Abstract
A newly isolated white rot fungal strain KU-RNW027 was identified as Trametes polyzona, based on an analysis of its morphological characteristics and phylogenetic data. Aeration and fungal morphology were important factors which drove strain KU-RNW027 to secrete two different ligninolytic enzymes as manganese peroxidase (MnP) and laccase. Highest activities of MnP and laccase were obtained in a continuous shaking culture at 8 and 47 times higher, respectively, than under static conditions. Strain KU-RNW027 existed as pellets and free form mycelial clumps in submerged cultivation with the pellet form producing more enzymes. Fungal biomass increased with increasing amounts of pellet inoculum while pellet diameter decreased. Strain KU-RNW027 formed terminal chlamydospore-like structures in cultures inoculated with 0.05 g/L as optimal pellet inoculum which resulted in highest enzyme production. Enzyme production efficiency of T. polyzona KU-RNW027 depended on fungal pellet morphology as size, porosity, and formation of chlamydospore-like structures.
Keywords: Chlamydospore-like structures, fungal pellet, ligninolytic enzymes, porosity, Trametes polyzona
1. Introduction
Manganese peroxidase (MnP, Mn(II): hydrogen-peroxide oxidoreductase, EC 1.11.1.13) is an extracellular heme-containing glycoprotein that drives a reaction in the presence of Mn2+ by catalyzing H2O2-dependent oxidation to Mn3+. Products form complexes with dicarboxylic acids such as malonic acid and are subsequently oxidized to a wide variety of aromatic compounds [1]. Laccase (benzenediol: dioxygen oxidoreductase, EC 1.10.3.2) belongs to the family of multicopper oxidases that catalyze the oxidation of many aromatic substances coupled with reducing oxygen to water [2]. These enzymes play significantly important roles in ligninolytic systems, fungal fruiting body formation, virulence factors of fungal pathogenicity, hyphal septa sealing, and pigmentation under stress responses [3–6]. Their abilities to oxidize a wide variety of recalcitrant aromatic compounds make MnP and laccase attractive candidates for eco-friendly applications including toxic aromatic dye decolorization [7,8], delignification and bleaching of pulp and paper [9], oxidative hair coloring [10], biocatalysts for material modification [11], wood protection [12], developing bactericidal paint [13], synthesis of iodinated phenolic compounds [14] and xenobiotic compounds degradation as pharmaceutical products [15,16], polycyclic aromatic hydrocarbons [17], fungal toxins [18], and pesticides [19].
Efficient production of MnP and laccase involves the combination of several factors including a high producing fungal strain, cultivation system, proper culture media, and culture condition. Influence of aeration on enzyme production by different fungal strains has been reported by several research groups. Aeration significantly affected growth, fungal pellet size, and enzyme production of Dichomitus squalens cultivated in a stirred tank with bubble column reactors [20]. However, aeration by agitation had no significant influence on laccase production by Trametes versicolor in a stirred tank bioreactor [21].
Two major fungal morphologies as pellets and free-form mycelial clumps appeared during submerged cultivation. Factors affecting fungal pellet formation comprised fungal strain, inoculum amount, aeration or agitation speed, volume of liquid medium, size of container, initial pH, and medium composition [10,22–29]. Fungal morphology in pellet form was important for production of various enzymes including glucoamylase [26], pectinase [28], phthalate-degrading enzymes [25], and ligninolytic enzymes [20].
Our preliminary study demonstrated that Trametes polyzona KU-RNW027, a newly discovered isolate from Thailand, exhibited high MnP and laccase activities in submerged fermentation and decolorized various synthetic dyes. Different culture conditions had diverse effects on fungal morphology and enzyme production. However, no reports address the phenomenon of ligninolytic enzyme production by T. polyzona and how different fungal pellet morphology affects enzyme production. Therefore, this study focused on the relationship between fungal morphology grown in submerged culture and pellet morphology on MnP and laccase production of T. polyzona KU-RNW027. Abilities regarding the decolorization of various toxic aromatic dyes were also evaluated.
2. Materials and methods
2.1. Fungal strain
A white rot fungus designated as KU-RNW027 was isolated from Klong Naka Wildlife Sanctuary, Ranong, Thailand. The mycelium from the fungal fruiting body was aseptically purified on potato dextrose agar (PDA) supplemented with 0.01% streptomycin. The fungus was maintained on PDA at 4 °C and subcultured every 3 months. Long-term preservation was achieved under 20% glycerol at –20 °C.
2.2. Morphological studies
Morphological characteristics of a mature fruiting body recultivated on a wooden log of Agastya (Sesbania grandiflora (L.) Pers.) were studied. Fungal mycelium was precultivated on a solid substrate of maize seeds before inoculation into 1 cm diameter holes in the log. Incubation was conducted at 25–35 °C and 65–75% humidity. Macroscopic characteristics of the fruiting body and microscopic characteristics of its basidiospores were recorded.
2.3. Molecular phylogeny
Genomic DNA was extracted from the fungal pellets cultivated in Kirk’s liquid medium [30]. Fungal pellets were filtered and ground in liquid nitrogen. Five hundred microliters of lysis buffer (pH 8.0), containing 400 mM Tris-HCl, 60 mM EDTA, 150 mM NaCl, and 1% SDS was added and incubated on ice for 30 min. One hundred and fifty microliters of 5 M potassium acetate (pH 4.8) was added and the mixture was further incubated on ice for 15 min before centrifuging at 13,000 rpm for 2 min. The supernatant was transferred into a new tube and an equal volume of isopropanol was added and centrifuged at 13,000 rpm for 2 min. Genomic DNA was washed with 70% ethanol and dry DNA pellets were resuspended in water.
Sequences of the small subunit (SSU) rRNA gene, large subunit (LSU) rRNA gene and internal transcribed spacer (ITS) region were determined by PCR amplification using forward (f) and reverse (r) primers shown in Table 1. All PCR mixtures contained 100 ng fungal genomic DNA in 50 µL reaction volume with a reaction buffer (10 mM Tris-HCl, 3.0 mM magnesium chloride, pH 8.3) and 0.4 µM of each primer and one unit of Taq DNA polymerase (Thermo Fisher Scientific, Waltham, MA, USA). PCR was performed with initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 62 °C (ITS and SSU) or 60 °C (LSU) for 30 s and extension at 72 °C for 2 min. A final extension at 72 °C for 10 min was included. PCR products were visualized on 0.9% w/v agarose gel electrophoresis. Purified PCR products were sequenced on both strands by using a commercial service (Macrogen, Seoul, Korea).
Table 1.
