Abstract
Aims/Introduction
To investigate the effect of endurance training on hippocampus DJ‐1 and cannabinoid receptor type 2 (CB 2) protein and blood glucose concentration in diabetic rats.
Materials and Methods
A total of 32 rats were randomly divided into diabetic (D), diabetic and exercise (DE), exercise (E) and control (C) groups. The endurance training was carried out five times per week for 6 weeks. The hippocampus DJ‐1 and CB 2 were measured using an enzyme‐linked immunosorbent assay method.
Results
The level of DJ‐1 in the D group was significantly higher than the other groups (P ≤ 0.01). However, the level of DJ‐1 was not significantly different between the C, E and DE groups. In addition, the level of CB 2 was significantly lower in the D group compared with the other groups (P ≤ 0.01). Blood glucose was significantly higher in the D group compared with the DE group (P ≤ 0.05). Furthermore, a significant positive correlation between the level of DJ‐1 and blood glucose was observed (r = 0.67, P ≤ 0.001). There was also a significant inverse correlation between the level of CB 2 and blood glucose (r = −0.77, P ≤ 0.001).
Conclusions
The results of this study suggest that the level of DJ‐1 and CB 2 might change in response to diabetes, and regular aerobic exercise could mediate the effect of DJ‐1 and CB 2 on diabetes‐induced neurodegenerative diseases.
Keywords: Metabolic syndrome, Neurodegenerative diseases, Physical activity
Introduction
The gradual and progressive loss of neural cells in neurodegenerative diseases leads to nervous system dysfunctions1, 2, 3. According to the National Institute of Neurological Disorders and Stroke, approximately 50 million Americans are affected each year from more than 600 neurological disorders1. Several factors are associated with neurodegenerative diseases, including α‐synuclein, parkin, PINK1, dardarin (leucine‐rich repeat kinase 2) and DJ‐14. Protein deglycase DJ‐1 is a widely distributed and highly expressed protein in the brain and extracerebral tissues4. Although the exact mechanism and roles of DJ‐1 are not fully known, it is believed that DJ‐1 is associated with metabolic syndrome5, and various cellular processes including response to oxidative stress, transcriptional regulation, mitochondrial regulation, ribonucleic acid‐binding, androgen‐receptor signaling, spermatogenesis and fertilization4, 6. In addition, DJ‐1 has been shown to play an important role in neuronal dynamics and modulation of neurodegeneration by reducing oxidative stress, inflammation and neurotoxicity7. A higher concentration of DJ‐1 has been observed in response to a high level of glucose in renal glomerular mesangial cells and β‐cells of the islets of Langerhans1, 6. An abnormal increase in blood glucose has been reported in most neurodegenerative diseases, such as Alzheimer's disease8, Parkinson's disease9 and Huntington's disease10, and dysfunction of DJ‐1 has also been reported in diabetes mellitus in a previous study6.
Cannabinoid receptors (CBs) are a cluster of receptors that exist in all tissues of the body and are involved in various physiological processes, including apoptosis, pain reduction and memory regulation11, 12. These receptors include two isoforms: cannabinoid receptor type 1 (CB1), and cannabinoid receptor type 2 (CB2). CB2 is expressed in most tissues of the central nervous system13 and is believed to be involved in nerve‐protecting mechanisms14. It has been shown that CB2 modifies the side‐effects of neurodegenerative diseases, and improves brain function by modulating the production of amyloid plaques and reduction of the destructive effects of inflammatory reactions and oxidative stress15. The negative correlation between the long‐term increase in blood glucose and CB2 suggests that hyperglycemia might decrease the level of CB2 in the nervous system13. Therefore, it seems plausible that the levels of DJ‐1 and CB2 might change in neurodegenerative diseases or diabetes.
Diabetes mellitus is defined as a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action or both6, 16, and had been shown to cause several health complications including cardiovascular diseases, retinopathy, nephropathy and neuropathy17. High blood glucose has harmful effects on specific areas of the brain, such as the hippocampus, and could cause memory loss, learning and problem‐solving difficulties, and mental and motor disorders8. Increased blood glucose is one of the major mechanisms involved in nerve degeneration, which has been observed in most neurodegenerative diseases, such as Alzheimer's disease and Parkinson's disease18.
Medications used to control diabetes and subsequent conditions might have moderate‐to‐severe side‐effects19, 20. Therefore, non‐pharmacological treatments are preferred by neuroscientists and other health professionals. A change in lifestyle and increase in daily energy expenditure is the most common non‐invasive treatment for diabetes patients20, 21, 22. Previous studies have shown that physical activity increases the metabolism at rest and during activity, resulting in positive changes in blood glucose23, 24. In addition, physical activity is likely to prevent diabetes‐related nerve damage25, 26 by modulating inflammatory responses27, improving anti‐oxidant responses28 and decreasing blood glucose levels29.
