Abstract
Chronic overconsumption of animal fats causes a variety of health problems, including diabetes mellitus and obesity. Underlying molecular mechanisms encompass leptin resistance, a decrease in rewarding effects of physical activities, xanthine oxidase‐induced oxidative stress in vasculature and peripheral tissue, impaired activation of incretin signaling, deviation in food preference, and dysbiosis of gut microbiota. Based on our clinical observation that daily intake of brown rice effectively ameliorates bodyweight gain, impaired glucose tolerance/insulin resistance and dependence on fatty foods in obese, prediabetes men, a line of research on brown rice (rice bran)‐derived γ‐oryzanol in mice experiments, cultured cells and human clinical trials is underway in our laboratory. Our works in mice showed that γ‐oryzanol, an ester mixture of ferulic acid and several kinds of phytosterols, acts as a molecular chaperone, thereby attenuating the strong preference for animal fats through suppression of endoplasmic reticulum stress in the hypothalamus. In pancreatic islets from both high‐fat diet‐induced and streptozotocin‐induced diabetic mice, γ‐oryzanol ameliorates endoplasmic reticulum stress and protects β‐cells against apoptosis. Noticeably, γ‐oryzanol also acts as a potent inhibitor against deoxyribonucleic acid methyltransferases in the brain reward system (striatum) in mice, thereby attenuating, at least partly, the preference for a high‐fat diet through the epigenetic modulation of striatal dopamine D2 receptor. Because dopamine D2 receptor signaling in the brain reward system is considerably attenuated in obese humans and rodents, γ‐oryzanol might represent a unique property to ameliorate both hedonic and metabolic dysregulation of feeding behavior, highlighting a promising prophylactic avenue to protect against metabolic derangement.
Keywords: γ‐Oryzanol, Brown rice, Obesity disease
Introduction
It has been well highlighted that chronic and excessive intake of animal‐derived fats causes a variety of metabolic hazards, including obesity, diabetes mellitus, dyslipidemia, atherosclerotic diseases and cognitive impairment (Figure 1). Some underlying mechanisms consist of defects in circadian organization and resultant disorders of physiological output1, exaggerated oxidative stress through the enzyme xanthine oxidase2, so‐called lipotoxicity in a variety of organs3, hyperphagia caused by leptin insensitivity in the brain hypothalamus4, and a decrease in rewarding effects of locomotion via hyperleptinemia through dopamine neurons in the midbrain5.
Figure 1.
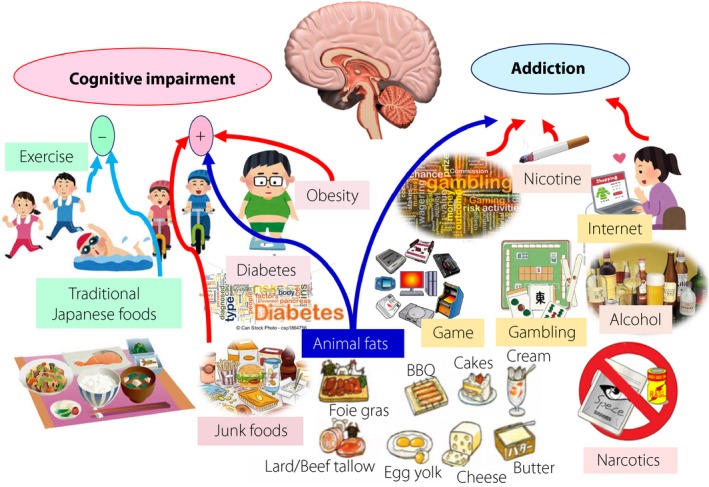
Cognitive impairment and addictive behavior, two major factors of brain dysfunction in a super aging society. Chronic and excessive intake of animal fat‐rich diets causes a variety of metabolic hazards, including diabetes mellitus and obesity. Furthermore, an animal fat‐rich diet apparently increases the risk for cognitive impairment and addiction to fatty foods.
