Abstract
A 69‐year‐old man started taking the dipeptidyl peptidase‐4 inhibitor, vildagliptin. One week later, C‐reactive protein and plasma immunoglobulin E levels were markedly elevated, and the vildagliptin was stopped. After the patient's laboratory findings were normalized, we decided to restart vildagliptin with the patient's agreement. The next day, he had a high fever, and C‐reactive protein and procalcitonin levels were elevated. Although we failed to find a focus of infection, we started antibiotics therapy. Two days later, the high fever had improved, and the C‐reactive protein level had decreased. A drug lymphocyte stimulation test showed a positive result for vildagliptin. We examined various kinds of cytokine and infection markers just before and after the treatment with vildagliptin. Finally, we diagnosed the patient with vildagliptin‐induced drug fever, probably based on the increase of various inflammatory cytokine levels and the response to this. Taken together, we should be aware of the possibility of vildagliptin inducing drug fever and/or acute inflammation.
Keywords: Acute inflammation, Drug fever, Vildagliptin
Introduction
It is known that after the ingestion of food, two incretin hormones, glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic peptide (GIP), are released from the gastrointestinal tract, and that such hormones stimulate insulin secretion from pancreatic β‐cells and regulate glucose homeostasis1. However, GLP‐1 and GIP are rapidly degraded and inactivated by dipeptidyl peptidase‐4 (DPP‐4)2. To suppress such inactivation of DPP‐4, DPP‐4 inhibitors were developed. DPP‐4 inhibitors augment endogenous active‐GLP‐1 and GIP levels, which facilitate glucose‐dependent insulin secretion, suppress glucagon secretion and increase pancreatic β‐cell mass3, 4, 5. At present, DPP‐4 inhibitors are very often used for the treatment of type 2 diabetes because of the low risk of side‐effects, such as hypoglycemia. Here, we present a patient with type 2 diabetes who suffered from drug fever and acute inflammation from hypercytokinemia triggered by the dipeptidyl peptidase‐4 inhibitor, vildagliptin.
Case Report
A 69‐year‐old man who had a 2‐year history of hepatocellular carcinoma in complete remission, and a 2‐month history of hypertension and type 2 diabetes was admitted to our hospital (Kawasaki Medical School General Medical Center, Okayama, Japan) due to left thalamic hemorrhage. At that time, he was taking 4 mg/day of benidipine hydrochloride for the treatment of hypertension, and 40 mg/day of gliclazide for type 2 diabetes. On admission, his vital signs were as follows: temperature 36.7°C, blood pressure 152/74 mmHg, heart rate 76 b.p.m. and oxygen saturation 98%. Abdominal computed tomography on admission did not detect hepatocellular carcinoma, but computed tomography imaging findings showed the presence of liver cirrhosis. Other laboratory data were also compatible with liver cirrhosis.
First, to reduce brain osmotic pressure, the patient was treated with glycerol therapy for 8 days. During the acute phase of thalamic hemorrhage, we carried out insulin therapy. Then, we stopped insulin therapy and started 100 mg/day of the DPP‐4 inhibitor, vildagliptin, and 500 mg/day of metformin. This was the first time this patient took these drugs. Two days later, the C‐reactive protein (CRP) level was elevated with no apparent reason, although white blood cell (WBC) and neutrophil levels were not increased. To be safe, we stopped vildagliptin and metformin. As shown in Figure 1, after stopping them, the CRP level promptly decreased, suggesting that the administration of these drugs was likely involved in the change of CRP level in this patient.
Figure 1.
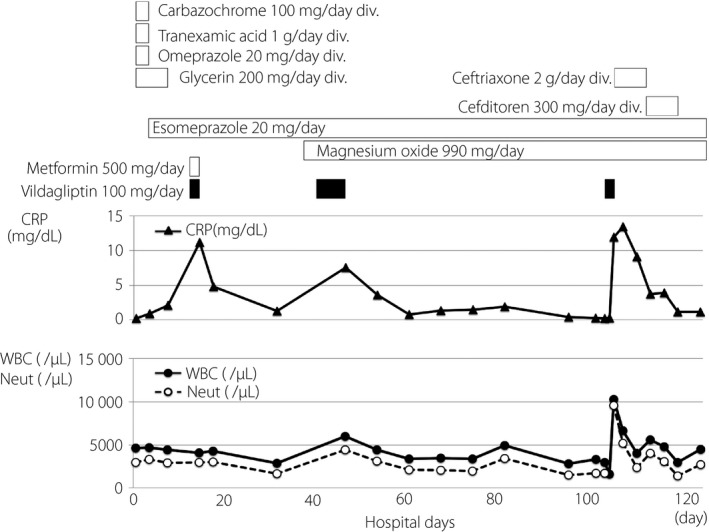
Clinical time‐course for the present patient. After starting vildagliptin, the C‐reactive protein (CRP) level (▲) was elevated twice. Therefore, we decided to restart vildagliptin with the patient's agreement. The next day, when he had taken a total of 150 mg vildagliptin, he had a high fever and the CRP level was elevated once more. Closed circles (●) indicate white blood cells (WBC) and open circles (○) indicate neutrophils (Neut). div, drip infusion into vein.