The effect of exercise on diabetes has been widely studied24, 30. However, the mechanisms for the effect of exercise on the modulation of nerve damage from diabetes is not clear31. Given the important role of DJ‐1 and CB2 in neurodegeneration, the aim of the current study was to investigate the effect of endurance training on the level DJ‐1 and CB2 in diabetic rats.
Methods
A total of 46 male Wistar rats (aged 8–10 weeks) were kept in groups of three in polycarbonate cages in a room with an average temperature of 22 ± 2°C and dark–light cycles of 12:12 h. Animals were handled by one researcher at the animal house of Lorestan University of Medical Sciences, and had free access to water and standard rat food (Pars Khorakdam Co, Tehran, Iran). All experiments in the current study followed the ethical principles approved by the Animal Ethics Committee of Lorestan University (reference number: LU. ECRA. 2017.1).
Rats were randomly, but unequally, assigned into diabetic (D; n = 13), diabetic and exercise (DE; n = 13), exercise (E; n = 10) and control (C; n = 10) groups. The number of rats was higher in the D and DE groups to account for diabetes‐induced mortality. Exercise familiarization in the E and DE groups included walking on a treadmill for 10–15 min at 5–10 m/s five times before the start of the experiment.
Diabetes was induced after 12 h of food deprivation by intraperitoneal injection of streptozotocin (STZ) solution (Sigma, St. Louis, MO, USA), 50 mg/kg dissolved in 0.5 mol/L fresh citrate buffer, pH 5.032. Rats in the E and C groups received an equivalent volume of citrate buffer. Two rats in the DE group died within 48 h after the injection of STZ solution. 48 h after inducing diabetes, blood samples were collected from the tail of the remaining rats and analyzed using the glucometer (Roche Diagnostics K.K., Tokyo, Japan). Rats with a blood glucose level >300 mg/dL were considered diabetic and were included in the study. Given that blood glucose levels in all injected rats were >300 mg/dL, diabetes was confirmed in all rats in the D and DE groups. Blood glucose levels were measured every 14 days to monitor changes in blood glucose during the intervention period (Figure 1).
Figure 1.
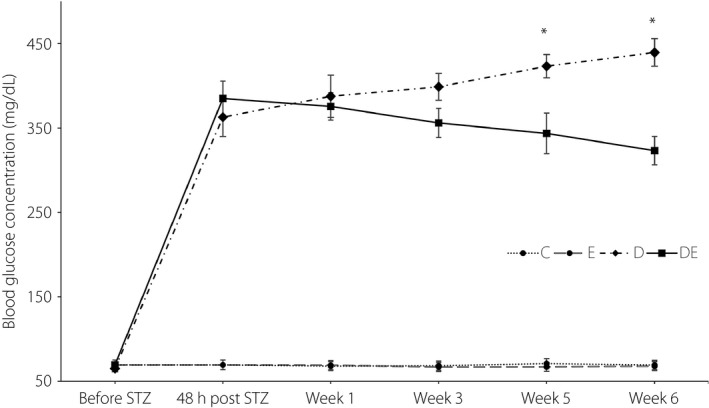
Blood glucose levels during the intervention. *Significantly different from the diabetic and exercise (DE) group. C, control group; D, diabetic group; E, exercise group; STZ, streptozotocin.
Rats in the E and DE groups were trained on a treadmill (Azarakhsh animal treadmill; Pishro Andisheh Technology Engineering Co., Tehran, Iran) at the intensity of 50–55% of maximum aerobic capacity33. Both groups completed five training sessions per week for 6 weeks, and every training session started with 3 min of warm‐up and was completed with 3 min of cool down. Intensity and volume training sessions increased gradually over the intervention period. In the first week, training consisted of 10 min of running on a treadmill at 10 m/min. In the second week, training duration increased to 20 min, while the intensity remained at 10 m/min. In the third week, training increased to 20 min of running at 15 m/min. In week 4, 5 and 6, rats trained for 30 min at 15, 18 and 18 m/min respectively. When rats failed to maintain the training intensity, they were encouraged to run by tapping on a Perspex box, and as necessary, electrical stimulation through a metal grid at the end of the treadmill. Rats received a maximum of 10 shocks of 0.5‐s duration over the exercise period. Rats in the E and DE groups did not show any signs of severe fatigue or locomotor impairment during the intervention period. However, a previous study has suggested that severe fatigue increases blood lactate levels and might increase the side‐effects of diabetes34.
Blood glucose was measured before STZ injection, 48 h after STZ injection, and at the end of week 1, week 3 and week 5, and 24 h after completion of the study in week 6. For the accuracy of blood glucose analysis, access to food was restricted 12 h before blood glucose assessment.