Chronic and excessive intake of animal‐derived fats drastically attenuates the action of leptin to the brain, a hormone secreted from adipocytes (also known as leptin resistance), thereby leading to difficulties in bodyweight reduction. In the brain, the arcuate nucleus in the hypothalamus is one of the main control centers for appetite regulation through input of a variety of hormones and the autonomic nerve system (also known as a regulatory system of metabolic hunger)6. Noticeably, excessive intake of animal fats provokes microglial inflammation and cellular stresses, such as endoplasmic reticulum (ER) stress and oxidant stress in the hypothalamus, paralyzing the regulatory system of metabolic hunger, thus creating a condition where the brain cannot correctly judge the energy intake appropriate for the body7. It is as if the brain is ‘hacked.’ When mice are fed animal fat‐rich foods, activated microglia infiltrates into the hypothalamus within a couple of days8. Subsequently, hypothalamic damage and immune cell migration is aggravated, resulting in the condition of chronic inflammation8. It is intriguing, however, to note that microglial inflammation occurring in the hypothalamus of mice associated with excess animal fat intake is drastically improved by daily exercise on a regular basis9.
The mechanistic similarity between the addiction to animal fats and that to narcotics, nicotine, alcohol, gaming and gambling is of great interest10. Among various types of dependence, a plausible mechanism where the intake of stimulants increases would be similar to that where the recognition threshold of the brain reward system gradually increases, and consequently, the brain cannot obtain the same satisfaction and pleasure as before. Experimentally, in obese mice housed on a high animal fat diet, the level that the brain reward system can respond to animal fat gradually rises, reminiscent of the case of dependence on cocaine and heroin11. It is therefore possible to speculate that the mice fall into a vicious cycle where no matter how much they eat, they cannot be satisfied. In rat experiments using narcotics, nicotine and alcohol, when these addictive substances were forcibly blocked, the robust dependence rapidly disappears within 3 days. However, the dependence on animal fats was not improved even 2 weeks after the diet was removed, suggesting that animal fat is more addictive than narcotics11.
Role of Hypothalamic ER Stress in Dependence on Animal Fats
When wild‐type C57/B6 mice are food‐deprived for 24 h and given both high carbohydrate and high animal fat foods placed side‐by‐side, they choose preferentially high carbohydrate food in order to avoid protracted hypoglycemia12. In contrast, in a similar experiment using mice with high animal fat diet‐induced obesity, obese mice still chose high animal fat food. Such a phenomenon can be explained by the notion that the mice became unable to judge how many calories they need and which food they should choose under the chronic excess intake of animal fat.
From a series of mouse experiments by the authors’ research group, it was clarified that exaggerated ER stress in the hypothalamus strongly impacts on the preference for animal fats13. For example, mice were allowed to choose between standard laboratory chow and high animal fat food, and then mice were dosed with 4‐phenyl butyric acid that functions as a molecular chaperone (also known as an ER stress reliever). Consequently, the proportion of mice choosing high animal fat foods was significantly decreased, and the metabolic phenotype in obesity and hyperglycemia was concomitantly alleviated13. These results raise the possibility that a vicious cycle is formed in which the excess intake of animal fat increases ER stress in the hypothalamus, and in turn, the exaggerated ER stress further strengthens the preference for animal fat13.
γ‐Oryzanol Functions as a ER Stress Reliever in the Hypothalamus
Brown rice (rice bran) is highlighted as a natural complete diet, which abundantly contains a variety of vitamins, minerals, anti‐oxidizing substances, trace elements, proteins, lipids and dietary fibers (carbohydrates)14. Brown rice is also characterized as a low glycemic index food, inhibiting postprandial hyperglycemia15. Noticeably, the Chinese character for ‘bran’ includes a part that means ‘health.’ In contrast, the Chinese character connecting ‘white’ and ‘rice’ means ‘nothing remains.’ From the results of a cross‐over interventional trial using brown rice for male patients with metabolic syndrome living in Okinawa Prefecture, Japan, named the BRAVO study, a series of metabolically‐beneficial benefits to dysfunction of vascular endothelium, liver steatosis, overweight, and furthermore, to the dependence on animal fat‐rich diets, such as junk foods and fast foods, were shown16.
