Abstract
Aims/Introduction
To evaluate the effect of probiotic supplements on insulin resistance in pregnant women with diet‐controlled gestational diabetes mellitus.
Materials and Methods
A randomized, double‐blind, placebo‐controlled trial was carried out between June 2016 and February 2017. Pregnant women with diet‐controlled gestational diabetes mellitus were enrolled in the study at 24–28 weeks‐of‐gestation and randomized to receive either probiotic supplements containing Bifidobacterium and Lactobacillus or a placebo daily for four consecutive weeks. Primary outcomes were mean differences in insulin resistance (homeostatic model assessment for insulin resistance), fasting insulin and fasting plasma glucose between the two groups. Secondary outcomes were changes in maternal weight after the intervention.
Results
Data from 28 patients in the probiotic group and 29 in the placebo group were analyzed. The changes in metabolic parameters after randomization showed significant improvement in glucose metabolism in the probiotic group compared with the placebo group, including fasting plasma glucose (0.68 ± 5.88 vs 4.620 ± 7.78 mg/dL, mean difference −3.94 mg/dL, 95% confidence interval −7.62, −0.27, P = 0.034), fasting plasma insulin (1.11 ± 1.71 vs 3.77 ± 1.70 mIU/L, mean difference −2.67 mIU/L, 95% confidence interval −3.57, −1.76, P = 0.001) and homeostatic model assessment for insulin resistance (0.25 ± 0.37 vs 0.89 ± 0.46, mean difference −0.63, 95% confidence interval −0.86, −0.41, P = 0.001). Weight gain during randomization was similar between the two groups.
Conclusions
Four weeks of probiotic supplements in women with diet‐controlled gestational diabetes in the late second and early third trimester lowered fasting glucose and increased insulin sensitivity. Probiotic supplements may be considered as an adjunct treatment for glycemic control in these patients.
Keywords: Gestational diabetes mellitus, Insulin resistance, Probiotics
Introduction
Gestational diabetes mellitus (GDM) is a major medical complication during pregnancy, occurring in 9.3–25.5% of pregnant women1. Increasing maternal insulin resistance, peaking at 24–28 weeks‐of‐gestation, in combination with inadequate β‐cell function, results in glucose intolerance. High levels of maternal blood glucose can lead to adverse pregnancy outcomes, including pre‐eclampsia2, unplanned cesarean delivery3, macrosomia, birth trauma and neonatal hypoglycemia4. Based on previous evidence, optimal glycemic control has been shown to reduce complications of mothers, fetuses and neonates5, 6. All pregnant women with GDM should be counseled regarding appropriate diet and exercise. However, approximately 11% of women with GDM fail to adequately control their glucose levels with diet and exercise alone, and require medication7.
Probiotics are defined as living microorganisms that, when consumed in adequate amounts, will provide health benefits to the host8. Most of them are prepared in the form of yogurt, fermented foods and capsule supplements. Ingestion of probiotics is generally considered safe in both pregnant women and their fetuses9, 10. The addition of these beneficial microorganisms help balance and increase the abundance of good microorganisms in the digestive tract. Indeed, the impact of probiotics on various outcomes has been shown, including dermatological diseases11, gastrointestinal diseases12, 13, 14 and metabolic disorders15, 16. Recently, several studies from different countries (i.e., Iran, Ireland and Turkey) have shown the favorable effects of probiotic supplements with diverse compositions and durations of therapy on glycemic control in pregnant women with GDM17, 18, 19, 20, 21, 22, 23. However, the impact of probiotics in South‐East Asian populations has not been reported. Dietary traditions, geographic locations and human genetics can influence gut microbial composition among different ethnic groups24, 25, 26. Furthermore, some studies have shown an abundance of Bacteroides/Bifidobacterium and Prevotella species in the digestive tracts of Asian populations24. Thus, the effectiveness of probiotic supplements might differ based on the background microbiota compositions of the population and should be investigated in various geographic regions. Therefore, the purpose of the present study was to evaluate the effects of probiotic supplements on metabolic parameters, including fasting glucose and insulin resistance, in pregnant women with GDM in Thailand.
Methods
Study design
A double‐blind, placebo‐controlled, randomized clinical trial was carried out between June 2016 and February 2017 at the Antenatal Care Clinic, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. This study was reviewed and approved by the Committee on Human Rights Related to Research Involving Human Subjects, Faculty of Medicine, Ramathibodi Hospital, and complied with the Declaration of Helsinki. Written informed consent was obtained from all participants. The study was registered at the Thai clinical trials registry, number 20170606002.