Body mass of rats was measured at the beginning of the study and the end of week 2, week 4 and week 6, using a digital scale with the accuracy of 0.001 kg (Seca, Hamburg, Germany). Body mass measurements were completed in the following week of every blood glucose assessment to reduce the effect of food deprivation and stress of blood sampling on body mass.
On completion of the intervention period, rats were anesthetized by intraperitoneal injection of ketamine and xylazine, 24 h after the last exercise session. The hippocampus was stored in a −80°C freezer for further analysis. For the analysis of DJ‐1 and CB2, 100 mg of tissue was rinsed with 1× phosphate‐buffered saline, homogenized in 1 mL of 1× phosphate‐buffered saline and stored overnight at −20°C. Two freeze–thaw cycles were carried out to break the cell membranes, and the homogenates were centrifuged for 5 min at 5,000 g at 2–8°C. The supernatant was removed and assayed immediately. DJ‐1 and CB2 protein levels in the hippocampus were measured using an enzyme‐linked immunosorbent assay kit (Cusabio DJ‐1 kit with a sensitivity of 0.078 ng/mL; Tokyo, Japan; and Biomatik CB2 kit with a sensitivity of 0.058 ng/mL; Cambridge, Canada). Antibodies specific for DJ‐1 and CB2 molecules were pre‐coated on a microplate. Standards and samples were pipetted into the wells, and any target molecule present was bound by the immobilized antibody. After removing any unbound substances, biotin‐conjugated antibodies specific for DJ‐1 and CB2 molecules were added to the wells. After washing, avidin‐conjugated horseradish peroxidase was added to the wells. After a wash to remove any unbound avidin enzyme reagent, a substrate solution was added to the wells and color developed in proportion to the intensity of the target molecules bound in the initial step. The color development was stopped and the intensity of the color was measured. All measurements were carried out in duplicates and the average value was used for statistical analysis.
Five rats from the D group and two rats from the DE group died during the experiment. Three rats (one from the DE group and two from the E group) did not complete 85% of the duration of the overall training sessions time and were excluded from the study. On completion of the study, eight rats from the DE group, and eight rats from the D and E groups satisfied the training and diabetes requirements, and were included in the study. To achieve an equal number of rats in each group, two rats from the C group were randomly excluded at the end of the study.
Data were analyzed using SPSS 25.0 (SPSS Inc., Chicago, IL, USA) and reported as mean ± standard deviation. The normality and homogeneity of data were analyzed using the Shapiro–Wilk and Levene's test, respectively. A one‐way analysis of covariance (ancova) was used to compare the level of hippocampus DJ‐1 and CB2 of rats in the D, DE, E and C groups. The body mass of rats at the end of the study was selected as a covariate to partial out the effect of rats’ body mass on the level of DJ‐1 and CB2. The correlation between DJ‐1, CB2 and blood glucose was assessed with a bivariate Pearson correlation coefficient.
A within‐between (groups × time) repeated measures anova was used to compare differences in bodyweight and blood glucose between groups through the study period. The probability level of statistical significance was set at P ≤ 0.05. Effect sizes were calculated using partial eta squared () with values of 0.2, 0.6 and >1.2 considered to be a small, medium and large effects35. A Bonferroni post‐hoc test was used to compare the difference between groups.
Results
The concentration of hippocampus DJ‐1 is presented in Figure 2. The ancova showed that, after accounting for the effects of bodyweight, there was a statistically significant effect of condition on the level of DJ‐1 at the end of the 6‐weeks intervention (F 3,27 = 7.84, P = 0.001, = 0.466). The results of the Bonferroni post‐hoc test are shown in Figure 2.
Figure 2.
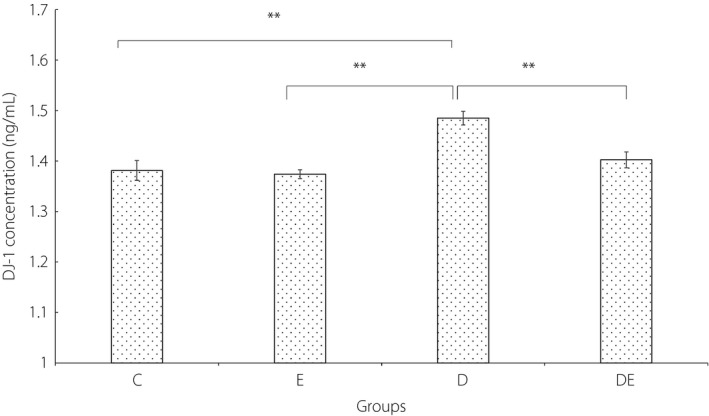
The concentration of DJ‐1 in the hippocampus of rats. *Significantly different, P ≤ 0.05. **Significantly different, P ≤ 0.01. C, control group; D, diabetic group; DE, diabetic and exercise group; E, exercise group.