Oligonucleotide primers.
| Primer | Target region | Primer sequence | References |
|---|---|---|---|
| SSU1f | SSU rRNA gene | 5′-CTGGTTGATCCTGCCAGTAGTCATA-3′ | Ruivo et al. [31] |
| SSU2r | SSU rRNA gene | 5′-ATGATCCTTCCGCAGGTTCAC-3′ | |
| LRORf | LSU rRNA gene | 5′-ACCCGCTGAACTTAAGC-3′ | Moncalvo et al. [32] |
| LR7r | LSU rRNA gene | 5′-TACTACCACCAAGATCT-3′ | |
| ITS1f | ITS region | 5′-TCCGTAGGTGAACCTGCGG-3′ | White et al. [33] |
| ITS4r | ITS region | 5′-TCCTCCGCTTATTGATATGC-3′ |
Sequence alignments and calculation of similarity were conducted using the Clustal W program [34]. A phylogenetic tree was constructed from ITS sequences with the Mega6 program using the maximum likelihood method with 1000 bootstrap resampling replicates [35].
2.4. Effect of aeration on enzyme production and fungal growth
Ligninolytic enzyme production capability was studied for strain culture under three different aeration conditions. Production was performed for 7 days at room temperature in Kirk’s liquid medium with 0.85 mM veratryl alcohol using 15 discs of mycelial tips [9]. Three different aeration conditions of 7-day cultivation were investigated for efficient enzyme production as 7-day continuous shaking, 3-day static followed by shaking for 4 days and 7-day static condition. MnP, laccase, and lignin peroxidase (LiP) activities were measured daily as well as fungal growth. All experiments were performed in triplicate. The optimal condition was selected for further study.
2.5. Effect of inoculum morphology on enzyme production and fungal growth
Two cultivation methods were followed to achieve different forms of inoculum morphology both methods were performed at room temperature. The first employed 15 discs, 5 mm in diameter from the growing edge of mycelia pre-grown on PDA as inoculum in 250 mL Erlenmeyer flasks containing 50 mL of Kirk’s liquid medium. Mycelial dry weight (MDW) of inoculum was 0.05 g/L. Cultures were incubated with shaking for 7 days. The second method prepared fungal pellets used as inoculum. Five discs of mycelial tips were pre-grown for 3 days in 50 mL Kirk’s liquid medium to allow pellet formation. The pellets were washed with 0.85 M NaCl. Then, pellets at 0.05 g/L were used as inoculum for further enzyme production for 7 days. Morphological structure and size of free-form mycelial clumps and pellets were compared to investigate their correlation with enzyme production.
2.6. Fungal morphology correlation with enzyme production
Amounts of pellet inoculum were varied depending on biomass at 0.025, 0.05, 0.1, 0.25, and 0.5 g/L and fungal growth morphology of the pellets was observed at different inoculum amounts under a stereomicroscope and a scanning electron microscope (SEM). Cross sections of pellets were studied under light microscope. Ligninolytic enzymes were assayed. Correlations between enzyme production and fungal morphology including pellet morphology and size of pellet were investigated to determine the influence of fungal morphology on ligninolytic enzyme production efficiency.
2.7. Enzyme assay
MnP, LiP, and laccase activities were assayed following the previously described methods [8,36,37]. MnP and laccase activities were determined by monitoring the oxidation of 2,6-dimethoxyphenol (2,6-DMP) at 469 nm. LiP activity was evaluated by monitoring the oxidation of veratryl alcohol at 310 nm. All enzyme activities were assayed following absorbance changes within 3 min. Reaction rates were converted into quantitative amounts of enzyme. Control reactions were also carried out in parallel. One unit (U) of either MnP, LiP or laccase was defined as 1 µmol of substrate oxidized per min. Enzyme activities were calculated by fungal growth and expressed as U/mg MDW.
2.8. Determination of fungal growth
Fungal growth was determined by mycelial dry weight. The culture was collected using a plankton net, was washed with distilled water and then oven-dried at 70 °C. MDW was measured using a chemical balance.
2.9. Sample preparation for scanning electron microscope
Fungal pellets at day 7 were fixed immediately in 2% (v/v) glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) for 24 h at 4 °C. Samples were washed three times with the buffer, then dehydrated with 10, 30, 50, 70, 90, and 100% ethanol at 15 min intervals and dried following critical point drying procedure. Dried samples were sputter-coated with gold and observations were made with a scanning electron microscope Quanta 450 (FEI Technol. Inc., Hillsboro, OR).
2.10. Preparation of cross-section samples
Fungal pellets were fixed in glutaraldehyde as described above. Buffer-washed samples were dehydrated using acetone in increasing concentrations of 10, 30, 50, 70, 90, and 100% with 15 min intervals. The samples were serially incubated in acetone: Spurr’s resin (2:1, 1:1, and 1:2) at 4 h intervals and finally kept in pure Spurr’s resin. The polymerized resin was sectioned with a Leica UC7 ultramicrotome (Leica Microsystems, Wetzlar, Germany) and stained with toluidine blue for 1 min. Morphology of the cross-sectioned pellets was observed under light microscope (Nikon Instech Co., Ltd., Tokyo, Japan).
2.11. Ability of dye decolorization
A plug of mycelial tip with 0.5 cm diameter was inoculated on the surface of a 5 mL PDA test tube containing 50 mg/L of each synthetic toxic dye. Five azo dyes as Remazol golden yellow RGB gran (RGY), Remazol brilliant yellow 4GL gran (RBY), Remazol red RGB gran (RR), Remazol brilliant red F3B gran (RBR), Remazol navy blue RGB 150% gran (RNB), and one anthraquinone dye as Remazol brilliant blue R (RBBR) were used. Dye decolorization was observed up to 28 days. Un-inoculated PDA agar supplemented with each dye was used as control.
2.12. Statistical analysis
All experiments were conducted in triplicate. A pairwise multiple comparison procedure following Student-Newman-Keuls post hoc tests was used to identify sample means that were significantly different from each other. SigmaPlot 11 (Systat Software Inc., San Jose, CA, USA) was used to calculate statistical probabilities. A P-value <0.05 indicated statistically significant differences between means of maximum MnP and laccase activities, fungal growth and pellet size at a 95% confidence interval.