Participants
Newly diagnosed women with GDM between 24–28 weeks‐of‐gestation who attended the Antenatal Care Clinic and met the inclusion criteria were invited to participate in the study. GDM was diagnosed based on the International Association of Diabetes and Pregnancy Study Groups criteria27 as follows: fasting plasma glucose ≥92 mg/dL at the first prenatal visit, or an abnormal glucose tolerance test at 24–28 weeks‐of‐gestation using a 75‐g oral glucose load (defined as one or more of the following abnormal glucose values: fasting plasma glucose ≥92 mg/dL, 1‐h ≥180 mg/dL, 2‐h ≥153 mg/dL). Other inclusion criteria included: (i) singleton pregnancy; (ii) maternal age of 18–45 years; (iii) normal fetal structures or chromosomes based on ultrasound scanning during the second trimester and/or invasive prenatal diagnosis; and (iv) no history of chronic diseases, such as immunodeficiency, hypertension, pre‐gestational diabetes, kidney disease or liver disease. Exclusion criteria included: (i) consuming probiotic food products, such as yogurt, fermented foods and bean paste during the 2 weeks before enrollment; and (ii) exposure to antibiotics during the 4 weeks before enrollment.
Baseline characteristics
Age and pre‐pregnancy weight (from chart review or patient interview) were obtained. Height was measured at first visit, and body mass index was calculated from the formula: weight (kg)/height2 (m2). Additionally, educational level and family history of diabetes in first‐degree relatives were obtained. Physical activity was evaluated using questionnaires. Participants were categorized into three groups based on their 3‐day usual activity; that is, low, moderate and high physical activity. Low physical activity was defined as limited to chores, such as cooking, sewing and working on a computer. Moderate physical activity was defined as work requiring bodily movements, such as cleaning or taking care of children. High physical activity was defined as regular aerobic exercise, such as running, biking or swimming.
Randomization
A double‐blind randomized controlled trial was carried out in which participants who agreed to participate in the study were randomly assigned to take one capsule containing either a probiotic or placebo. Random allocation sequence was carried out in blocks of four by the statistician. Researchers arranged the enrollment and intervention assignment of participants. The capsules and their packages were unidentified to participants, researchers and primary investigators.
Interventions
All participants were randomly allocated to take one capsule containing either a probiotic or placebo once daily after the morning meal for four consecutive weeks. The probiotic used in this study was Infloran® (Laboratorio Farmaceutico SIT, Mede, Italy, and imported by DKSH, Bangkok, Thailand). It is commercially available at Ramathibodi Hospital, where it has been used for the treatment of acute non‐specific enterocolitis and chronic constipation. Each capsule contained 1,000 million CFU of Lactobacillus acidophilus and 1,000 million CFU of Bifidobacterium bifidum. This dose was similar to that utilized in previous studies18, 20. Each placebo capsule contained gelatin. Participants were counseled to avoid probiotic‐containing foods and supplements throughout the study period to minimize the confounding from other probiotics.
Participants were given a 2‐week package of either probiotic or placebo capsules and instructed to refrigerate them at 4°C. They were seen every 2 weeks at our antenatal care clinic for standard antenatal treatment, monitoring compliance with the treatment and monitoring for adverse effects from the interventions. Compliance monitoring was carried out by capsule count. Afterwards, a second 2‐week package of either probiotic or placebo capsules was given to the patients. Additionally, weekly telephone follow‐up calls were carried out to encourage compliance with the assigned intervention and to monitor for any possible adverse events.
All participants received counseling regarding diet control and lifestyle modification from nutrition counselors and nurses as per the standard of care at Ramathibodi Hospital. Additionally, a 24‐h dietary recall questionnaire for three consecutive days was completed after 2 weeks of intervention and used as the participant's representative diet during the study period. Daily caloric intake, along with carbohydrates, fat, protein and fiber consumption, were analyzed using the Thai nutritional database developed by the Institute of Nutrition, Mahidol University, Thailand (INMUCAL software).
Participants were discontinued from the study if one of the following occurred: (i) use of antibiotics or immunosuppressive drugs during the intervention; (ii) serious adverse effects from probiotics, such as diarrhea, nausea/vomiting or sepsis; and (iii) need for insulin.
