated with HbA1C.
Table III.
Individual Models Predicting HbA1C
| Parent report models |
|||||
|---|---|---|---|---|---|
| Estimate | Standard error | t | p value | ||
| 1 | Intercept | 6.72 | 0.29 | 23.51 | <.0001 |
| Age | 0.12 | 0.03 | 4.50 | <.0001 | |
| 2 | Intercept | 3.91 | 1.19 | 3.29 | .0013 |
| Age | 0.39 | 0.09 | 4.31 | <.0001 | |
| Child Seek | 1.28 | 0.47 | 2.71 | .0092 | |
| Age * Child Seek | −0.10 | 0.03 | −2.80 | .0074 | |
| 3 | Intercept | 3.44 | 1.54 | 2.23 | .028 |
| Age | 0.44 | 0.12 | 3.60 | .0005 | |
| Child Express | 1.27 | 0.55 | 2.32 | .0244 | |
| Age * Child Express | −0.10 | 0.04 | −2.40 | .02 | |
| 4 | Intercept | 6.38 | 2.14 | 2.98 | .0035 |
| Age | 0.18 | 0.17 | 1.05 | .2969 | |
| Parent Express | 0.15 | 0.67 | 0.23 | .82 | |
| Age * Parent Express | −0.004 | 0.05 | −0.07 | .9454 | |
| 5 | Intercept | 3.49 | 1.49 | 2.34 | .0208 |
| Age | 0.40 | 0.12 | 3.34 | .0012 | |
| Joint | 1.35 | 0.54 | 2.50 | .0159 | |
| Age * Joint | −0.09 | 0.04 | −2.18 | .0339 | |
Note. Child report models not shown; none were significant in predicting HbA1C.
HbA1C = hemoglobin A1C.
Discussion
The present study contributes to research on adherence to chronic illness management by identifying children’s involvement in decision-making as a multidimensional process that changes with development and is associated with treatment adherence and glycemic control in youth with T1D. The construct of DMI, grounded in social cognitive theory, adds to the literature by addressing a potential process through which children learn to make decisions about illness management, which may inform future intervention efforts to facilitate treatment adherence as children mature. In addition to the conceptual strengths, this study used a cohort sequential design to examine developmental trajectories. The primary benefit of this design, compared with the traditional longitudinal design, is shorter study duration (i.e., 2 years of data collection to generate the full longitudinal curve from 8 to 19 years), which results in reduced attrition and lower costs.
The results indicated that most aspects of decision-making involvement increased in a linear fashion with age. Several child report subscales (Parent Express, Child Seek, and Joint) had nonlinear relationships with age, with scores increasing until age 12–14 years and subsequently decreasing. Overall, these findings suggest that both parents and children perceived that they became more active in decision-making discussions with one another as children got older. These increases in engagement may reflect that decisions and discussions about illness management become more complex, as children mature and spend more time away from home. At the same time, children, but not parents, perceived that the parental role (i.e., parents expressing opinions/guidance and children seeking input from them) decreased after age 12–14 years. In contrast to parents, children may have responded based on a perception of or desire for more independence around mid-adolescence. Indeed, children’s reports of Child Seek, which measures the extent to which children seek opinions and information from parents, showed the steepest decline with age, ending at age 19 years at levels lower than where they started at age 8 years. As children gain more experience with illness management tasks and decision-making, they may perceive less of a need to pursue guidance from parents. This does not mean, however, that youth do not value and need parental input, especially for decisions that are more serious or deviate from typical situations (Lipstein, Muething, Dodds, & Britto, 2013; Miller, 2009).
Consistent with prior research (Rausch et al., 2012), adherence decreased across the developmental trajectory from age 8 to 19 years. In the separate parent report models predicting adherence, Child Seek, Child Express, and Joint predicted overall levels of adherence, such that children who sought parental advice/support, expressed an opinion, and engaged in joint decision-making behaviors with parents during illness management discussions were more adherent across the developmental spectrum from age 8 to 19 years. In the multivariate model that included these three subscales as predictors of adherence, the only one that remained significant was Child Express, indicating that the child’s active engagement in decision-making, and not the parent’s, was critical for adherence. When children are more actively engaged in decision-making interactions, it may increase the likelihood that parents and children are on the same page regarding aspects of illness management, such as recognition of symptoms and potential barriers to treatment. Having accurate information from children may enable parents to provide effective and timely guidance or support for T1D-related tasks or decisions. Alternatively, children’s verbal engagement in discussions may reflect an unmeasured third variable that may also be associated with adherence, such as empowerment over one’s illness or decision-making skills.