In addition, after accounting for the effects of bodyweight, there was a statistically significant effect of condition on the level of CB2 at the end of the 6‐week intervention (F 3,27 = 10.10, P < 0.001, = 0.550). The results of the Bonferroni post‐hoc test are shown in Figure 3.
Figure 3.
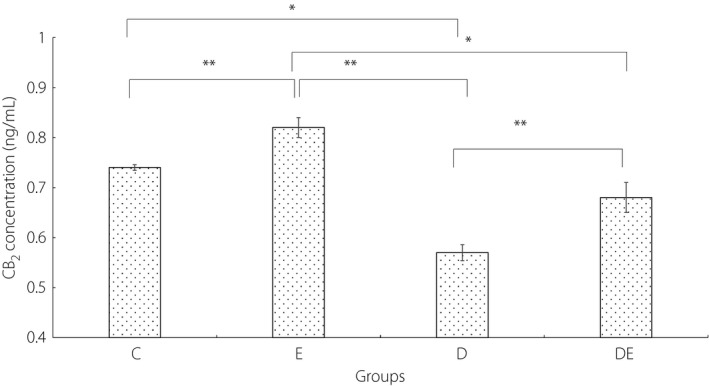
The concentration of cannabinoid receptor type 2 (CB2) in the hippocampus of rats. *Significantly different, P ≤ 0.05. **Significantly different, P ≤ 0.01. C, control group; D, diabetic group; DE, diabetic and exercise group; E, exercise group.
A 4 × 6 mixed model anova was used to investigate the impact of diabetes, exercise, and the combination of diabetes and exercise on blood glucose during a 6‐week study. In the current study, the blood glucose was measured six times (Figure 1). There was a significant main effect for time (F 3.25,91.11 = 7,130.97, P < 0.001, = 0.824). There was also a significant effect of group (F 3,28 = 328.80, P < 0.001, = 0.972). The D group showed significantly higher blood glucose than the DE (P = 0.21), E (P < 0.01) and C (P < 0.01) groups. Both the E and C groups showed significantly lower blood glucose than the DE group (P < 0.001). The difference in blood glucose between the C and E groups was not statistically significant (P = 1.00). There was also a significant interaction of group and time (F 3,28 = 75.61, P < 0.001, = 0.890).
A 4 × 4 mixed model anova was used to investigate the impact of diabetes, exercise, and the combination of diabetes and exercise on bodyweight of rats during the 6‐week study. Changes of body mass are presented in Figure 4. There was no significant main effect for time (F 2.45,68.52 = 2.216, P = 0.106, = 0.073). There was also a significant effect of group (F 3,28 = 10.44, P < 0.001, = 0.528). Both the D and DE groups showed a significantly higher body mass than the E and C groups (P < 0.05). There was no significant difference between the bodyweight of the D group and the DE group, and between the E group and the C group. There was also a significant interaction of group and time (F 7.34,68.52 = 77.46, P < 0.001, = 0.892).
Figure 4.
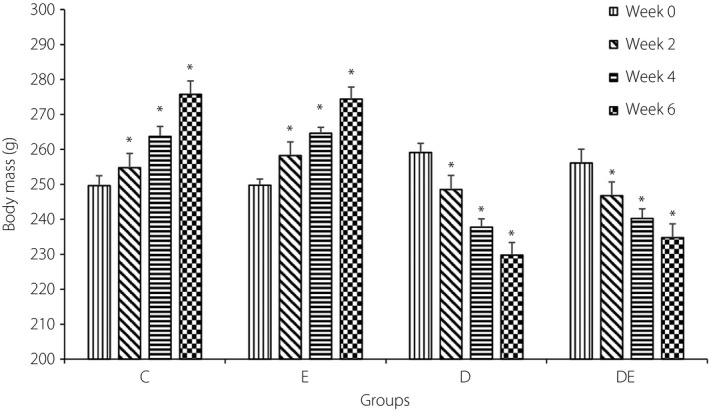
Average body mass of rats in the four groups. *Significantly different from week 0. C, control group; D, diabetic group; DE, diabetic and exercise group; E, exercise group.
Analysis of the results also showed a significant correlation between the level of DJ‐1 and blood glucose in week 6 (r 30 = 0.67, P < 0.001). There was also a significant inverse correlation between the level of CB2 and blood glucose in week 6 (r 30 = −0.77, P < 0.001). In addition, there was a significant inverse correlation between the level of CB2 and DJ‐1 in week 6 (r 30 = −0.62, P < 0.001).
Discussion
Previous studies have reported an increase in the level of DJ‐1 and a decrease in the level of CB2 in response to an abiding increase in blood glucose13, 36. The effect of regular physical activity on the level of DJ‐1 and CB2 in diabetes patients is not fully understood. However, studies have shown an increase in the level of DJ‐1 in response to lifestyle intervention, and have suggested that the increase in DJ‐1 could prevent metabolic syndrome5.