Outcome measures
Primary outcomes of the present study were mean differences in the changes of metabolic parameters between the two groups during the intervention, including fasting plasma glucose, insulin and insulin resistance index. At baseline and 4 weeks after randomization, blood samples were obtained after an overnight fast. Fasting plasma glucose and fasting plasma insulin were measured (see below). Homeostatic model assessment for insulin resistance (HOMA‐IR), an index of fasting insulin resistance, was calculated from the formula: fasting plasma insulin (U/mL) × fasting plasma glucose (mmol/L)/22.528, 29. For the present study, plasma glucose was measured by hexokinase/glucose‐6‐phosphate dehydrogenase (Architect c Systems; Abbott Core Laboratories, Abbott Park, IL, USA), and fasting plasma insulin was measured immunometrically using chemiluminescence detection (Immulite 2000; Siemens, Erlangen, Germany; coefficients of variance <5%).
The mean difference in maternal weight gain across the study period was a secondary outcome. Although not a pre‐specified planned outcome of the study, pregnancy outcomes were collected from medical records. These included total gestational weight gain, birth weight and rates of neonatal hypoglycemia.
Sample size
Based on similar randomized clinical trials in which HOMA‐IR was used as an outcome measure18, assuming types I and II error rates of 5% (α = 0.05) and 20% (β = 0.2; power = 80%), respectively, the sample size for each group was calculated at 30 women.
Statistical analysis
Data were analyzed using the SPSS statistical software package version 22 IBM (International Business Machines Corp.), Armonk, New York, USA. The variables were expressed as means (standard deviation) and n (%). After testing data for normality, two‐sample t‐tests or Mann–Whitney tests were used to compare continuous variables between the two groups at baseline as appropriate. The χ2‐test and Fisher's exact tests were used to compare categorical data. Mean differences in the changes in metabolic parameters during the intervention period between the two groups were compared by independent t‐tests. P‐values <0.05 were considered statistically significant.
Results
A total of 122 potential participants were screened. Of these, 40 did not meet the inclusion criteria, and 22 declined to participate. This resulted in 60 newly diagnosed GDM pregnant women enrolled in the trial and randomized into either the probiotic or placebo group (Figure 1). Two pregnant women were discontinued from the probiotic group; one due to a subsequent diagnosis of systemic lupus erythematosus requiring steroid treatment, and the other due to antibiotic use during the study period. One woman discontinued from the placebo group due to a subsequent diagnosis of systemic lupus erythematosus requiring steroid treatment. As a result, data from the 28 patients in the probiotic group and 29 in the placebo group were analyzed at study completion. The CONSORT diagram of the study is shown in Figure 1.
Figure 1.
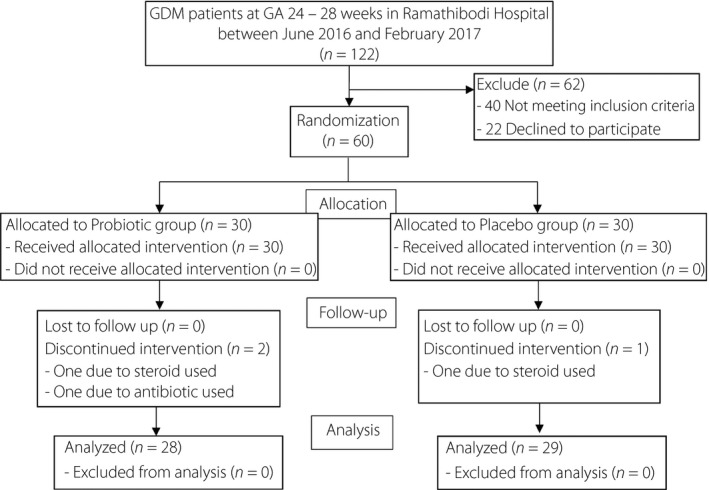
CONSORT 2010 flow diagram for ‘Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double‐blind randomized controlled trial.’
Baseline characteristics of participants in each group are shown in Table 1. The mean age, gestational age, pre‐pregnancy body mass index, body weight at enrollment and baseline physical activity levels were not significantly different between the probiotic and placebo groups. There were no statistically significant differences between the two groups in fasting plasma glucose, fasting plasma insulin and HOMA‐IR at baseline.
Table 1.