Based on the notion that Okinawan people of senior age are fond of eating brown rice, the authors’ research group focused their attention on γ‐oryzanol, a functional component specifically and abundantly contained in brown rice (rice bran)17. It was clarified by a line of mouse and cellular experiments in which γ‐oryzanol functions as a molecular chaperone that promotes protein folding and decreases ER stress, which considerably increases in the hypothalamus by the chronic, excessive intake of animal fats, thereby attenuating the dependence on animal fats, and improving fuel dyshomeostasis and insulin resistance13.
Because the scientific name of rice is Oryza sativa, γ‐oryzanol exactly symbolizes the main component of rice oil. γ‐Oryzanol was first extracted from brown rice by two Japanese researchers, Dr Tsuchiya and Dr Kaneko. Structurally, γ‐oryzanol is a ferulic acid ester compound of several kinds of triterpene alcohol and phytosterol (Figure 2)17. When orally dosed in mouse experiments, a part of γ‐oryzanol passes through the blood–brain barrier, keeping its ester binding as it is, and is distributed in the brain in considerably high concentrations18. It is well documented that C57/B6 mice show a strong preference for animal fats, as do humans12. In an experimental paradigm where a part of the carbohydrates of both standard laboratory chow and animal fat‐rich diets was substituted with brown rice powder or white rice powder with the same calories, only the mice of the group in which carbohydrates were substituted with brown rice had a significantly decreased preference for an animal fat diet (~20%)13. Consequently, the obesity and fuel dysmetabolism in mice were markedly improved. Such a result can be reproduced by an experiment where a part of the carbohydrates of both standard laboratory chow and animal fat‐rich diets is substituted with γ‐oryzanol7.
Figure 2.
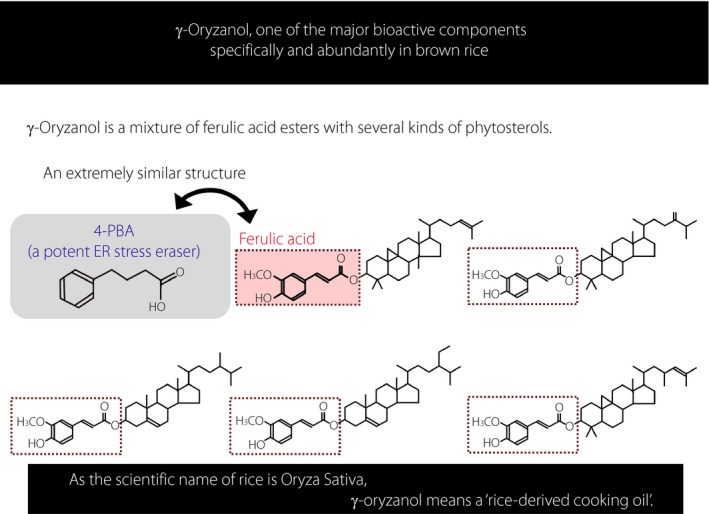
Structure and biochemical properties of γ‐oryzanol. γ‐Oryzanol is a functional component specifically and abundantly contained in brown rice. Structurally, γ‐oryzanol is a ferulic acid ester compound of several kinds of triterpene alcohol and phytosterol. Chemical properties of γ‐oryzanol are beneficial for its stable central action. The structure is resistant to esterase, and robust against heat, pressure and acidic environment. 4‐PBA, 4‐phenyl butyric acid; ER, endoplasmic reticulum.