DJ‐1 functions as a cytoplasmic redox‐dependent protein chaperone37, and the findings of recent studies suggest that DJ‐1 might be associated with cancers and neurodegenerative diseases38, 39, 40, 41. The role and mechanisms of the effect of DJ‐1 in neurological disorders are not fully understood, and the findings of previous studies on the effect of DJ‐1 concentration on neurodegenerative diseases are inconsistent. Some studies have shown a significant increase in levels of DJ‐1 in patients with Parkinson's disease and Alzheimer's disease2, whereas other studies reported lower levels of DJ‐1 and α‐synuclein in Parkinson's disease patients than in healthy people and those with Alzheimer's disease42.
In the current study, the level of DJ‐1 in the D group was significantly higher than the other three groups, suggesting that the levels of DJ‐1 in male rats might have increased 6 weeks after induced diabetes. This finding supports the results of previous studies that showed an increase in DJ‐1 in response to oxidative stress and hyperglycemia1, 43. The significant difference between the levels of DJ‐1 in the D group and DE group suggests that regular physical activity might have mediated the level of DJ‐1 in diabetic rats. The non‐significant difference between the levels of DJ‐1 in the E and C groups could suggest that exercise might have regulated the levels of DJ‐1 by reducing the levels of blood glucose in diabetic rats, but had no significant effect on the DJ‐1 levels in healthy rats. The significant positive correlation between the levels of DJ‐1 and blood glucose in the current study support this claim that increased levels of blood glucose might increase the level of DJ‐1. This increase in the level of DJ‐1 could be controlled by regular endurance training, possible by reducing the level of blood glucose.
The results of the present study also showed a significantly lower level of CB2 in the D group compared with the other groups. The mechanisms involved in reducing CB2 in diabetic rats are not yet clear; however, insufficiency of insulin function and increased levels of TGF‐β1 could have contributed to the reduced levels of CB2 in diabetic rats13. Barutta et al.13 showed that the level CB2 protein in diabetes patients with advanced nephropathy decreased in human and animal models as a result of increased levels of TGF‐β1. Additionally, Oddi et al.12, with induction of hemicerebellectomy in rats and injection of JWH‐015 (CB2 agonist) into cerebellar astrocytes, concluded that injection prevented the increase of nitric oxide synthase expression in astrocytes, reduced the oxidative stress in the neurons, increased levels of anti‐oxidative (Hsp70) and anti‐apoptotic (Bcl‐2) proteins, and prevented delayed apoptosis by signaling through the phosphoinositide 3‐kinase–protein kinase B pathway. Palazuelos et al.44 investigated the expression of CB2 in activated microglia cells in the brain of Huntington's mice and showed the upregulation of this receptor, and this phenomenon is compatible with inflammatory responses at the onset of the disease.
The results of some studies in other neurodegenerative disorders were not consistent with the results of the present study. For example, by examining the post‐mortem brains from patients with Alzheimer's disease, Benito et al.45 found that CB2 receptors are abundantly and selectively expressed in neuritic plaque‐associated astrocytes and microglia, respectively, in the hippocampus and endothelial cortex sections. These contradictions can be related to the different types of disease, protein measurement method, the antibody used to measure CB2 and extracted tissue type. The results of the current study showed a significant difference between the levels of CB2 in the D and DE groups. This significant difference suggests that the negative effect of high blood glucose on the concentration of CB2 could be reduced by regular endurance training. The significant inverse correlation between blood glucose and the level of CB2 in the current study supports this possibility.
Regular exercise might also have a positive effect on the health of the nervous system26, 46. The results of a previous study showed an improvement in brain functions after regular endurance training, especially in the hippocampus region46. The authors suggested that exercise might have improved the resistance to neurodegeneration caused by diabetes. In addition, some researchers showed an improvement in memory, a reduction of free electron levels, and an increase in the nerve growth factor and brain‐derived neurotrophic factor levels after regular exercise26, 47, 48. Aksu et al.49 reported that endurance exercise increases superoxide dismutase anti‐oxidant enzyme levels in different parts of the brain, such as the hippocampus, and leads to an increase in the anti‐oxidant capacity of the brain.
Some studies have been carried out to identify the mechanisms through which the beneficial results of exercising in improving the neurodegeneration in the brain could be understood. Some of these mechanisms include reduction of oxidative stress and inflammatory factors, improvement of the anti‐oxidant defense, increase in angiogenesis, increase in secretion of neurotrophin and catecholamine, and neurogenesis, especially in hippocampus region50, 51, 52. Therefore, it seems that among the mechanisms related to exercise, reducing oxidative stress, improving anti‐oxidant defense, and reducing inflammatory factors involved in changes in levels of DJ‐1 and CB2 proteins in the hippocampus of diabetic rats4, 6, 13. The results of the present study showed that endurance training resulted in a non‐significant decrease in DJ‐1 protein content in the E group. This non‐significant change could be attributed to an insufficient duration of the intervention period, and low duration and intensity of training sessions, or both.