Baseline characteristics of the participants
| Demographic data | Probiotic group (n = 28) | Placebo group (n = 29) | P‐value |
|---|---|---|---|
| Maternal age (years) | 32.50 ± 5.02 | 30.72 ± 5.05 | 0.19 |
| Gestational age at study (weeks) | 27.29 ± 2.42 | 27.97 ± 2.54 | 0.31 |
| Pre‐pregnancy BMI (kg/m2) | 22.74 ± 3.73 | 22.04 ± 3.12 | 0.45 |
| Body weight at study (kg) | 63.49 ± 10.75 | 62.88 ± 9.33 | 0.82 |
| Physical activity, n (%) | |||
| Low | 22 (78.6) | 24 (82.8) | 0.69 |
| Moderate | 6 (21.4) | 5 (17.2) | |
| High | 0 (0) | 0 (0) | |
| Gestation, n (%) | |||
| Primigravida | 10 (35.7) | 13 (44.8) | 0.25 |
| Multiparity | 18 (64.3) | 16 (55.2) | |
| History of diabetes in first‐degree relatives, n (%) | 6 (21.4) | 7 (24.1) | 0.36 |
| Physical activity, n (%) | |||
| Low | 22 (78.6) | 24 (82.8) | 0.69 |
| Moderate | 6 (21.4) | 5 (17.2) | |
| High | 0 (0) | 0 (0) | |
| Education, n (%) | |||
| Less than college degree | 5 (17.9) | 2 (6.9) | 0.69 |
| College degree or higher | 23 (82.1) | 27 (93.1) | |
BMI, body mass index.
The primary outcomes, which were mean differences in the changes of fasting plasma glucose, fasting plasma insulin and HOMA‐IR, after the intervention between the two groups, are provided in Table 2. The changes in metabolic parameters after randomization showed significantly improved glucose metabolism in the probiotic group compared with the placebo group, including fasting plasma glucose (0.68 ± 5.88 vs 4.620 ± 7.78 mg/dL, mean difference (MD) −3.94 mg/dL, 95% confidence interval [CI] −7.62, −0.27), P = 0.034), fasting plasma insulin (1.11 ± 1.71 vs 3.77 ± 1.70 mIU/L, MD −2.67 mIU/L, 95% CI −3.57, −1.76, P = 0.001) and HOMA‐IR (0.25 ± 0.37 vs 0.89 ± 0.46, MD −0.63, 95% CI −0.86, −0.41, P = 0.001).
Table 2.
Changes in glucose metabolism indices, weight gain and caloric intake of the participants
| Results | Probiotic group (n = 28) | Placebo group (n = 29) | Mean difference (95% CI) | P‐value |
|---|---|---|---|---|
| Fasting plasma glucose (mg/dL) | ||||
| Pre‐intervention | 82.96 ± 6.70 | 83.68 ± 8.30 | −0.72 (−4.74, 3.29) | |
| Post‐intervention | 83.92 ± 6.48 | 88.31 ± 8.74 | −4.39 (−8.48, −0.28) | |
| Mean change | 0.68 ± 5.88 | 4.62 ± 7.78 | −3.94 (−7.62, −0.27) | 0.03 |
| Fasting insulin (mIU/mL) | ||||
| Pre‐intervention | 8.77 ± 4.56 | 6.76 ± 3.98 | 2.01 (−0.26, 4.28) | |
| Post‐intervention | 9.88 ± 4.15 | 10.53 ± 5.33 | −0.65 (−3.20, 1.89) | |
| Mean change | 1.11 ± 1.71 | 3.77 ± 1.70 | −2.67 (−3.57, −1.76) | 0.01 |
| HOMA‐IR | ||||
| Pre‐intervention | 1.82 ± 0.99 | 1.44 ± 0.94 | 0.38 (−0.14, 0.90) | |
| Post‐intervention | 2.07 ± 0.94 | 2.34 ± 1.30 | −0.27 (−0.88, 0.34) | |
| Mean change | 0.25 ± 0.37 | 0.89 ± 0.46 | −0.63 (−0.86, −0.41) | 0.01 |
| Weight (kg) | ||||
| Pre‐intervention | 63.49 ± 10.75 | 62.88 ± 9.33 | 0.61 (−4.72, 5.95) | |
| Post‐intervention | 65.18 ± 11.42 | 64.64 ± 10.11 | 0.54 (−4.11, 6.08) | |
| Mean change | 1.69 ± 0.64 | 1.76 ± 0.78 | −0.06 (−0.51, 0.39) | 0.78 |
| Caloric intake (kcal) | 2,167.38 ± 253.99 | 2,175.40 ± 243.84 | −8.02 (−140.17, 124.12) | 0.90 |
| Carbohydrate (g) | 277.42 ± 32.59 | 275.60 ± 29.83 | 1.82 (−14.75, 18.40) | 0.78 |
| Fat (g) | 80.59 ± 14.12 | 81.57 ± 14.81 | −0.98 (−8.66, 6.70) | 0.79 |
| Protein (g) | 83.32 ± 13.97 | 84.81 ± 12.90 | −1.49 (−8.62, 5.64) | 0.83 |
| Dietary fiber (g) | 11.50 ± 3.59 | 12.93 ± 6.83 | −1.43 (−4.34, 1.48) | 0.20 |
| Distribution of carbohydrate (%) | 51.27 ± 3.89 | 50.80 ± 3.46 | 0.47 (−1.48, 2.43) | 0.27 |
| Distribution of fat (%) | 33.39 ± 3.71 | 33.59 ± 3.28 | −0.19 (−2.05, 1.67) | 0.54 |
| Distribution of protein (%) | 15.35 ± 1.47 | 15.60 ± 1.65 | −0.25 (−1.08, 0.59) | 0.33 |
CI, confidence interval; HOMA‐IR, homeostatic model assessment for insulin resistance.