Similar to what was found in the parent report models, Child Seek, Child Express, and Joint were associated with better adherence in the separate child report models, but so was Parent Express, which reflects parental provision of guidance and information during decision-making interactions. This finding is consistent with prior research demonstrating the importance of continued parental involvement for illness management in youth with T1D (King et al., 2014; Wiebe et al., 2014; Wu et al., 2014). In the multivariate child report model that included Child Seek, Child Express, Parent Express, and Joint as predictors of adherence, the only decision-making involvement subscale that remained significant was Joint. This finding is consistent with prior cross-sectional research examining associations of DMI with adherence (Miller & Jawad, 2014) and with other studies showing that collaborative maternal involvement in diabetes care is important for adherence and that teamwork interventions have a positive impact on diabetes-related outcomes (Laffel et al., 2003; Nansel, Iannotti, & Liu, 2012; Nansel et al., 2009; Wiebe et al., 2005). Working together to make T1D-related decisions appears to be an important way for children and adolescents to learn effective self-management and decision-making skills.
The findings suggest several clinical implications related to enhancing children’s involvement in discussions and decisions about T1D. Parents can be counseled to seek their child’s opinions about T1D decisions at an early age, which may set the stage for increased participation as the child matures. It is important for parents to know that there are multiple ways for children to be involved in decisions and that joint decision-making provides the opportunity to practice, while still receiving support and guidance from parents. Providers can also support youth involvement in decision-making during office visits by encouraging children and adolescents to share their opinions and concerns about T1D management and decisions (Miller et al., 2017). From an intervention standpoint, it is important to note that while aspects of involvement were associated with overall levels of adherence, involvement did not mitigate the declines in adherence that were seen with age. Therefore, strategies to facilitate children’s involvement in decision-making may boost adherence but are unlikely to prevent the declines in adherence that are typically seen during adolescence in youth with T1D. Multicomponent interventions targeting behavioral, social, emotional, and family processes related to diabetes management are needed to impact adherence, as well as the ultimate outcome of improved glycemic control (Hood, Rohan, Peterson, & Drotar, 2010).
The findings from the present study should be considered with several limitations in mind. First, the study relied on self-report measures of adherence, which may inflate adherence scores and may not converge with objective measures (Modi et al., 2006). However, the results did indicate that several DMIS parent report subscales were associated with glycemic control. Second, there was a small sample size for each cohort, which may influence the robustness of the findings. Third, there may have been selection bias, such that families with high levels of conflict or other relational difficulties may have declined enrollment in a study about parent–child interactions regarding illness management. Fourth, the sample consisted of largely Caucasian participants, and parents were primarily mothers; findings may not be generalizable to father–child interactions about illness management or to racial and ethnic minority families. Finally, the DMIS assesses decision-making interactions related to one sample of behavior identified by the dyad, which may or may not be representative of how parents and youth typically interact with one another when management decisions or problems arise. However, the majority of both youth and parents in this sample indicated that their behaviors were typical of other discussions about illness management.
Future research is needed to understand reasons for the discrepant trajectories of DMI based on parent versus youth report, which may inform anticipatory guidance provided to families regarding children’s involvement in T1D management decisions as they mature. Second, additional studies should examine whether the impact of DMI on adherence can be explained by more effective T1D-related decision-making skills. There may also be moderating effects of additional variables that were not assessed in this study. For example, joint decision-making between parents and youth may not be helpful when parents themselves demonstrate ineffective decision-making. Third, the DMIS can be used to measure youth’s involvement in specific single-event decisions, which would reduce decision variability (i.e., all participants would be responding with respect to the same target decision) and allow for the assessment of decision-specific outcomes. For example, the study team is currently examining youth DMI regarding the decision to add CGM to the treatment regimen for T1D and assessing whether DMI impacts CGM satisfaction, self-efficacy, and adherence. Finally, future research should develop and evaluate intervention strategies to facilitate active involvement of children and adolescents in medical decision-making from an early age and determine whether parent-, child-, or provider-directed intervention efforts (or a combination) are most effective at increasing youth engagement, and, ultimately, impacting health behaviors and outcomes as children mature.