3. Results
3.1. Identification of fungal strain KU-RNW027
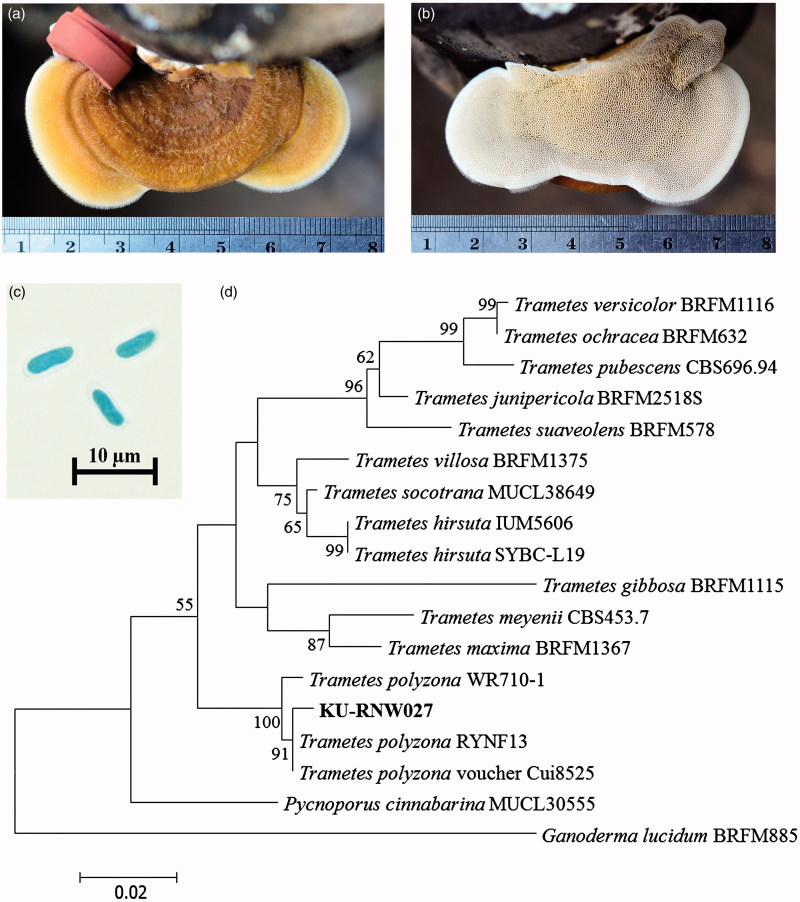
The white rot fungal strain KU-RNW027 developed shelf-like polypore basidiomata on the wooden log of Agastya after 1 month. Morphological characteristics of the mature fruiting body included a basidiocarp of reniform to subcircular shape 3.0–5.0 cm wide, 5.0–8.0 cm long, and 0.5–0.7 cm thick (Figure 1(a)). Its upper surface was yellowish brown with radial furrows, fine velvety hairs, and no stipe. Irregularly shaped pores were creamy in color and darkened to yellowish brown when bruised. There were 3–5 pores per mm2 (Figure 1(b)). The basidiospores were 7.0–9.0 × 2.0–3.0 µm as curved cylindrical sausage-like shapes with smooth walls and a white spore print (Figure 1(c)). These characteristics suggested that KU-RNW027 belonged to the genus Trametes [38,39].
Figure 1.
Identification of fungal strain KU-RNW027. Upper side of basidiomata (a) and lower side of basidiomata (b) grown on Agastya after one month. Micrograph of basidiospores (c) and phylogenetic tree (d) of related species in genus Trametes based on alignment of 555 nucleotides of the internal transcribed spacer (ITS) region. Bootstrap values (%) were generated from maximum likelihood analysis. Confidence values above 50% obtained from 1000-replicate bootstrap are indicated at the branch nodes. The scale bar indicates number of base substitutions per site.
Molecular taxonomy using fungal SSU rDNA, LSU rDNA, and ITS region confirmed its genus and showed closest similarity to T. polyzona. SSU rDNA of 1656 bp showed 99% similarity to a complete sequence of 18S SSU rDNA of T. polyzona strain OH-184-SP. LSU rDNA of 1362 bp showed 100% similarity to a partial sequence of 25S LSU rDNA of T. polyzona voucher Cui8525, while ITS region 561 bp fragment showed 99% similarity to a complete sequence of ITS region of T. polyzona strain RYNF13.
A phylogenetic tree was constructed using the ITS sequence of genus Trametes [40]. ITS sequence of Pycnoporus cinnabarinus MUCL30555 and Ganoderma lucidum BRFM885 were used as the outgroup. The best phylogenetic tree was obtained using a maximum parsimony method (Figure 1(d)) Strain KU-RNW027 was clustered in the same position as T. polyzona RYNF13, voucher Cui8525, and WR710-1 [17,41].
Based on both morphology and genetic information, strain KU-RNW027 was classified as Trametes polyzona KU-RNW027. DNA sequences of SSU rDNA, LSU rDNA, and ITS sequence of T. polyzona KU-RNW027 were deposited in GenBank under accession numbers LC190466, LC190465, and LC190464, respectively.