After 4 weeks of intervention, weight gain between the participants in the probiotic and placebo group was not significantly different (1.69 ± 0.64 vs 1.76 ± 0.78 kg, MD −0.06 kg, 95% CI −0.51, 0.39, P = 0.781). In addition, dietary intake obtained from 3‐day food record at 2 weeks after treatment initiation, including the amount of carbohydrates, fat, protein and fiber intake, was not different between the two groups.
Participant compliance in each group was analyzed and did not differ significantly (probiotic 96.42% vs placebo 93.10%, P = 0.65). Furthermore, there were no any adverse effects, such as diarrhea, nausea/vomiting or sepsis in each group.
Pregnancy outcomes are shown in Table 3. Total pregnancy weight gain was comparable in both groups. Neonatal birthweight and rates of neonatal hypoglycemia were not significantly different between the probiotic group and placebo group.
Table 3.
Pregnancy outcomes of the participants
| Pregnancy outcomes | Probiotic group (n = 28) | Placebo group (n = 29) | P‐value |
|---|---|---|---|
| Total weight gain (kg) | 10.72 ± 4.00 | 11.82 ± 4.80 | 0.35 |
| Birth weight (g) | 3,120.36 ± 411.09 | 3,123.45 ± 369.81 | 0.98 |
| Neonatal hypoglycemia, n (%) | 5 (17.86) | 7 (24.13) | 0.35 |
Discussion
In the present study of pregnant women with GDM in Thailand, probiotic supplementation for 4 weeks during the late second to early third trimester resulted in favorable metabolic changes on fasting plasma glucose, fasting plasma insulin and HOMA‐IR, compared with placebo. These effects occurred without significant differences in caloric intake, dietary macronutrient composition, fiber intake or weight gain between the placebo and probiotic groups. In addition, probiotic supplementation was shown to be well tolerated and safe in the participants. These results support the beneficial effects of probiotics on glucose metabolism in women with GDM.
In pregnancy, insulin resistance increases, especially around the late second trimester and beyond. The present data are in agreement with this, as fasting glucose, insulin and HOMA‐IR increased during the 4‐week period in the placebo group. Probiotic supplementation in the current study resulted in significantly less increase in insulin resistance, and more favorable fasting glucose and insulin levels, compared with placebo. A few previous studies have also shown a favorable effect on fasting glucose levels. Dolatkhah et al.18 studied 64 pregnant women with newly diagnosed GDM who were randomized to daily probiotic supplements (4‐strain bacteria: L. acidophilus, Bifidobacterium, Streptococcus thermophilus and Lactobacillus delbrueckii bulgaricus) or placebo for 8 weeks. Fasting glucose levels showed a greater decline in the intervention group compared with the control group. In another study of 60 pregnant women in Iran, probiotic treatment for 6 weeks also resulted in significant decreases in fasting plasma glucose compared with placebo (−9.2 mg/dL vs +1.1 mg/dL, P < 0.001)20. However, not all studies have found effects on fasting glucose levels. For example, one of the largest studies was carried out by Lindsay et al.19 in 149 pregnant women with GDM who were randomized to either a daily probiotic (Lactobacillus salivarius) or placebo capsule for 4–6 weeks. There were no significant differences in fasting glucose levels between the two groups after the intervention. Consequently, it is not surprising that a recent meta‐analysis including four randomized controlled studies (288 women) did not find significant effects of probiotics on fasting glucose levels30. It is possible that the lack of positive effects in fasting glucose levels seen in some studies could be due to a relatively mild degree of hyperglycemia in these women with diet‐controlled GDM, and probiotics could not further lower glucose levels, as some of them might have been in the normal range at baseline. More consistent findings, however, were shown for HOMA‐IR, a marker of insulin resistance18, 19, 20, 21. The meta‐analysis of these studies found a significant reduction in HOMA‐IR as a result of probiotic supplementation, compared with placebo, with a mean difference of −0.6930. This effect size was remarkably similar to the findings in the present study. Thus, the present data are in agreement with these previous studies and collectively further support the role of probiotics for improving glucose metabolism in GDM.