After the patient moved to the rehabilitation department, we started 100 mg/day of vildagliptin. Approximately 1 week later, the CRP level was markedly elevated to 7.54 mg/dL, but the patient did not have any symptoms, such as a high fever (Figure 1). WBC and neutrophil levels were also moderately increased. We stopped vildagliptin, because the cause of increased CRP was unknown and plasma immunoglobulin E (IgE) level was elevated to 5,310 IU/mL (normal range: 28–138 IU/mL). As shown in Figure 1, after stopping vilgagliptin, CRP, WBC and neutrophil levels were decreased, suggesting that vildagliptin triggered some inflammatory change in this patient. He had no past history of drug or food allergy. Anti‐nuclear antibody, rheumatoid factor and anti‐cyclic citrullinated peptide antibody were all within the normal range.
After the patient's laboratory findings were normalized, we decided to restart vildagliptin with the patient's agreement. Table 1 shows laboratory data the next day after to restarting vildagliptin. As shown in Figure 1, the next day the patient had a high fever. The CRP level was markedly elevated to 11.95 mg/dL compared with its level just 2 days before (0.22 mg/dL; Figure 1; Table 1). The WBC level and percentage of neutrophil were also increased to 10,300/μL and 77.7%, respectively, compared with those levels just 2 days before (2,980/μL and 56.4%; Table 1). In addition, the procalcitonin level was also significantly elevated to 36.7 ng/mL. The creatinine level (1.13 mg/dL) was slightly higher compared with normal range, probably due to dehydration, but creatinine levels at all other time‐points were within the normal range. The patient's chest and abdominal computed tomography showed no findings, except for the possibility of diverticulitis of colon, and we did not detect any obvious focus. In addition, we did not detect any bacterial infection in the blood or urine culture. As we could not eliminate the possibility of the patient having an infection, we started antibiotics therapy. Two days later, the high fever and clinical symptoms had improved, and laboratory data including CRP levels had decreased (Figure 1). As shown in Table 1, 18 days later, CRP, WBC and neutrophil were 1.23 mg/dL, 4,470/μL and 71.9%, respectively, all of which were almost within normal ranges.
Table 1.
Laboratory data on the next day after restarting vildagliptin, and cytokine profiles before and after drug fever and acute inflammation triggered by vildagliptin
| Variable | Result | Reference range | Variable | Result | Reference range |
|---|---|---|---|---|---|
| Peripheral blood | Blood biochemistry | ||||
| White blood cells (/μL) | 10,300 | 3,500–9,500 | AST (U/L) | 86 | 10–35 |
| Blast (%) | 0.0 | 0.0–0.0 | ALT (U/L) | 55 | 7–42 |
| Promyelo (%) | 0.0 | 0.0–0.0 | γ‐GTP (U/L) | 126 | 5–60 |
| Myelo (%) | 0.0 | 0.0–0.0 | LDH (U/L) | 251 | 120–240 |
| Meta (%) | 0.0 | 0.0–0.0 | ALP (U/L) | 326 | 110–360 |
| Band (%) | 32.0 | 2.0–10.0 | Creatinine (mg/dL) | 1.13 | 0.60–1.10 |
| Seg (%) | 61.0 | 50.0–70.0 | BUN (mg/dL) | 31 | 8–22 |
| Eosino (%) | 0.0 | 1.0–5.0 | CRP (mg/dL) | 11.95 | <0.30 |
| Baso (%) | 0.0 | 0.0–1.0 | Sodium (mEq/L) | 139 | 137–146 |
| Mono (%) | 2.0 | 1.0–6.0 | Potassium (mEq/L) | 4.3 | 3.6–5.0 |
| Lymph (%) | 5.0 | 20.0–40.0 | Chloride (mEq/L) | 109 | 101–110 |
| Red blood cells (×104/μL) | 442 | 410–540 | Amylase (U/L) | 182 | 42–118 |