3.2. Effect of aeration on ligninolytic enzyme production and fungal growth
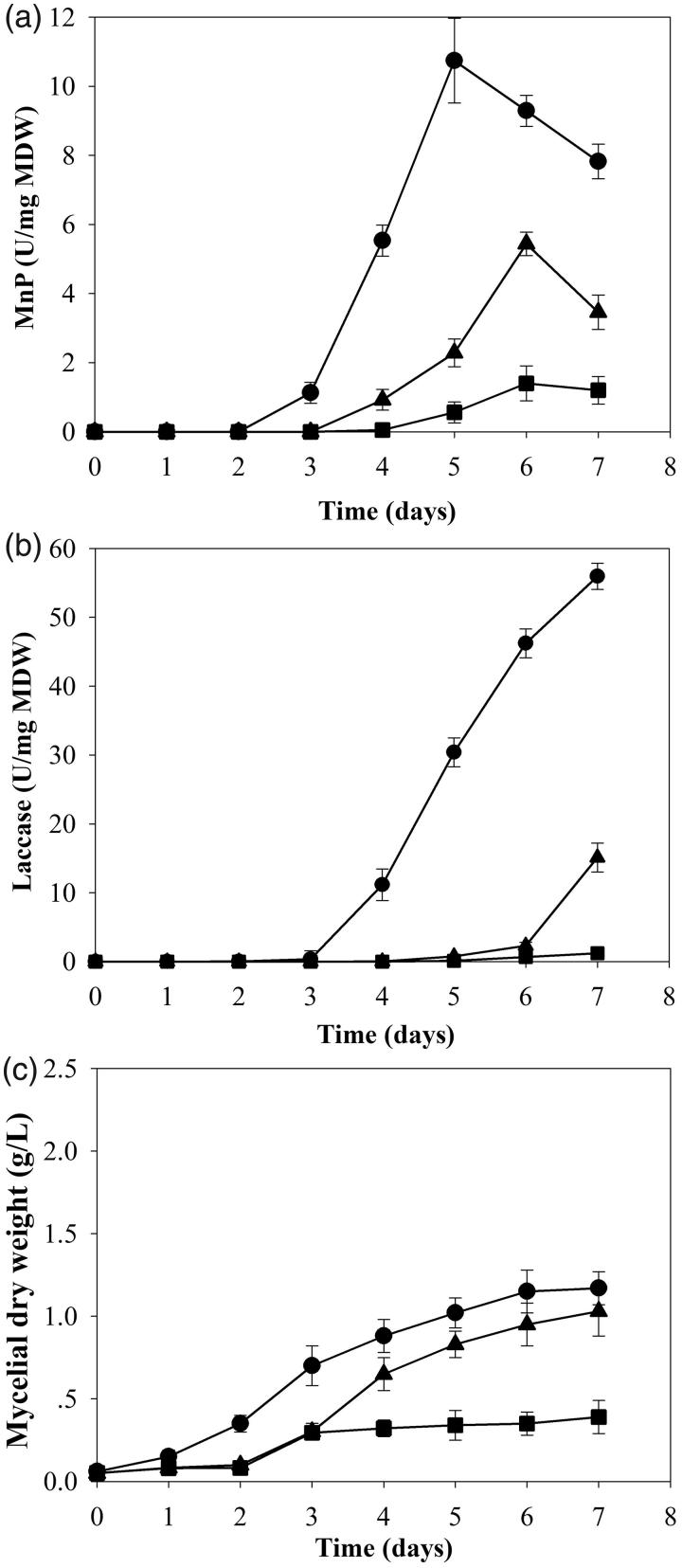
Comparison of enzyme production was performed with cultures grown from 15 discs of mycelial tips in 50 mL broth under three different conditions of continuous shaking, 3-day static followed by 4-day shaking and static conditions. Cultures grown in continuous shaking conditions for 7 days gave the highest activities of MnP and laccase (Figure 2(a,b)). No activity of LiP was detected in all conditions. The continuous shaking culture also produced enzymes faster than the other two conditions. Enzymes were detected on day 2 and 3. Efficiencies of cells in producing MnP and laccase in continuous shaking culture were highest at 10.74 and 55.95 U/mg MDW, respectively at 2 and 4 times higher than the 3-day static followed by 4-day shaking condition. Moreover, they were 8 and 47 times higher than the static culture (Figure 2(a,b)).
Figure 2.
Effect of aeration in shaking condition on production of manganese peroxidase (a), laccase (b) and growth (c) of Trametes polyzona KU-RNW027 in Kirk’s liquid medium.  ; Continuous shaking culture,
; Continuous shaking culture,  ; Static 3 days followed by shaken culture,
; Static 3 days followed by shaken culture,  ; Static culture. Error bars show standard deviation (n = 3).
; Static culture. Error bars show standard deviation (n = 3).
Aeration also affected fungal growth of strain KU-RNW027 (Figure 2(c)). Growth under continuous shaking was the fastest and obtained the highest biomass of 1.13 g/L at day 7. The shaking condition was selected for further study.
3.3. Effect of inoculum morphology on enzyme production and fungal growth
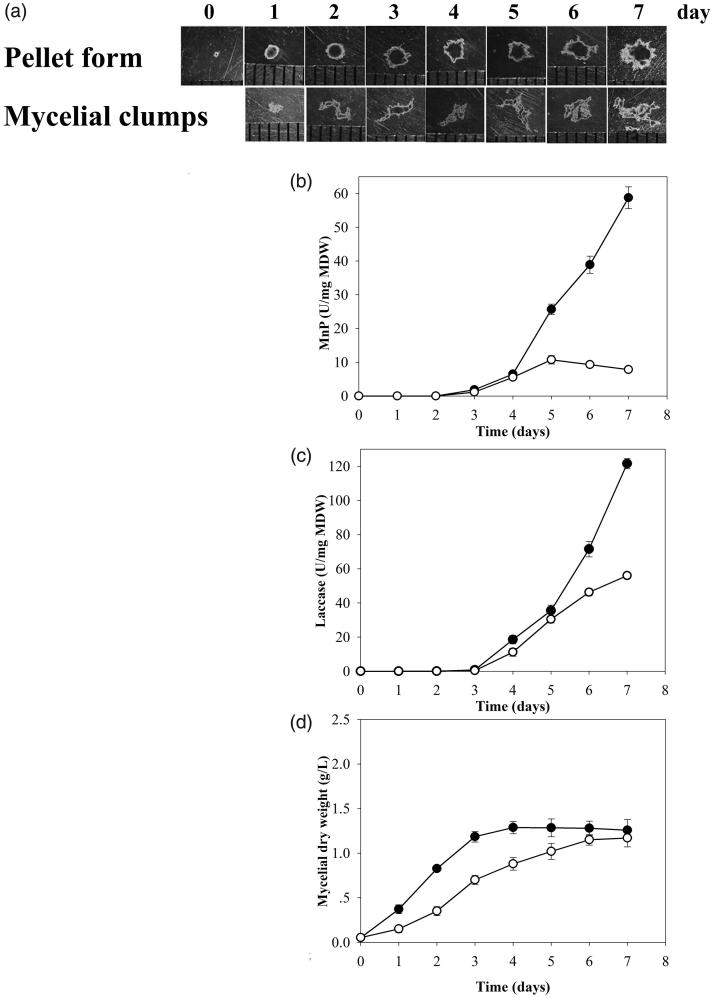
After cultivation using 15 discs of mycelial tips as inoculum in the shaking condition, the fungus grew as free-form mycelial clumps. However, when using fungal pellets as inoculum and a smaller number of 5 discs under the same conditions, the fungus grew in pellet form. The mycelial clumps had different shapes and sizes. In contrast, using pellets as inoculum resulted in the fungus maintaining a homogenous spherical shaped pellet form after 7 days (Figure 3(a)).
Figure 3.
Stereomicrographs of pellets and mycelial clumps observed daily (a). Effects of fungal morphology of pellet and mycelial clumps on manganese peroxidase (b), laccase (c), and activities by Trametes polyzona KU-RNW027 in Kirk’s liquid medium and fungal growth (d).  ; Pellet form,
; Pellet form,  ; Mycelial clump form. Error bars show standard deviation (n = 3).
; Mycelial clump form. Error bars show standard deviation (n = 3).