Using a HEK293 cell line, the authors showed that γ‐oryzanol significantly suppresses the transcriptional activity in the ER stress responsive element that is induced by tunicamycin13. Furthermore, experiments using a fetal mouse brain‐derived nerve cell primary culture verified that γ‐oryzanol remarkably suppresses the gene expression of ER stress‐related molecules induced by tunicamycin13. Taken together, we confirmed that γ‐oryzanol surely functions as a chaperone in a viable cell system.
γ‐Oryzanol Improves Dysfunction of Pancreatic Islets Associated with Glucolipotoxicity
In mouse experiments, our research team showed that γ‐oryzanol improves dysfunction of pancreatic islets associated with glucolipotoxicity through enhancing glucose‐stimulated insulin secretion (GSIS) and also inhibiting the excessive secretion of glucan18. We clarified that such a metabolically beneficial effect of γ‐oryzanol on pancreatic islets is explained by the following two molecular mechanisms. First, γ‐oryzanol functions as a chaperone and alleviates the exaggerated ER stress in pancreatic islets (Figure 3)18. Second, at both transcription and translation levels, γ‐oryzanol inhibits the dopamine type 2 receptor (D2R) signaling in pancreatic islets, which is augmented by animal fat‐rich diets19.
Figure 3.
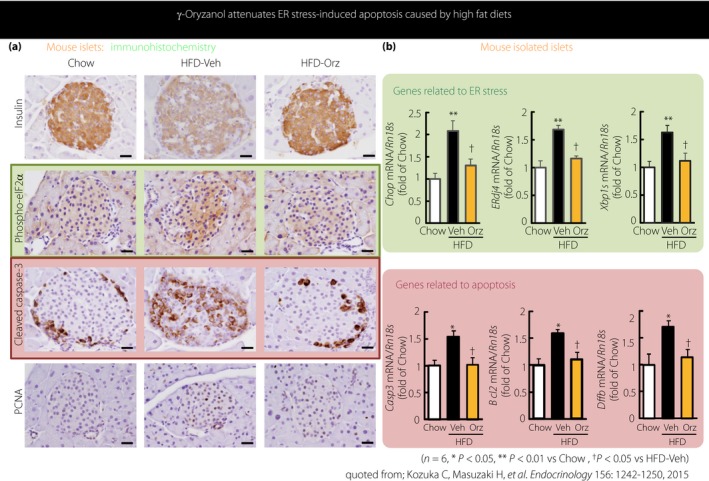
γ‐Oryzanol attenuates endoplasmic reticulum (ER) stress‐induced apoptosis caused by high‐fat diets. (a) Immunostaining images. (b) Messenger ribonucleic acid (mRNA) expression for ER stress‐related genes (upper panel) and for apoptosis‐related genes (lower panel). Levels of mRNA expression for Chop, ERdj4, Xbp1s, Casp3, Bcl2 and Dffb in isolated pancreatic islets are shown by quantitative real‐time polymerase chain reaction and normalized with ribosomal 18S. Data are expressed as mean ± standard error of the mean (n = 6 in each group), *P < 0.05, **P < 0.01 vs standard laboratory diet (Chow), † P < 0.05 vs high‐fat diet plus vehicle‐treated group (HFD‐Veh). Bcl2, the BCL‐2 families determine the commitment of cells to apoptosis; Casp‐3, caspase‐3, sequential activation of caspases plays a central role in apoptosis; Chop, Chop is a C/EBP homologous protein, also known as growth arrest‐ and deoxyribonucleic acid damage‐inducible gene 153 (GADD153); Dffb, Dffb triggers both deoxyribonucleic acid fragmentation and chromatin condensation during apoptosis; ERdj4, ERdj4 is a soluble endoplasmic reticulum DnaJ family protein that interacts with endoplasmic reticulum‐associated degradation machinery; HFD‐Orz, high‐fat diet plus γ‐oryzanol‐treated group; PCNA, proliferating cell nuclear antigen; Phospho‐eIF2, phosphorylation of the eukaryotic initiation factor 2 α subunit; Xbp1s, Xbp1 is a transcription factor that regulates the expression of genes in the cellular stress response. Reproduced with permission from Endocrinology 201518. Copyright and all rights reserved.