| Hemoglobin (g/dL) | 12.5 | 13.0–16.5 | Procalcitonin | 36.7 | 0.00–0.05 |
| Platelets (×104/μL) | 3.4 | 15.0–35.0 | Plasma glucose (mg/dL) | 125 | 70–110 |
| Cytokines | 2 days before | Just after a high fever | 18 days later |
|---|---|---|---|
| IFN‐γ (<0.1 IU/mL) | <0.1 | 0.5 | <0.1 |
| TNF‐α (0.6–2.8 pg/mL) | 1.4 | 16.8 | 1.3 |
| IL‐1β (<10 pg/mL) | <10 | <10 | 11 |
| IL‐6 (<4.0 pg/mL) | 6.3 | 362 | 12.3 |
| IL‐8 (<2.0 pg/mL) | 14.1 | 89.4 | 16.5 |
| IL‐10 (<5 pg/mL) | <2 | 20 | <2 |
| IL‐12 (<7.8 pg/mL) | <7.8 | <7.8 | <7.8 |
| IL‐18 (126 ± 44.5 pg/mL) | 218 | 1,690 | 217 |
| WBC (3,500 – 9,500/μL) | 2,980 | 10,300 | 4,470 |
| Neut. (52.0–80.0%) | 56.4 | 77.7 | 71.0 |
| CRP (<0.30 mg/dL) | 0.22 | 11.95 | 1.23 |
| IgG (1,000–1,800 mg/dL) | 2,426 | 2,066 | 2,478 |
| IgA (110–490 mg/dL) | 566 | 509 | 617 |
| IgM (65–260 mg/dL) | 136 | 120 | 136 |
| IgE (28–138 IU/mL) | 4,859 | 3,779 | 5,301 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C‐reactive protein; IgA, immunoglobulin A; IgE, immunoglobulin E; IgG, immunoglobulin G; IgM, immunoglobulin M; IL, interleukin; LDH, lactate dehydrogenase; Neut., neutrophil; TNF‐α, tumor necrosis factor‐α; WBC, white blood cells; γ‐GTP, γ‐glutamyltranspeptidase.
The drug lymphocyte stimulation test showed a positive result for vildagliptin (961 c.p.m., stimulation index (S.I.) 183%, control 524 c.p.m.). We examined various kinds of cytokine and infection markers just before and after the treatment with vildagliptin (Table 1). Tumor necrosis factor‐α, interferon‐γ and interleukin (IL)‐6, all of which are known as endogenous pyrogens, were elevated to 16.8 pg/mL (normal range: 0.6–2.8 pg/mL), 0.5 IU/mL (<0.1 IU/mL) and 362 pg/mL (<4.0 pg/mL), respectively. IL‐18 was elevated to 1,690 pg/mL, whereas IL‐12 level was within the normal range (<7.8 pg/mL). Furthermore, IL‐10, which is known to be stimulated by IL‐18, was elevated to 20 pg/mL (<5 pg/mL). Therefore, we diagnosed the patient with vildagliptin‐induced drug fever, which was probably based on the increase of IL‐18 level and its reactive response.
Informed consent was obtained from the patient for publication of the present study.
Discussion
DPP‐4 is expressed on the cell surface of several cell types and has various functions, although these effects are still largely unknown6. It is known that DPP‐4 is involved in T‐cell activation. In general, IL‐18 is released from T‐cell lymphocytes, and an excess amount of IL‐18 causes immunodeficiency for rheumatoid arthritis, sepsis and allergy7, 8, 9. The increased IL‐18 levels together with increased IL‐12 levels indicate the activation of Th1‐mediated immune responses. In contrast, increased IL‐18 levels without an increase of IL‐12 levels indicates the activation of Th2‐mediated immune responses. Such activation increases IL‐10 levels, which finally leads to allergic inflammation, as observed in the present patient.
It has been suggested that DPP‐4 inhibition could influence the immune system. Indeed, it is known that the frequency of various kinds of infection is higher in DPP‐4 inhibitor users compared with users of other antidiabetic drugs10. In general, procalcitonin is a marker of inflammatory response, and is stimulated by bacterial products (endotoxins/lipopolysaccharide) and cytokines (IL‐1, IL‐2, IL‐6, tumor necrosis factor‐α). Therefore, we believe that vildagliptin triggered not only drug fever, but also acute inflammation through some other mechanism in this patient.