As dietary intake, including calories, macronutrients and fiber consumption, and weight gain were comparable between the two groups, the improvement in metabolic parameters likely involved other mechanistic pathways. Potential mechanisms by which probiotics improve glucose metabolism include reduction in oxidative stress and inflammation, reduction in intestinal permeability, and increased secretion of incretins31, 32, 33, 34. In a study of 60 GDM women, 6 weeks of probiotic supplementation led to a significant reduction in serum high‐sensitivity C‐reactive protein and a significant increase in total anti‐oxidant capacity levels, along with reduction in fasting glucose levels23. Probiotics have also been shown to increase the expression of adhesion proteins within the intestinal epithelium and to reduce intestinal permeability, leading to less systemic inflammation that might contribute to less insulin resistance35. In addition, production of short‐chain fatty acids, propionate and butyrate were facilitated by probiotics, leading to enhanced glucagon‐like peptide‐1 (GLP‐1) secretion32. GLP‐1 is one of the incretin hormones that stimulates insulin secretion and delays gastric emptying, leading to improved glucose levels; indeed, GLP‐1 receptor agonists are widely used as antidiabetic medications36. Other mechanisms involved could be downregulation of fasting‐induced adipose factor, leading to increased triglyceride deposition in adipocytes37, suppression of the release of adenosine monophosphate‐activated protein kinase, which leads to increased glucose uptake and insulin secretion38, 39, and the ability to convert choline to trimethylamine, which indirectly affects the storage of triglycerides in the liver40. Collectively, these data help explain how probiotics might exert beneficial effects on glucose metabolism.
In the present study, the participants had diet‐controlled GDM; hence, their fasting glucose values were relatively close to the normal range. However, a mean difference in fasting glucose levels of 3.9 mg/dL between the probiotic and placebo groups could still be clinically meaningful. According to the Hyperglycemia and Adverse Pregnancy Outcome study41, every 4‐mg/dL increase in fasting glucose levels >75 mg/dL was associated with an incremental risk of macrosomia and primary cesarean section. It is possible that probiotics might be beneficial in women with more severe degrees of hyperglycemia, such as those requiring insulin, but this remains to be investigated. In addition, probiotic supplementation has been shown in some studies to provide benefits beyond the pregnancy period. In a study by Laitinen et al.17, probiotic supplementation coupled with dietary counseling in the first trimester exerted positive effects on glucose and insulin levels, as well as HOMA‐IR, that persisted over the 12‐month postpartum period. Furthermore, probiotic supplementation was also shown to be associated with favorable changes in breast milk composition42, and moderated excessive weight gain in the offspring from the fetal period until 24–48 months‐of‐age43. Finally, favorable effects of probiotics on lipid profiles of pregnant women with GDM have also been shown20. As the intrauterine environment can affect the long‐term metabolic health of the offspring, future research should explore longitudinal effects of probiotics use during pregnancy in both mothers and their children.
Although this study had the strength of being a double‐blind randomized controlled study, there were some limitations. We did not have data on the gut microbiota composition of the participants at baseline or after the intervention to evaluate the impact of the intervention on the intestinal microbial community and relate them to changes in metabolic parameters. In addition, we did not have information on changes in GLP‐1 or short‐chain fatty acids levels, which could help elucidate the mechanisms linking probiotic use and improved insulin resistance. This should be explored in future research. Although some studies used longer treatment periods18, 19, 20, 21, 23, the intervention period in the present was just 4 weeks, and we used two‐strain probiotics, whereas some investigators used probiotics with four strains18. The decision to use the two‐strain probiotic was driven by their availability and approval at our institution for treatment of other conditions. Therefore, the optimum duration to achieve maximum metabolic effects without adverse outcomes, along with the most suitable probiotic compositions, should be explored in the future. As the present study only enrolled diet‐controlled women with GDM, the results might not be generalized to other groups of patients, such as those with more severe hyperglycemia requiring insulin. In addition, we did not observe any effects on pregnancy outcomes, as the study was not powered to capture these. Whether improved maternal metabolic parameters associated with probiotics use will result in favorable pregnancy outcomes should be further investigated.