Pellet formation only appeared when small numbers of mycelium were inoculated, and resulted in greater activities of MnP and laccase at 4.8 and 2 times higher, respectively compared with the mycelial clump form (Figure 3(b,c)). These results suggested that morphology of the fungal formation had an impact on its ligninolytic enzyme production capability with the pellet form showing greater potential for MnP and laccase. Biomass of the fungus in pellet form was 1.28 g/L, slightly more than the mycelial clumps at 1.17 g/L (Figure 3(d)).
3.4. Fungal morphology and its correlation with enzyme production
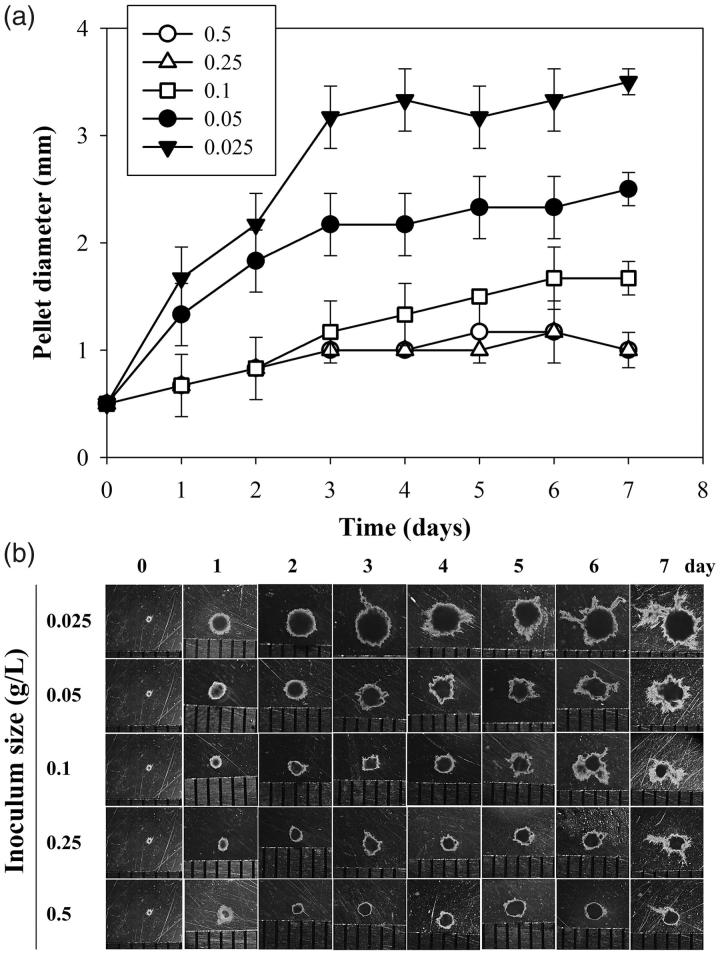
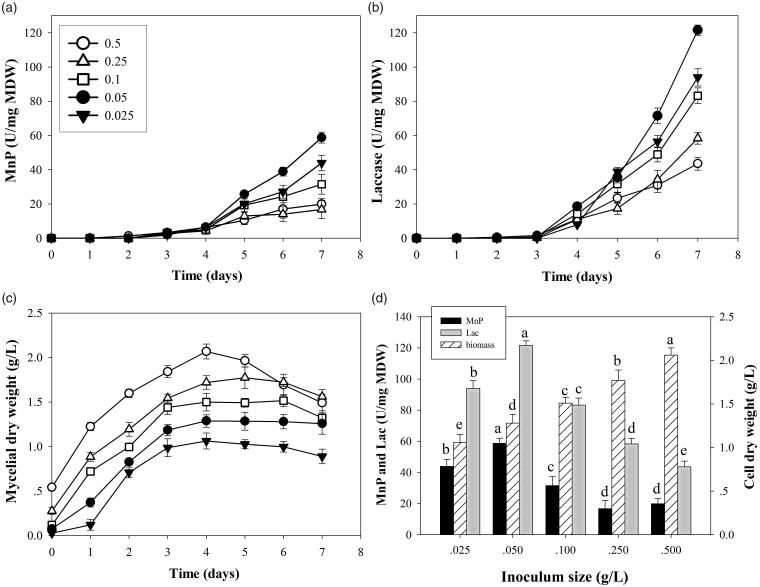
The quality of pellets as inoculum also affected enzyme productivity. Various amounts of pellet inoculum at 0.025, 0.05, 0.1, 0.25, and 0.5 g/L resulted in different pellet diameters (Figure 4). Largest pellet diameter of 3.5 mm was observed in cultures with 0.025 g/L inoculum. Cultures with 0.25 and 0.5 g/L inoculum gave smallest pellet diameter of 1.0 mm. Inoculum amounts of 0.025, 0.05, 0.1, 0.25, and 0.5 g/L produced pellet diameters at 3.5, 2.5, 1.7, 1.0, and 1.0 mm, respectively (Figure 4(a)). Mycelial appendages were elongated in the pellets after 3 days of cultivation and reverse correlation with inoculum amount was observed (Figure 4(b)).
Figure 4.
Time course of Trametes polyzona KU-RNW027 fungal pellet growth with different inoculum amounts. Average diameter (mm) of pellets grown with different inoculum amounts (a) and morphology under stereomicroscope (b),  ; 0.025 g/L,
; 0.025 g/L,  ; 0.05 g/L,
; 0.05 g/L,  ; 0.1 g/L,
; 0.1 g/L,  ; 0.25 g/L,
; 0.25 g/L,  ; 0.5 g/L. Error bars show standard deviation (n = 10).
; 0.5 g/L. Error bars show standard deviation (n = 10).