In addition, the findings of the current study showed a strong correlation between the levels of DJ‐1 and CB2 with blood glucose. The level of glucose in the DE group was significantly lower than in the D group, suggesting that the prescribed exercise regimen resulted in a significant reduction of blood glucose after inducing diabetes. This reduction in blood glucose was accompanied by a decrease of DJ‐1 and an increase of CB2 in the hippocampus. If an increase in DJ‐1 and a decrease in CB2 levels in response to diabetes was associated with neurodegenerative disease, then the inclusion of regular exercise could reduce the blood glucose and lower the risk of developing neurodegenerative diseases. Previous studies suggested that the reduction of blood glucose during exercise and post‐exercise might have beneficial effects on neuronal protective processes including anti‐oxidative and anti‐inflammatory systems, in addition, insulin sensitivity increases with exercise29, 51, 53. It is possible that exercise could affect the levels of DJ‐1 and CB2, and consequently the neurodegeneration process, by a reduction of the blood glucose concentration. It is shown that insulin treatment could improve the function of sensory neurons in diabetic specimens with developed neuronal disorders54, 55, 56. Although the relationship between an increase in blood glucose with neurodegeneration was not assessed in the current study, the strong positive relationship between DJ‐1 and CB2 with glucose could provide a valuable insight into a possible mechanism for the prevention of diabetes‐induced neurodegeneration. Previous studies examined the role of DJ‐1 and CB2 in the function of neural cells and neurodegenerative diseases, and suggested that these proteins play roles in regulating free electrons, inflammatory factors and apoptosis12, 36. In addition, studies have shown that nerve cells in diabetic rats might have mitochondrial disorders, increased inflammatory factors and oxidative stress57, 58.
In addition, studies have shown beneficial effects of exercise, such as improvement of the anti‐oxidant system, increased mitochondrial biogenesis, reduction of inflammatory factors and reduced blood glucose53. Thus, changes in levels of DJ‐1 and CB2 proteins due to endurance activity might be the result of improving the anti‐oxidant defense system, producing fewer free electrons, reducing inflammatory cytokines or reducing blood glucose. Considering the strong correlation between levels of DJ‐1 and CB2 proteins with blood glucose levels, the effects of these proteins on neuronal protection in diabetes patients might be associated with blood glucose levels. However, it should be noted that this hypothesis was not explored in the present study, and more studies are required to investigate the effect of DJ‐1 and CB2 on the neural health of diabetes patients.
An increase in blood glucose above normal levels could have significant effects on hippocampus DJ‐1 and CB2. However, inclusion of regular physical activity might mediate the effect of diabetes on DJ‐1 and CB2 by reducing blood glucose level.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
We would like to acknowledge Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences for providing the facility and equipment.
J Diabetes Investig 2019; 10: 43–50
References
- 1. Brown RC, Lockwood AH, Sonawane BR. Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect 2005; 113: 1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choi J, Sullards MC, Olzmann JA, et al Oxidative damage of DJ‐1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem 2006; 281: 10816–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sajjad MU, Green EW, Miller‐Fleming L, et al DJ‐1 modulates aggregation and pathogenesis in models of Huntington's disease. Hum Mol Genet 2014; 23: 755–766. [DOI] [PubMed] [Google Scholar]
- 4. Lev N, Roncevic D, Ickowicz D, et al Role of DJ‐1 in Parkinson's disease. J Mol Neurosci 2006; 29: 215–225. [DOI] [PubMed] [Google Scholar]
- 5. Yamane T, Murao S, Kozuka M, et al Serum DJ‐1 level is positively associated with improvements in some aspects of metabolic syndrome in Japanese women through lifestyle intervention. Nutr Res 2014; 34: 851–855. [DOI] [PubMed] [Google Scholar]
- 6. Eberhard D, Lammert E. The role of the antioxidant protein DJ‐1 in Type 2 diabetes mellitus In: Ariga H, Iguchi‐Ariga SMM. (eds). DJ‐1/PARK7 Protein. Berlin, Germany: Springer, 2017; 173–186. [DOI] [PubMed] [Google Scholar]
- 7. Ariga H, Takahashi‐Niki K, Kato I, et al Neuroprotective function of DJ‐1 in Parkinson's disease. Oxid Med Cell Longev 2013; 2013: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toth C. Diabetes and neurodegeneration in the brain. Handb Clin Neurol 2014; 126: 489–511. [DOI] [PubMed] [Google Scholar]