In summary, the present study showed that two‐strain probiotic supplements for 4 weeks in diet‐controlled Thai women with GDM in the late second to early third trimester exerted beneficial effects on fasting glucose levels and markers of insulin resistance without any adverse effects. As GDM is a global problem with maternal and offspring consequences, the data of the present study should be replicated in various populations, with optimization of strains and duration of probiotic use. The present results suggest that probiotic supplements may be considered as an adjunctive treatment for glycemic control in women with GDM.
Disclosure
SR receives grant support from Merck Sharp and Dohme, research equipment support from ResMed, and speaker honoraria from Sanofi, Novo Nordisk and Medtronic. The other authors declare no conflict of interest.
Acknowledgment
This study was funded by The Thailand Research Fund (TRF).
J Diabetes Investig 2019; 10: 163–170
Clinical Trial Registry
Thai Clinical Trials Registry 20170606002
References
- 1. Sacks DA, Hadden DR, Maresh M, et al Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel‐recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 2012; 35: 526–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol 2004; 191: 1655–1660. [DOI] [PubMed] [Google Scholar]
- 3. Ehrenberg HM, Durnwald CP, Catalano P, et al The influence of obesity and diabetes on the risk of cesarean delivery. Am J Obstet Gynecol 2004; 191: 969–974. [DOI] [PubMed] [Google Scholar]
- 4. Metzger BE, Lowe LP, Dyer AR, et al Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 5. Crowther CA, Hiller JE, Moss JR, et al Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005; 352: 2477–2486. [DOI] [PubMed] [Google Scholar]
- 6. Hartling L, Dryden DM, Guthrie A, et al Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta‐analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med 2013; 159: 123–129. [DOI] [PubMed] [Google Scholar]
- 7. Meshel S, Schejter E, Harel T, et al Can we predict the need for pharmacological treatment according to demographic and clinical characteristics in gestational diabetes? J Matern Fetal Neonat Med 2016; 29: 2062–2066. [DOI] [PubMed] [Google Scholar]
- 8. Reid G. Food and Agricultural Organization of the, United Nation and the WHO. The importance of guidelines in the development and application of probiotics. Curr Pharm Des 2005; 11: 11–16. [DOI] [PubMed] [Google Scholar]
- 9. Dugoua JJ, Machado M, Zhu X, et al Probiotic safety in pregnancy: a systematic review and meta‐analysis of randomized controlled trials of Lactobacillus, Bifidobacterium, and Saccharomyces spp. J Obstet Gynaecol 2009; 31: 542–552. [DOI] [PubMed] [Google Scholar]
- 10. Didari T, Solki S, Mozaffari S, et al A systematic review of the safety of probiotics. Expert Opin Drug Saf 2014; 13: 227–239. [DOI] [PubMed] [Google Scholar]
- 11. Cao L, Wang L, Yang L, et al Long‐term effect of early‐life supplementation with probiotics on preventing atopic dermatitis: a meta‐analysis. J Dermatolog Treat 2015; 26: 537–540. [DOI] [PubMed] [Google Scholar]
- 12. Sullivan A, Nord CE. The place of probiotics in human intestinal infections. Int J Antimicrob Agents 2002; 20: 313–319. [DOI] [PubMed] [Google Scholar]
- 13. Saez‐Lara MJ, Gomez‐Llorente C, Plaza‐Diaz J, et al The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. Biomed Res Int 2015; 2015: 505878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ganji‐Arjenaki M, Rafieian‐Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol 2017; 233: 2091–2103. [DOI] [PubMed] [Google Scholar]
- 15. Palacios T, Vitetta L, Coulson S, et al The effect of a novel probiotic on metabolic biomarkers in adults with prediabetes and recently diagnosed type 2 diabetes mellitus: study protocol for a randomized controlled trial. Trials 2017; 18: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li C, Li X, Han H, et al Effect of probiotics on metabolic profiles in type 2 diabetes mellitus: a meta‐analysis of randomized, controlled trials. Medicine 2016; 95: e4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laitinen K, Poussa T, Isolauri E. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr 2009; 101: 1679–1687. [DOI] [PubMed] [Google Scholar]