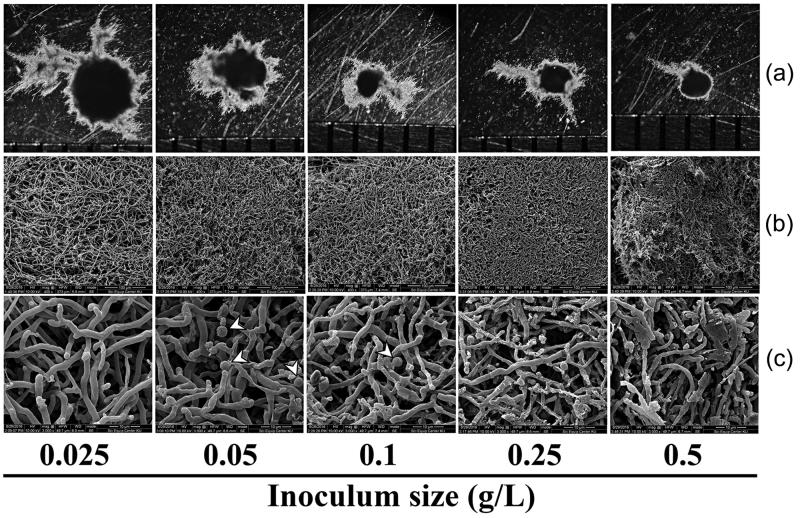
Highest enzyme activities of MnP and laccase at 58.7 and 121.5 U/mg MDW, respectively were obtained in cultures of inoculum at 0.05 g/L (Figure 5(a,b)). At this inoculum amount, pellets at 7 days of cultivation had 2.5 mm diameter (Figure 4(a,b)). Fungal biomass increased with increasing amount of pellet inoculum (Figure 5(c)) and pellets obtained from cultures with large quantities of inoculum were denser with thinner mycelia (Figure 6). Production of MnP and laccase in dense inoculum decreased (Figure 5(d)). Notably, culture inoculum amounts of 0.05 and 0.1 g/L showed mycelial differentiation, forming spherical cells 3–4 µm in diameter. This structure was similar to chlamydospore (Figure 6(c)) and was not found in the other conditions. More chlamydospore-like structures were observed in cultures with 0.05 g/L inoculum. Visualization of pellet cross-sections confirmed that porosity decreased at higher inoculum amounts (Figure 7(a,b)) and cell debris was more frequently seen, in particular for 0.5 g/L (Figure 7(b)). These findings suggested that inoculum amount of pellets critically affected fungus growth and resulted in different sizes of pellets and loose or dense mycelium, porosity, chlamydospore-like structures, and cell debris. Efficiency of cells in producing enzymes was different (Figure 5(d)). Pellets larger or smaller than 2.5 mm diameter significantly reduced enzyme productivity. Results suggested that pellet size, pellet porosity and chlamydospore-like structure formation were all variables affecting fungal cell efficiency in ligninolytic enzyme production.
Figure 5.
Effects of pellet inoculum size on productivities of manganese peroxidase (a) and laccase (b) of Trametes polyzona KU-RNW027, and fungal growth (c) in Kirk’s liquid medium.  ; 0.025 g/L,
; 0.025 g/L,  ; 0.05 g/L,
; 0.05 g/L,  ; 0.1 g/L,
; 0.1 g/L,  ; 0.25 g/L,
; 0.25 g/L,  ; 0.5 g/L. Comparison of values with different pellet inoculum amounts (d). Error bars show standard deviation (n = 3).
; 0.5 g/L. Comparison of values with different pellet inoculum amounts (d). Error bars show standard deviation (n = 3).
Figure 6.
Morphology of pellets of Trametes polyzona KU-RNW027 at 7-day incubation with different amounts of pellet inoculum as 0.025, 0.05, 0.1, 0.25, and 0.5 g/L. pellet morphology under stereomicroscope (a), structure of fungal pellet under SEM (400×) (b), and structure of fungal pellet under SEM (3,000×) (c). Arrows indicate terminal chlamydospore-like structures.
Figure 7.
Visualization of cross-sectioned pellets of Trametes polyzona KU-RNW027 at inoculum amounts 0.025, 0.05, and 0.5 g/L. cross-section of whole pellets (a), and fungal hyphae inside the pellets showing different porosity (b). Scale bar =100 µM.
3.5. Abilities of dye decolorization
Trametes polyzona KU-RNW027 completely decolorized five azo dyes and one anthraquinone dye (Figure 8). Decolorization was observed at 3 days in a deep tube agar containing 50 mg/L of each dye. Complete decolorization of RNB was found at 21 days, while other dyes required 28 days. Results indicated that white rot fungus, T. polyzona KU-RNW027 can be applied in bioremediation applications of aromatic compounds, especially synthetic dyes.
Figure 8.
Decolorization abilities of Trametes polyzona KU-RNW027 on deep tube agar containing 50 mg/L of each dye, Remazol brilliant yellow 4GL gran (RBY), Remazol brilliant blue R (RBBR), Remazol brilliant red F3B gran (RBR), Remazol golden yellow RGB gran (RGY), Remazol navy blue RGB 150% gran (RNB), and Remazol red RGB gran (RR).
4. Discussion
A white rot fungus was identified as T. polyzona KU-RNW027 according to its morphology and molecular taxonomy. This fungus showed high potential in decolorizing five azo dyes and one anthraquinone dye which are toxic commercial dyes that pollute the environment. The fungus produced two kinds of ligninolytic enzymes as MnP and laccase but not LiP. These two enzymes play important roles in degrading aromatic compounds including dyes [8,42].
Aeration was important for enzyme production by this fungus. Increasing oxygen by aeration or agitation resulted in significant increase of metabolic products [26,28]. However, Perenniporia tephropora produced MnP and laccase in cultures first grown in static condition for 3 days to obtain mycelium, followed by shaking for 7 days. Efficiency of cells under this condition was higher than static or continuous shaking [9]. Aspergillus niger preferred shaking condition to producing pectinase [28], while Ganoderma lucidum produced laccase more rapidly under shaking than static condition [43]. T. polyzona KU-RNW027 showed highest efficiency in producing MnP and laccase under continuous shaking from the first day, unlike P. tephropora. Optimal oxygen concentration for ligninolytic enzyme production was different for each strain.
Morphological characteristics of fungus grown in submerged culture related to cell capability for ligninolytic enzyme production. Fungal morphology appeared as two main types, viz mycelium clumps and pellets [23]. Using two different methods of inoculum preparation, mycelium clumps and pellets of T. polyzona KU-RNW027 were obtained. High inoculum amounts of fungal mycelium tips led T. polyzona KU-RNW027 to form mycelium clumps, whereas hyphal formation at low inoculum levels appeared as pellets [44]. Cultures that grew in the form of mycelium clumps were less efficient in producing MnP and laccase than those growing as pellets, concurring with the efficiency of laccase production by Flammulina velutipes [10]. MnP and laccase activities detected after 7 days cultivation of T. polyzona KU-RNW027 grown in pellet form were 4.8 and 2 times higher than from mycelial clumps, respectively.