- 9. Santiago JA, Potashkin JA. Integrative network analysis unveils convergent molecular pathways in Parkinson's disease and diabetes. PLoS One 2013; 8: e83940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma TC, Buescher JL, Oatis B, et al Metformin therapy in a transgenic mouse model of Huntington's disease. Neurosci Lett 2007; 411: 98–103. [DOI] [PubMed] [Google Scholar]
- 11. Li Y, Kim J. CB2 cannabinoid receptor knockout in mice impairs contextual long‐term memory and enhances spatial working memory. Neural Plast 2016; 2016: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oddi S, Latini L, Viscomi M, et al Distinct regulation of nNOS and iNOS by CB 2 receptor in remote delayed neurodegeneration. J Mol Med 2012; 90: 371–387. [DOI] [PubMed] [Google Scholar]
- 13. Barutta F, Piscitelli F, Pinach S, et al Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes 2011; 60: 2386–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmöle A‐C, Lundt R, Ternes S, et al Cannabinoid receptor 2 deficiency results in reduced neuroinflammation in an Alzheimer's disease mouse model. Neurobiol Aging 2015; 36: 710–719. [DOI] [PubMed] [Google Scholar]
- 15. Cassano T, Calcagnini S, Pace L, et al Cannabinoid receptor 2 signaling in neurodegenerative disorders: from pathogenesis to a promising therapeutic target. Front Neurosci 2017; 11: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37(Suppl 1): S81–S90. [DOI] [PubMed] [Google Scholar]
- 17. Dyck PJ, Kratz K, Karnes J, et al The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population‐based cohort The Rochester Diabetic Neuropathy Study. Neurology 1993; 43: 817–824. [DOI] [PubMed] [Google Scholar]
- 18. Toth C. Diabetes and neurodegeneration in the brain Handbook of Clinical Neurology, Vol. 126 Amsterdam, Netherlands: Elsevier, 2014; 489–511. [DOI] [PubMed] [Google Scholar]
- 19. Nathan DM, Buse JB, Davidson MB, et al Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheen AJ. Drug treatment of non‐insulin‐dependent diabetes mellitus in the 1990s. Drugs 1997; 54: 355–368. [DOI] [PubMed] [Google Scholar]
- 21. Herman WH, Hoerger TJ, Brandle M, et al The cost‐effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005; 142: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boulé NG, Haddad E, Kenny GP, et al Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta‐analysis of controlled clinical trials. JAMA 2001; 286: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 23. Little JP, Gillen JB, Percival ME, et al Low‐volume high‐intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol 2011; 111: 1554–1560. [DOI] [PubMed] [Google Scholar]
- 24. Colberg SR, Sigal RJ, Fernhall B, et al Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010; 33: e147–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang P, Li S, Shao M, et al Calorie restriction and endurance exercise share potent anti‐inflammatory function in adipose tissues in ameliorating diet‐induced obesity and insulin resistance in mice. Nutr Metab 2010; 7: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Radak Z, Toldy A, Szabo Z, et al The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int 2006; 49: 387–392. [DOI] [PubMed] [Google Scholar]
- 27. Pedersen BK. The anti‐inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem 2006; 42: 105–117. [DOI] [PubMed] [Google Scholar]
- 28. Coskun O, Ocakci A, Bayraktaroglu T, et al Exercise training prevents and protects streptozotocin‐induced oxidative stress and β‐cell damage in rat pancreas. Tohoku J Exp Med 2004; 203: 145–154. [DOI] [PubMed] [Google Scholar]
- 29. Hay‐Smith EJ, Herderschee R, Dumoulin C, et al Comparisons of approaches to pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev 2011: CD009508. [DOI] [PubMed] [Google Scholar]
- 30. Ivy JL. Role of exercise training in the prevention and treatment of insulin resistance and non‐insulin‐dependent diabetes mellitus. Sports Med 1997; 24: 321–336. [DOI] [PubMed] [Google Scholar]
- 31. Jellinger KA. Recent advances in our understanding of neurodegeneration. J Neural Transm (Vienna) 2009; 116: 1111–1162. [DOI] [PubMed] [Google Scholar]
- 32. Ne'eman Z, Barash V, Rosenmann E, et al Localization of glycogen in the placenta of diabetic rats: a light and electron microscopic study. Placenta 1987; 8: 201–208. [DOI] [PubMed] [Google Scholar]
- 33. Chae CH, Jung SL, An SH, et al Treadmill exercise improves cognitive function and facilitates nerve growth factor signaling by activating mitogen‐activated protein kinase/extracellular signal‐regulated kinase1/2 in the streptozotocin‐induced diabetic rat hippocampus. Neuroscience 2009; 164: 1665–1673. [DOI] [PubMed] [Google Scholar]
- 34. Chen SR, Pan HL. Hypersensitivity of spinothalamic tract neurons associated with diabetic neuropathic pain in rats. J Neurophysiol 2002; 87: 2726–2733. [DOI] [PubMed] [Google Scholar]
- 35. Hopkins WG. A New View of Statistics 2016. Available from: http://sportsci.org/resource/stats/ Accessed November 25, 2017.