It has been shown that augmented signaling of D2R in pancreatic islets inhibits GSIS and resultantly elevates the blood glucose level20. However, to date, detailed molecular mechanisms are not fully elucidated. In this context, the authors’ finding that excess intake of animal fat does increase D2R signaling locally in pancreatic islets would exemplify a novel facet of metabolic hazard caused by overconsumption of animal fats19. Furthermore, we showed that γ‐oryzanol acts directly on islets and enhances GSIS through the activation of the cyclic adenosine monophosphate/protein kinase A pathway. Elevated expression levels of molecules involved in D2R signaling in islets from high animal fat diet‐fed mice are normalized by oral administration of γ‐oryzanol. Experiments with ribonucleic acid interference against D2R and D2R ligands also suggest that γ‐oryzanol suppresses D2R signaling and augments GSIS. The unexpected role of γ‐oryzanol on D2R signaling in pancreatic islets might shed light on a natural food‐based novel antidiabetic approach in humans.
γ‐oryzanol as a Epigenetic Controller in the Brain Reward System
The signal of the brain reward system is conveyed by dopamine neurons. Clinically, an apparent decrease in D2R activity in the striatum, one of the key nuclei in the brain reward system, is shown in obese people who abuse cocaine10, 21, 22. In accordance with this notion, functional magnetic resonance imaging shows that postprandial activation of the striatum, as evidenced by an increase in blood flow, is markedly diminished in obese people23. These findings indicate that obese people cannot appropriately accept the reward signal in the brain during meals.
As a molecular mechanism responsible for the decrease in the D2R signal of the brain reward system accompanied by the addiction to animal fat‐rich foods, epigenetic modifications, such as deoxyribonucleic acid (DNA) hypermethylation in the promoter region (CpG island) of the D2R gene, would be suggested10, 24. For example, regarding the mechanism whereby visceral fat tends to accumulate in the excess of animal fat‐rich diets, the expression level of peroxisome proliferator‐activated receptor‐γ (PPARγ), a master transcriptional factor that controls the accumulation of subcutaneous fat depots, has been shown to decrease in mice on a high‐fat diet, where DNA hypermethylation in the promoter region of PPARγ gene is also involved25. It is therefore tempting to speculate that excess intake of animal fats inactivates a variety of genes involved in metabolic and endocrine homeostasis.
Recently, the authors showed that γ‐oryzanol acts on the brain reward system in obese mice fed an animal fat‐rich diet, and changes ‘the brain that cannot be satisfied’ to ‘the brain that can be satisfied’ as an epigenome controller26. In striatum from mice fed an animal fat‐rich diet, the expression level of D2R was significantly decreased through the considerable increase in DNA methylation of its promoter region. First, pharmacological inhibition of DNA methyltransferases by 5‐aza‐2′‐deoxycytidine in mice normalized the expression level of D2R in striatum with a concomitant decrease in the DNA methylation of its promoter region. In this experimental setting, the preference for an animal fat‐rich diet was significantly decreased in 5‐aza‐2′‐deoxycytidine‐treated mice. Second, oral administration of γ‐oryzanol also decreased the expression of DNA methyltransferases, thereby decreasing the DNA methylation in the promoter region of D2R and concomitantly restoring the expression level of D2R in striatum. Consistent with a series of in vivo findings, enzymatic in vitro assays also showed that γ‐oryzanol inhibited the activity of DNA methyltransferases26.
Together with our previous research in mice showing that γ‐oryzanol attenuates the preference for an animal fat‐rich diet through hypothalamic regulation of ER stress, γ‐oryzanol also represents a unique property to ameliorate both hedonic and metabolic dysregulation of feeding behavior (Figure 4). Because overeating in obese individuals shares, at least partly, common mechanisms with addiction to alcohol, nicotine and narcotics10, a natural food‐based approach toward the brain reward system is anticipated to safely treat obesity. In this paradigm, γ‐oryzanol is a promising anti‐obesity candidate with a distinct property of epigenetic modulator.