- 18. Dolatkhah N, Hajifaraji M, Abbasalizadeh F, et al Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J Health Popul Nutr 2015; 33: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lindsay KL, Brennan L, Kennelly MA, et al Impact of probiotics in women with gestational diabetes mellitus on metabolic health: a randomized controlled trial. Am J Obstet Gynecol 2015; 212: 496.e1–496.e11. [DOI] [PubMed] [Google Scholar]
- 20. Karamali M, Dadkhah F, Sadrkhanlou M, et al Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: a randomized, double‐blind, placebo‐controlled trial. Diabetes Metab 2016; 42: 234–241. [DOI] [PubMed] [Google Scholar]
- 21. Jafarnejad S, Saremi S, Jafarnejad F, et al Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: a randomized controlled clinical trial. J Nutr Metab 2016; 2016: 5190846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wickens KL, Barthow CA, Murphy R, et al Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br J Nutr 2017; 117: 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Badehnoosh B, Karamali M, Zarrati M, et al The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J Matern Fetal Neonatal Med 2017; 10: 1–9. [DOI] [PubMed] [Google Scholar]
- 24. Lee YK, Conway P, Pettersson S, et al ILSI Southeast Asia Region conference proceedings: the gut, its microbes and health: relevance for Asia. Asia Pac J Clin Nutr 2017; 26: 957–971. [DOI] [PubMed] [Google Scholar]
- 25. Jandhyala SM, Talukdar R, Subramanyam C, et al Role of the normal gut microbiota. World J Gastroenterol 2015; 21: 8787–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Voreades N, Kozil A, Weir TL. Diet and the development of the human intestinal microbiome. Front Microbiol 2014; 5: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Metzger BE, Gabbe SG, Persson B, et al International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 29. Matthews DR, Hosker JP, Rudenski AS, et al Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 30. Taylor BL, Woodfall GE, Sheedy KE, et al Effect of probiotics on metabolic outcomes in pregnant women with gestational diabetes: a systematic review and meta‐analysis of randomized controlled trials. Nutrients 2017; 9: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paszti‐Gere E, Szeker K, Csibrik‐Nemeth E, et al Metabolites of Lactobacillus plantarum 2142 prevent oxidative stress‐induced overexpression of proinflammatory cytokines in IPEC‐J2 cell line. Inflammation 2012; 35: 1487–1499. [DOI] [PubMed] [Google Scholar]
- 32. Yadav H, Lee JH, Lloyd J, et al Beneficial metabolic effects of a probiotic via butyrate‐induced GLP‐1 hormone secretion. J Biol Chem 2013; 288: 25088–25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ejtahed HS, Mohtadi‐Nia J, Homayouni‐Rad A, et al Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012; 28: 539–543. [DOI] [PubMed] [Google Scholar]
- 34. Kim YA, Keogh JB, Clifton PM. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr Res Rev 2017; 17: 1–17. [DOI] [PubMed] [Google Scholar]
- 35. Gomes AC, Bueno AA, de Souza RG, et al Gut microbiota, probiotics and diabetes. Nutr J 2014; 13: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 37. Backhed F, Ding H, Wang T, et al The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004; 101: 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Winder WW, Hardie DG. AMP‐activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 1999; 277: E1–E10. [DOI] [PubMed] [Google Scholar]
- 39. Boulange CL, Neves AL, Chilloux J, et al Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med 2016; 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dumas ME, Barton RH, Toye A, et al Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin‐resistant mice. Proc Natl Acad Sci USA 2006; 103: 12511–12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Catalano PM, McIntyre HD, Cruickshank JK, et al The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012; 35: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoppu U, Isolauri E, Laakso P, et al Probiotics and dietary counselling targeting maternal dietary fat intake modifies breast milk fatty acids and cytokines. Eur J Nutr 2012; 51: 211–219. [DOI] [PubMed] [Google Scholar]
- 43. Luoto R, Kalliomaki M, Laitinen K, et al The impact of perinatal probiotic intervention on the development of overweight and obesity: follow‐up study from birth to 10 years. Int J Obes 2010; 34: 1531–1537. [DOI] [PubMed] [Google Scholar]