- 36. Jain D, Jain R, Eberhard D, et al Age‐and diet‐dependent requirement of DJ‐1 for glucose homeostasis in mice with implications for human type 2 diabetes. J Mol Cell Biol 2012; 4: 221–230. [DOI] [PubMed] [Google Scholar]
- 37. Shendelman S, Jonason A, Martinat C, et al DJ‐1 is a redox‐dependent molecular chaperone that inhibits alpha‐synuclein aggregate formation. PLoS Biol 2004; 2: e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilson MA. The role of cysteine oxidation in DJ‐1 function and dysfunction. Antioxid Redox Signal 2011; 15: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kahle PJ, Waak J, Gasser T. DJ‐1 and prevention of oxidative stress in Parkinson's disease and other age‐related disorders. Free Radic Biol Med 2009; 47: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 40. Bonifati V, Rizzu P, van Baren MJ, et al Mutations in the DJ‐1 gene associated with autosomal recessive early‐onset parkinsonism. Science 2003; 299: 256–259. [DOI] [PubMed] [Google Scholar]
- 41. Sajjad MU, Green EW, Miller‐Fleming L, et al DJ‐1 modulates aggregation and pathogenesis in models of Huntington's disease. Hum Mol Genet 2013; 23: 755–766. [DOI] [PubMed] [Google Scholar]
- 42. Hong Z, Shi M, Chung KA, et al DJ‐1 and alpha‐synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain 2010; 133: 713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Surmeier DJ, Guzman JN, Sanchez‐Padilla J, et al The role of calcium and mitochondrial oxidant stress in the loss of substantia nigra pars compacta dopaminergic neurons in Parkinson's disease. Neuroscience 2011; 198: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palazuelos J, Aguado T, Pazos MR, et al Microglial CB2 cannabinoid receptors are neuroprotective in Huntington's disease excitotoxicity. Brain 2009; 132: 3152–3164. [DOI] [PubMed] [Google Scholar]
- 45. Benito C, Núñez E, Tolón RM, et al Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque‐associated glia in Alzheimer's disease brains. J Neurosci 2003; 23: 11136–11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab 2012; 16: 706–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aguiar AS Jr, Speck AE, Prediger RD, et al Downhill training upregulates mice hippocampal and striatal brain‐derived neurotrophic factor levels. J Neural Transm (Vienna) 2008; 115: 1251–1255. [DOI] [PubMed] [Google Scholar]
- 48. Rybak L, Somani S, Ravi R. Effect of exercise training on antioxidant system in brain regions of rat. Pharmacol Biochem Behav 1995; 50: 635–639. [DOI] [PubMed] [Google Scholar]
- 49. Aksu I, Topcu A, Camsari UM, et al Effect of acute and chronic exercise on oxidant‐antioxidant equilibrium in rat hippocampus, prefrontal cortex and striatum. Neurosci Lett 2009; 452: 281–285. [DOI] [PubMed] [Google Scholar]
- 50. Adlard PA, Perreau VM, Cotman CW. The exercise‐induced expression of BDNF within the hippocampus varies across life‐span. Neurobiol Aging 2005; 26: 511–520. [DOI] [PubMed] [Google Scholar]
- 51. Ruscheweyh R, Willemer C, Kruger K, et al Physical activity and memory functions: an interventional study. Neurobiol Aging 2011; 32: 1304–1319. [DOI] [PubMed] [Google Scholar]
- 52. van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 1999; 2: 266–270. [DOI] [PubMed] [Google Scholar]
- 53. Chen Y‐W, Li Y‐T, Chen YC, et al Exercise training attenuates neuropathic pain and cytokine expression after chronic constriction injury of rat sciatic nerve. Anesth Analg 2012; 114: 1330–1337. [DOI] [PubMed] [Google Scholar]
- 54. Lee JH, McCarty R. Glycemic control of pain threshold in diabetic and control rats. Physiol Behav 1990; 47: 225–230. [DOI] [PubMed] [Google Scholar]
- 55. Lee JH, McCarty R. Pain threshold in diabetic rats: effects of good versus poor diabetic control. Pain 1992; 50: 231–236. [DOI] [PubMed] [Google Scholar]
- 56. Millan MJ. The induction of pain: an integrative review. Prog Neurobiol 1999; 57: 1–164. [DOI] [PubMed] [Google Scholar]
- 57. Edwards JL, Vincent AM, Cheng HT, et al Diabetic neuropathy: mechanisms to management. Pharmacol Therapeut 2008; 120: 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Said G. Diabetic neuropathy—a review. Nat Rev Neurol 2007; 3: 331. [DOI] [PubMed] [Google Scholar]