Figure 4.
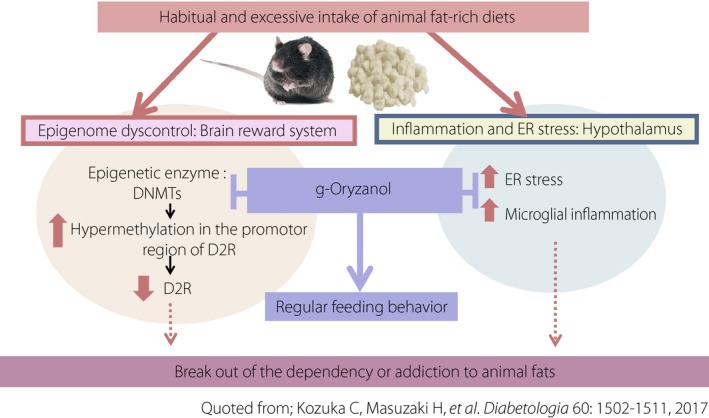
γ‐Oryzanol ameliorates addictive behavior to animal fat‐rich diets through hypothalamic and midbrain mechanisms. γ‐Oryzanol attenuates the preference for animal fat‐rich diet through hypothalamic regulation of endoplasmic reticulum (ER) stress. In contrast, γ‐oryzanol also decreases the expression of deoxyribonucleic acid methyltransferases (DNMTs) in the brain reward system (striatum), thereby decreasing deoxyribonucleic acid methylation in the promoter region of dopamine type 2 receptor (D2R) and concomitantly restoring the expression level of D2R in striatum. In this paradigm, γ‐oryzanol is a promising anti‐obesity candidate with a distinct property of epigenetic modulator.
Approach toward the Practical use and Social Implementation of γ‐oryzanol
A series of approaches aiming at the practical use and social implementation of γ‐oryzanol is underway. A fermented brown rice beverage richly containing γ‐oryzanol, GENMAI ORYZANO as the product name, has already been developed by the cross‐ministerial Strategic Innovation Promotion Program, a Japanese national strategic project, collaborating with Aizu Tenpo Jozo K.K. Its practical use and social implementation has been successful, and the product was commended with the Food Action Nippon Award (FAN) of the Ministry of Agriculture, Forestry and Fishery, Research Development New Technology Excellence Award 2015. A line of the accomplishment was also featured by Nature SPOTLIGHT (page 19, issue of 30 March 2017). As a result of the cross‐over interventional trial with GENMAI ORYZANO for 40 middle‐aged people with metabolic syndrome in Okinawa, significant improvement in metabolic derangement, dysbiosis of gut microbiota and related constipation, and robust preference for animal fats was observed (Yonamine et al. manuscript in preparation, 2018). Notably, such a line of a metabolically beneficial impact of GENMAI ORYZANO was significantly exaggerated in the case of those who did not habitually eat traditional Japanese foods or those whose balance of gut microbiota was aggravated before the trial.
Furthermore, our research team has succeeded in developing a novel delivery system that remarkably increases metabolically beneficial effects of γ‐oryzanol by use of nanoparticle technology in collaboration with SENTAN Pharma Inc. In fact, toward the clinical application of γ‐oryzanol, extremely low absorption efficiency from the intestine has been a difficult obstacle. Therefore, to overcome extremely low bioavailability of γ‐oryzanol with super high lipophilicity, we encapsulated γ‐oryzanol in polymer poly (DL‐lactide‐co‐glycolide) nanoparticles (Nano‐Orz), and evaluated its metabolically beneficial impact in genetically obese diabetic ob/ob mice, the best‐known most severe diabetic model in mice. Consequently, Nano‐Orz markedly ameliorated fuel dysmetabolism shown in ob/ob mice with an unexpected magnitude (approximately 1,000‐fold lower dose) compared with the regular γ‐oryzanol7. Surprisingly, such a conspicuous impact was achievable by its administration once every 2 weeks. Besides the excellent impact on dysfunction of the hypothalamus and pancreatic islets, Nano‐Orz markedly decreased ER stress and inflammation in the liver and adipose tissue7.