Supplementary Data
Supplementary data can be found at: http://www.jpepsy.oxfordjournals.org/.
Supplementary Material
Acknowledgments
The first author is grateful for her graduate school mentor, Denny Drotar, who taught her, and many others, the knowledge and skills to develop important and methodologically rigorous research to understand and improve the health and well-being of children and adolescents. The authors thank the children and parents who participated in this study. The authors also thank the Diabetes Center for Children and the Cystic Fibrosis Center at Children’s Hospital of Philadelphia for supporting this program of research.
Funding
This work was supported by grant number #1R01HD064638-01A1 awarded to the first author from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
Conflicts of interest: None declared.
References
- Anderson B. J., Ho J., Brackett J., Finkelstein D., Laffel L. M. (1997). Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. Journal of Pediatrics, 130, 257–265. [DOI] [PubMed] [Google Scholar]
- Bandura A. (2004). Health promotion by social cognitive means. Health Education and Behavior, 31, 143–164. [DOI] [PubMed] [Google Scholar]
- Bender B., Milgrom H., Rand C., Ackerson L. (1998). Psychological factors associated with medication nonadherence in asthmatic children. Journal of Asthma, 35, 347–353. [DOI] [PubMed] [Google Scholar]
- Berg C. A., Butner J. E., Butler J. M., King P. S., Hughes A. E., Wiebe D. J. (2013). Parental persuasive strategies in the face of daily problems in adolescent type 1 diabetes management. Health Psychology, 32, 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckloh L. M., Lochrie A. S., Antal H., Milkes A., Canas J. A., Hutchinson S., Wysocki T. (2008). Qualitative analysis of parents’ perspectives of family learning and knowledge. Diabetes Care, 31, 1516–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco P., La Greca A., Ireland S., Wick P., Freeman C., Agramonte R., Gutt M., Skyler J. (1990). Assessing adherence in IDDM: A comparison of two methods (Abstract). Diabetes, 39(Supp. 1), 108A. [Google Scholar]
- Hood K. K., Rohan J. M., Peterson C. M., Drotar D. (2010). Interventions with adherence-promoting components in pediatric type 1 diabetes. Diabetes Care, 33, 1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. S., Berg C. A., Butner J., Butler J. M., Wiebe D. J. (2014). Longitudinal trajectories of parental involvement in Type 1 diabetes and adolescents’ adherence. Health Psychology, 33, 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M., Obrosky D. S., Goldston D., Drash A. (1997). Major depressive disorder in youths with IDDM: A controlled prospective study of course and outcome. Diabetes Care, 20, 45–51. [DOI] [PubMed] [Google Scholar]
- Laffel L. M., Vangsness L., Connell A., Goebel-Fabbri A., Butler D., Anderson B. J. (2003). Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with Type I diabetes. Journal of Pediatrics, 142, 409–416. [DOI] [PubMed] [Google Scholar]
- Lewin A. B., La Greca A. M., Geffken G. R., Williams L. B., Duke D. C., Storch E. A., Silverstein J. H. (2009). Validity and reliability of an adolescent and parent rating scale of type 1 diabetes adherence behaviors: The Self-Care Inventory (SCI). Journal of Pediatric Psychology, 34, 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipstein E. A., Muething K. A., Dodds C. M., Britto M. T. (2013). “I'm the one taking it”: Adolescent participation in chronic disease treatment decisions. Journal of Adolescent Health, 53, 253–259. [DOI] [PubMed] [Google Scholar]
- Little R. J. A., Rubin D. B. (2002). Statistical analysis with missing data (2nd ed). Hoboken, NJ: Wiley-Interscience. [Google Scholar]
- Miller-Johnson S., Emery R., Marvin R., Clarke W., Lovinger R., Martin M. (1994). Parent-child relationships and the management of insulin-dependent diabetes mellitus. Journal of Consulting and Clinical Psychology, 62, 603–610. [DOI] [PubMed] [Google Scholar]
- Miller V. A. (2009). Parent-child collaborative decision making for the management of chronic illness: A qualitative analysis. Families, Systems, and Health, 27, 249–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. A., Drotar D. (2007). Decision making competence and adherence to treatment in adolescents with diabetes. Journal of Pediatric Psychology, 32, 178–188. [DOI] [PubMed] [Google Scholar]