It is known that IgE level is increased soon after the onset of a type I allergy. The present patient had a high IgE level, but his IgE level was high even before restarting vildagliptin. Therefore, it is not likely that he had a type I allergy. Considering the time‐course and alteration of cytokine levels, it is possible that he had a type IV allergy, but he had no skin rash, which was not compatible with a type IV allergy. These results suggest that his fever and cytokine reaction were induced by vildagliptin per se rather than a type I or IV allergic reaction. In addition, the elevated IgE level was not accompanied by an increase of eosinophils in the peripheral blood. Therefore, we assume that the elevated IgE level was simply due to the characteristics of this patient rather than due to treatment with vildagliptin.
The present case report had a limitation. First, in this case, the patient was treated with vildagliptin three times, and the first and second time he had high CRP levels without other symptoms, such as fever. However, when we decided to restart vildagliptin, he developed a high fever with elevated CRP levels. Therefore, we cannot exclude the possibility that such alteration was induced by some other effects rather than the direct effect of vildagliptin. Furthermore, we believe the present patient was affected by a hypercytokinemia response induced by vildagliptin. Second, we carried out a drug lymphocyte stimulation test only for vildagliptin among various DPP‐4 inhibitors. From this, we could not conclude that his cytokine effects were due to induced DPP‐4 inhibition. In addition, the results of the drug lymphocyte stimulation test for vildagliptin (961 c.p.m., S.I. 183%, control 524 c.p.m.) might not have been enough for a positive status for a drug allergy. Third, the patient had liver dysfunction on admission, and his past history was hepatocellular carcinoma in complete remission. On admission, his hepatic enzyme levels were as follows: aspartate transaminase 69 U/L, alanine transaminase 30 U/L, gamma‐glutamyltransferase 309 U/L and platelet number was 6.8 × 104/μL. Although the patient's liver injury was improved before restarting vildagliptin, there might have been something in the liver that could influence the inflammatory cytokine levels.
Vildagliptin is one of the DPP‐4 inhibitors that are very frequently used all over the world for the treatment of type 2 diabetes. Therefore, we should keep in mind the possibility that vildagliptin induces drug fever and/or acute inflammation when we examine one of the uncertain causes of high fever and/or elevated CRP levels during treatment with vildagliptin.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2019; 10: 182–185
References
- 1. Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab 2018; 20(Suppl 1): 5–21. [DOI] [PubMed] [Google Scholar]
- 2. Deacon CF. A review of dipeptidyl peptidase‐4 inhibitors. Hot topics from randomized controlled trials. Diabetes Obes Metab 2018; 20(Suppl 1): 34–46. [DOI] [PubMed] [Google Scholar]
- 3. Hamamoto S, Kanda Y, Shimoda M, et al Vildagliptin preserves the mass and function of pancreatic β cells via the developmental regulation and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetes Obes Metab 2013; 15: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirukawa H, Kaneto H, Shimoda M, et al Combination of DPP‐4 inhibitor and PPARγ agonist exerts protective effects on pancreatic β‐cells in diabetic db/db mice through the augmentation of IRS‐2 expression. Mol Cell Endocrinol 2015; 413: 49–60. [DOI] [PubMed] [Google Scholar]
- 5. Kaneto H, Obata A, Shimoda M, et al Promising diabetes therapy based on the molecular mechanism for glucose toxicity: usefulness of SGLT2 inhibitors as well as incretin‐related drugs. Curr Med Chem 2016; 23: 3044–3051. [DOI] [PubMed] [Google Scholar]
- 6. Gorrell MD, Gysbers V, McCaughan GW. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol 2001; 54: 249–264. [DOI] [PubMed] [Google Scholar]
- 7. Liew FY, Wei XQ, McInnes IB. Role of interleukin 18 in rheumatoid arthritis. Ann Rheum Dis 2003; 62(Suppl 2): ii48–ii50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grobmyer SR, Lin E, Lowry SF, et al Elevation of IL‐18 in human sepsis. J Clin Immunol 2000; 20: 212–215. [DOI] [PubMed] [Google Scholar]
- 9. Sanders NL, Mishra A. Role of interleukin‐18 in the pathophysiology of allergic diseases. Cytokine Growth Factor Rev 2016; 32: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Willemen MJ, Mantel‐Teeuwisse AK, Straus SM, et al Use of dipeptidyl peptidase‐4 inhibitors and the reporting of infections: a disproportionality analysis in the World Health Organization VigiBase. Diabetes Care 2011; 34: 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]