Recent studies have highlighted that modulation of gut microbiota by unrefined whole grains results in metabolically beneficial changes in plasma levels of short chain fatty acids through bacterial fermentation7. Notably, in most cases of human and rodent obesity, the diversity of gut microbiota is decreased, and the ratio of Firmicutes to Bacteroidetes is elevated27, 28. We showed that Nano‐Orz did decrease the Firmicutes‐to‐Bacteroidetes ratio, as well as a trend toward an increase in plasma short chain fatty acids levels in mice. Taken together, nanotechnology‐based developments of functional foods oriented toward γ‐oryzanol might shed light on the novel approach to obesity in humans7.
Perspective
Chronic and excessive intake of animal fats and the resultant dependence on fatty foods complexly act on the onset and aggravation of type 2 diabetes mellitus and obesity in humans. As we summarized in the present review, brown rice‐derived γ‐oryzanol can act on the brain hypothalamus and reward system, pancreatic islets, liver and adipose tissue, and gut microbiota with a unique and distinct property. Collectively, γ‐oryzanol effectively acts against a line of metabolic hazards provoked by chronic overconsumption of an animal fat‐rich diet (Figure 5). In this paradigm, γ‐oryzanol is a promising anti‐obesity, antidiabetic target in academic, clinical and industrial fields (obtained intellectual properties are as follows. Japanese patents: No. 6098973 [2017], No. 6143215 [2017], No. 6281919 [2018], No. 6182540 [2017]; International patent 13F088‐PCT‐EP, ZL 201380067472.2 [China]).
Figure 5.
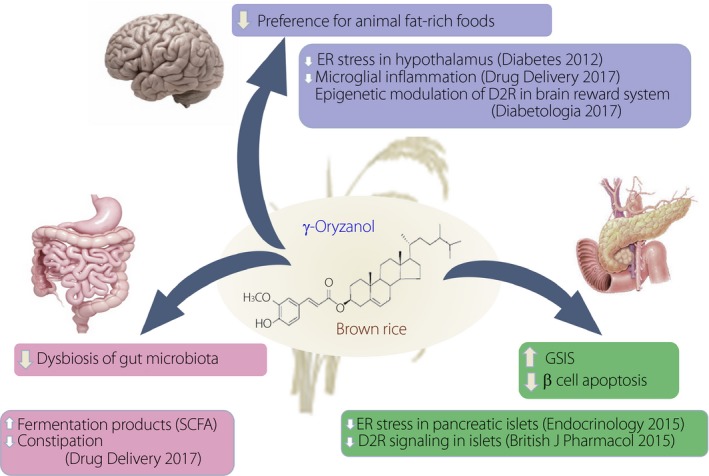
Metabolically beneficial impact of γ‐oryzanol throughout the body. γ‐Oryzanol acts on the brain hypothalamus and reward system, pancreatic islets, liver and adipose tissue, and gut microbiota with a unique and distinct property. D2R, dopamine type 2 receptor, ER stress, endoplasmic reticulum stress; GSIS, glucose‐stimulated insulin secretion; SCFA, short chain fatty acid.