Further experiments using different amounts of pellets as inoculum for enzyme production are required to provide more information regarding morphology formation and correlation between morphology and cell efficiency on enzyme production. Higher amounts of pellets used as inoculum produced smaller pellet diameter at the end of 7-day incubation. With large inoculum of 0.25 and 0.5 g/L, the fungus grew to pellet size of 1.0 mm. Stereomicroscope and SEM imagery confirmed that cross sections of dense pellets showed low porosity. Inadequate transfer of oxygen and nutrients were suggested to occur in dense pellets [45]. Limitations of oxygen and substrate transfer resulted in autolysis of fungal cells inside the core of the pellet [46]. Therefore, dense hyphal pellets of 0.25 and 0.5 g/L resulted in more cell debris with reduced enzyme production. At the small inoculum value of 0.025 g/L, fungal pellets grew to 3.5 mm diameter after 7 days with loose hyphae. Large pellets resulted in a low-oxygen area in the core with limited oxygen penetration depth [45]. Consequently, large pellets of 0.025 g/L also showed poor efficiency in enzyme production. At inoculum size of 0.05 g/L, cultures produced highest MnP and laccase production. Pellets observed on day 7 had 2.5 mm diameter and formed more terminal chlamydospore-like structures. The formation of chlamydospore-like structures suggested their involvement as reservoirs to deliver enzymes into the medium [47].
Morphology of the fungal pellets significantly influenced oxygen and substrate transfer [29,44,48]. Our results confirmed that in T. polyzona KU-RNW027, oxygen supply was a critical factor for fungal growth and ligninolytic enzyme production. This fungus required continuous shaking condition and the pellet form gave higher enzyme productivity than mycelial clumps. Our results also suggested that pellet size and morphology of the growth during incubation were critical factors. Inoculum amount impacted on the morphology of pellet growth to obtain optimal pellet size, porosity and chlamydospore-like structure. All these factors related to oxygen balance, substrate transfer and enzyme reservoirs regarding fungal efficiency for MnP and laccase production.
Funding Statement
This work was supported by the Thailand Research Fund through the Royal Golden Jubilee Ph.D. program (grant number PHD/0214/2553).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Hofrichter M. Review: lignin conversion by manganese peroxidase (MnP). Enzyme Microb Technol. 2002;30:454–466. [Google Scholar]
- 2.Riverra-Hoyos CM, Edwin DM, Raul AP, et al. . Fungal laccases. Fungal Biol Rev. 2013;27:67–82. [Google Scholar]
- 3.Teerapatsakul C, Parra R, Bucke C, et al. . of Ganoderma sp. KU-Alk4, regulated by different glucose concentration in alkaline media. World J Microbiol Biotechnol. 2007;23:1519–1527. [Google Scholar]
- 4.Fang W, Fernandes ÉKK, Roberts DW, et al. . A laccase exclusively expressed by Metarhizium anisopliae during isotropic growth is involved in pigmentation, tolerance to abiotic stresses and virulence. Fungal Genet Biol. 2010;47:602–607. [DOI] [PubMed] [Google Scholar]
- 5.Ujor VC, Monti M, Peiris DG, et al. . The mycelial response of the white-rot fungus, Schizophyllum commune to the biocontrol agent, Trichoderma viride. Fungal Biol. 2012;116:332–341. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Chen H, Chen M, et al. . Cloning and functional analysis of a laccase gene during fruiting body formation in Hypsizygus marmoreus. Microbiol Res. 2015;179:54–63. [DOI] [PubMed] [Google Scholar]
- 7.Osma JF, Toca-Herrera JL, Rodríguez-Couto S. Biodegradation of a simulated textile effluent by immobilised-coated laccase in laboratory-scale reactors. Appl Catal A. 2010;373:147–153. [Google Scholar]
- 8.Teerapatsakul C, Parra R, Keshavarz T, et al. . Repeated batch for dye degradation in an airlift bioreactor by laccase entrapped in copper alginate. Int Biodeterior Biodegradation. 2017;120:52–57. [Google Scholar]
- 9.Teerapatsakul C, Chitradon L. Physiological regulation of an alkaline-resistant laccase produced by Perenniporia tephropora and efficiency in biotreatment of pulp mill effluent. Mycobiology. 2016;44:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito K, Ikeda R, Endo K, et al. . Isolation of a novel alkaline-induced laccase from Flammulina velutipes and its application for hair coloring. J Biosci Bioeng. 2012;113:575–579. [DOI] [PubMed] [Google Scholar]
- 11.Fillat A, Gallardo O, Vidal T, et al. . Enzymatic grafting of natural phenols to flax fibres: development of antimicrobial properties. Carbohydr Polym. 2012;87:146–152. [DOI] [PubMed] [Google Scholar]
- 12.Schubert M, Engel J, Thöny-Meyer L, et al. . Protection of wood from microorganisms by laccase-catalyzed iodination. Appl Environ Microbiol. 2012;78:7267–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grover N, Borkar IV, Dinu CZ, et al. . Laccase- and chloroperoxidase-nanotube paint composites with bactericidal and sporicidal activity. Enzyme Microb Technol. 2012;50:271–279. [DOI] [PubMed] [Google Scholar]
- 14.Ihssen J, Schubert M, Thöny-Meyer L, et al. . Laccase catalyzed synthesis of iodinated phenolic compounds with antifungal activity. Plos One. 2014;9:e89924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen X, Jia Y, Li J. Enzymatic degradation of tetracycline and oxytetracycline by crude manganese peroxidase prepared from Phanerochaete chrysosporium. J Hazard Mater. 2010;177:924–928. [DOI] [PubMed] [Google Scholar]
- 16.Mir-Tutusaus JA, Sarrà M, Caminal G. Continuous treatment of non-sterile hospital wastewater by Trametes versicolor: how to increase fungal viability by means of operationalstrategies and pretreatments. J Hazard Mater. 2016;318:561–570. [DOI] [PubMed] [Google Scholar]
- 17.Teerapatsakul C, Pothiratana C, Chitradon L, et al. . Biodegradation of polycyclic aromatic hydrocarbons by a thermotolerant white rot fungus Trametes polyzona RYNF13. J Gen Appl Microbiol. 2016;62:303–312. [DOI] [PubMed] [Google Scholar]
- 18.Alberts JF, Gelderblom WCA, Botha A, et al. . Degradation of aflatoxin B(1) by fungal laccase enzymes. Int J Food Microbiol. 2009;135:47–52. [DOI] [PubMed] [Google Scholar]