- Miller V. A., Feudtner C., Jawad A. F. (2017). Children’s decision making involvement about research participation: Associations with perceived fairness and self-efficacy. Journal of Empirical Research on Human Research Ethics, 122, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. A., Harris D. (2012). Measuring children’s decision making involvement regarding chronic illness management. Journal of Pediatric Psychology, 373, 292–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. A., Jawad A. (2014). Relationship of youth involvement in diabetes-related decisions to treatment adherence. Journal of Clinical Psychology in Medical Settings, 212, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y., Raudenbush S. W. (2000). Tests for linkage of multiple cohorts in an accelerated longitudinal design. Psychological Methods, 5, 44–63. [DOI] [PubMed] [Google Scholar]
- Modi A. C., Lim C. S., Yu N., Geller D., Wagner M. H., Quittner A. L. (2006). A multi-method assessment of treatment adherence for children with cystic fibrosis. Journal of Cystic Fibrosis, 5, 177–185. [DOI] [PubMed] [Google Scholar]
- Nansel T. R., Iannotti R. J., Liu A. (2012). Clinic-integrated behavioral intervention for families of youth with type 1 diabetes: Randomized clinical trial. Pediatrics, 129, e866–e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nansel T. R., Rovner A. J., Haynie D., Iannotti R. J., Simons-Morton B., Wysocki T., Anderson B., Weissberg-Benchell J., Laffel L. (2009). Development and validation of the Collaborative Parent Involvement Scale for youths with type 1 diabetes. Journal of Pediatric Psychology, 34, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quittner A. L., Modi A. C., Lemanek K. L., Ievers-Landis C. E., Rapoff M. A. (2008). Evidence-based assessment of adherence to medical treatments in pediatric psychology. Journal of Pediatric Psychology, 33, 916–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch J. R., Hood K. K., Delamater A., Shroff Pendley J., Rohan J. M., Reeves G., Dolan R., Drotar D. (2012). Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care, 35, 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricker J. H., Delamater A. M., Hsu J. (1998). Correlates of regimen adherence in cystic fibrosis. Journal of Clinical Psychology in Medical Settings, 5, 159–172. [Google Scholar]
- Seiffge-Krenke I. (2002). “Come on, say something, Dad!”: Communication and coping in fathers of diabetic adolescents. Journal of Pediatric Psychology, 27, 439–450. [DOI] [PubMed] [Google Scholar]
- Vonesh E. F., Chinchilli V. M. (1997). Linear and nonlinear models for the analysis of repeated measurements. New York, NY: Marcel Dekker, Inc. [Google Scholar]
- White F. (1996). Parent-adolescent communication and adolescent decision-making. Journal of Family Studies, 2, 41–56. [Google Scholar]
- Wiebe D. J., Berg C. A., Korbel C., Palmer D. L., Beveridge R. M., Upchurch R., Lindsay R., Swinyard M. T., Donaldson D. L. (2005). Children's appraisals of maternal involvement in coping with diabetes: Enhancing our understanding of adherence, metabolic control, and quality of life across adolescence. Journal of Pediatric Psychology, 30, 167–178. [DOI] [PubMed] [Google Scholar]
- Wiebe D. J., Chow C. M., Palmer D. L., Butner J., Butler J. M., Osborn P., Berg C. A. (2014). Developmental processes associated with longitudinal declines in parental responsibility and adherence to type 1 diabetes management across adolescence. Journal of Pediatric Psychology, 39, 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. P., Rausch J., Rohan J. M., Hood K. K., Pendley J. S., Delamater A., Drotar D. (2014). Autonomy support and responsibility-sharing predict blood glucose monitoring frequency among youth with diabetes. Health Psychology, 33, 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki T., Meinhold P., Abrams K. C., Barnard M. U., Clarke W. L., Bellando B. J., Bourgeois M. J. (1992). Parental and professional estimates of self-care independence of children and adolescents with IDDM. Diabetes Care, 15, 43–52. [DOI] [PubMed] [Google Scholar]
- Wysocki T., Taylor A., Hough B., Linscheid T., Yeates K., Naglieri J. (1996). Deviation from developmentally appropriate self-care autonomy. Diabetes Care, 19, 119–125. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wang L. (2009). Statistical power analysis for growth curve models using SAS. Behavior Research Methods, 41, 1083–1094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.