- 19.Purnomo AS, Mori T, Kamei I, et al. . Application of mushroom waste medium from Pleurotus ostreatus for bioremediation of DDT-contaminated soil. Int Biodeterior Biodegradation. 2010;64:397–402. [Google Scholar]
- 20.Babic J, Pavko A. Enhanced enzyme production with the pelleted form of D. squalens in laboratory bioreactors using added natural lignin inducer. J Ind Microbiol Biotechnol. 2012;39:449–457. [DOI] [PubMed] [Google Scholar]
- 21.Tavares APM, Coelho MAZ, Agapito MSM, et al. . Optimization and modeling of laccase production by Trametes versicolor in a bioreactor using statistical experimental design. Appl Biochem Biotechnol. 2006;134:233–248. [DOI] [PubMed] [Google Scholar]
- 22.Fomina M, Gadd GM. Influence of clay minerals on the morphology of fungal pellets. Mycol Res. 2002;106:107–117. [Google Scholar]
- 23.Papagianni M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv. 2004;22:189–259. [DOI] [PubMed] [Google Scholar]
- 24.Liao W, Liu Y, Frear C, et al. . A new approach of pellet formation of a filamentous fungus - Rhizopus oryzae. Bioresour Technol. 2007;98:3415–3423. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y, Song H. Effect of fungal pellet morphology on enzyme activities involved in phthalate degradation. J Microbiol. 2009;47:420–424. [DOI] [PubMed] [Google Scholar]
- 26.Lin P, Scholz A, Krull R. Effect of volumetric power input by aeration and agitation on pellet morphology and product formation of Aspergillus niger. Biochem Eng J. 2010;49:213–220. [Google Scholar]
- 27.Haroune L, Saibi S, Bellenger J, et al. . Evaluation of the efficiency of Trametes hirsuta for the removal of multiple pharmaceutical compounds under low concentrations relevant to the environment. Bioresour Technol. 2014;171:199–202. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim D, Weloosamy H, Lim S. Effect of agitation speed on the morphology of Aspergillus niger HFD5A-1 hypha and its pectinase production in submerged fermentation. World J Biol Chem. 2015;6:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu HS, Lin BC. Effect of oxygen transfer and pellet size for producing of glucosamine using Aspergillus sydowii BCRC 31742 cultivated in a fermenter. J Food Process Technol. 2017;8:697. doi: 10.4172/2157-7110.1000697 [DOI] [Google Scholar]
- 30.Tien M, Kirk TK. Lignin-degrading enzyme from Phanerochaete chrysosporium: purification, characterization and catalytic properties of unique H2O2 requiring oxygenase. Proc Natl Acad Sci USA. 1984;81:2280–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruivo CCC, Lachance M, Rosa CA, et al. . Candida bromeliacearum sp. nov. and Candida ubatubensis sp. nov., two yeast species isolated from the water tanks of Canistropsis seidelii (Bromeliaceae). Int J Syst Evol Microbiol. 2005;55:2213–2217. [DOI] [PubMed] [Google Scholar]
- 32.Moncalvo JM, Lutzoni FM, Rehner SA, et al. . Phylogenetic relationships of agaric fungi based on nuclear large subunit ribosomal DNA sequences. Syst Biol. 2000;49:278–305. [DOI] [PubMed] [Google Scholar]
- 33.White TJ, Bruns T, Lee S, et al. . Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: Innis MA, Gelfand DH, Sninsky JJ, et al. editors. PCR Protocols: a guide to methods and applications. New York: Academic Press; 1990. p. 315–322. [Google Scholar]
- 34.Thompson JD, Higgins DG, Gibson TJ. Improved sensitivity of profile searches through the use of sequence weights and gap excision. Comput Appl Biosci. 1994;10:19–29. [DOI] [PubMed] [Google Scholar]
- 35.Tamura K, Peterson D, Peterson N, et al. . MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony. Mol Biol Evol. 2011;28:2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondo R, Harazono K, Sakai K. Bleaching of hardwood kraft pulp with manganese peroxidase secreted from Phanerochaete sordida YK-624. Appl Environ Microbiol. 1994;60:4359–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirk TK, Tien M, Kersten PJ, et al. . Lignin peroxidase from fungi: Phanerochaete chrysosporium. Method Enzymol. 1990;188:159–171. [Google Scholar]
- 38.Chandrasrikul A, Suwnnarit P, Sangwanit U, et al. . Diversity of mushrooms and macrofungi in Thailand. Bangkok: Kasetsart University Press; 2008. [Google Scholar]
- 39.Raksawong P, Flegel TW. Thai mushrooms and other fungi. National Center for Genetic Engineering and Biotechnology (BIOTEC). Bangkok: National Science and Technology Development Agency, 2001. [Google Scholar]
- 40.Welti S, Moreau P, Favel A, et al. . Molecular phylogeny of Trametes and related genera, and description of a new genus Leiotrametes. Fungal Divers. 2012;55:47–64. [Google Scholar]
- 41.Chairin T, Nitheranont T, Watanabe A, et al. . Purification and characterization of the extracellular laccase produced by Trametes polyzona WR710–1 under solid-state fermentation. J Basic Microbiol. 2014;54:35–43. [DOI] [PubMed] [Google Scholar]
- 42.Bilal M, Muhammad A, Roberto P, et al. . Immobilized ligninolytic enzymes: an innovative and environmental responsive technology to tackle dye-based industrial pollutants – a review. Sci Total Environ. 2017;576:646–659. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Chaozhi T, Guangli Y, et al. . A novel membrane-surface liquid co-culture to improve the production of laccase from Ganoderma lucidum. Biochem Eng J. 2013;80:27–36. [Google Scholar]
- 44.Espinosa-Ortiz EJ, Rene ER, Pakshirajan K, et al. . Fungal pelleted reactors in wastewater treatment: applications and perspectives. Chem Eng J. 2016;283:553–571. [Google Scholar]
- 45.Bizukojc M, Gonciarz J. Influence of oxygen on lovastatin biosynthesis by Aspergillus terreus ATCC 20542 quantitatively studied on the level of individual pellets. Bioprocess Biosyst Eng. 2015;38:1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Enshasy H, Kleine J, Rinas U. Agitation effects on morphology and protein productive fractions of filamentous and pelleted growth forms of recombinant Aspergillus niger. Process Biochem. 2006;41:2103–2112. [Google Scholar]
- 47.Jiménez-Tobon G, Kurzatkowski W, Rozbicka B, et al. . In situ localization of manganese peroxidase production in mycelial pellets of Phanerochaete chrysosporium. Microbiology (Reading, Engl). 2003;149:3121–3127. [DOI] [PubMed] [Google Scholar]
- 48.Hille A, Neu TR, Hempel DC, et al. . Oxygen profiles and biomass distribution in biopellets of Aspergillus niger. Biotechnol Bioeng. 2005;92:614–623. [DOI] [PubMed] [Google Scholar]