Achieving a novel strategy to modify dietary habits through focusing on functional components derived from traditional Japanese foods is highly anticipated. In this context, a series of metabolically beneficial actions of γ‐oryzanol might shed light on a novel, natural food‐based preventive medicine for type 2 diabetes and obesity disease. Our research team is currently investigating the potential benefit of γ‐oryzanol in cognitive impairment, physical inactivity, and addiction to alcohol, nicotine and dietary animal fats, all of which are often associated with type 2 diabetes and obesity.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We are grateful to Professor Keiko Abe (Department of Applied Biological Chemistry, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan) for invaluable advice and continued support. We thank Chikako Noguchi, Mamiko Hirata, Hoshino Kaneshiro, Tsugumi Uema and Ikuko Asato for secretarial assistance, and also we acknowledge Tomoko Ikematsu, Ayano Kinjo, Yuko Murayama, Ikumi Nomura and Chie Horiguchi for technical assistance. This work was supported in part by Grants‐in‐Aid from the Japan Society for the Promotion of Science (JSPS; KAKENHI; 15K19520 and 24591338), Council for Science, Technology and Innovation (CSTI, Japanese Government), Cross‐ministerial Strategic Innovation Promotion Program (SIP, Japanese Government), ‘Technologies for Creating Next‐Generation Agriculture, Forestry and Fisheries’, New Energy and Industrial Technology Development Organization (NEDO, Japanese Government), and a Grant from the Okinawa prefecture for promotion of advanced medicine (Okinawa Prefecture, Japan).
J Diabetes Investig 2019; 10: 18–25
References
- 1. Pivovarova O, Jürchott K, Rudovich N, et al Changes of dietary fat and carbohydrate content alter central and peripheral clock in humans. J Clin Endocrinol Metab 2015; 100: 2291–2302. [DOI] [PubMed] [Google Scholar]
- 2. Sunagawa S, Shirakura T, Hokama N, et al. Activity of xanthine oxidase in plasma correlates with indices of insulin resistance and liver dysfunction in patients with type 2 diabetes mellitus and metabolic syndrome: A pilot exploratory study. J Diabetes Investig 2019; 10: 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taira S, Shimabukuro M, Higa M, et al Lipid deposition in various sites of skeletal muscle and liver exhibits a positive correlation with visceral fat accumulation in middle‐aged Japanese men with metabolic syndrome. Intern Med 2013; 52: 1561–1571. [DOI] [PubMed] [Google Scholar]
- 4. Tanaka T, Masuzaki H, Yasue S, et al Central melanocortin signaling restores skeletal muscle AMP‐activated protein kinase phosphoylation in mice fed a high fat diet. Cell Metab 2007; 5: 395–402. [DOI] [PubMed] [Google Scholar]
- 5. Fernandes MF, Matthys D, Hryhorczuk C, et al Leptin suppresses the rewarding effects of running via STAT3 signaling in dopamine neurons. Cell Metab 2015; 22: 741–749. [DOI] [PubMed] [Google Scholar]
- 6. Waterson MJ, Horvath TL. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab 2015; 22: 962–970. [DOI] [PubMed] [Google Scholar]
- 7. Kozuka C, Shimizu‐Okabe C, Takayama C, et al Marked augmentation of PLGA nanoparticle‐induced metabolically‐beneficial impact of γ‐oryzanol on fuel dyshomeostasis in genetically obese‐diabetic ob/ob mice. Drug Delivery 2017; 24: 558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thaler JP, Yi CX, Schur EA, et al Obesity is associated with hypothalamic injury in rodents and humans. J Clin Investig 2012; 122: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yi CX, Al‐Massadi O, Donelan E, et al Exercise protects against high‐fat diet‐induced hypothalamic inflammation. Physiol Behav 2012; 106: 485–490. [DOI] [PubMed] [Google Scholar]
- 10. DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci 2012; 15: 1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epstein DH, Shaham Y. Cheesecake‐eating rats and the question of food addiction. Nat Neurosci 2010; 13: 529–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okamoto S, Sato T, Tateyama M, et al Activation of AMPK‐regulated CRH neurons in the PVH is sufficient and necessary to induce dietary preference for carbohydrate over fat. Cell Rep 2018; 22: 706–721. [DOI] [PubMed] [Google Scholar]
- 13. Kozuka C, Yabiku K, Sunagawa S, et al Brown rice and its component, gamma‐oryzanol, attenuate the preference for high‐fat diet by decreasing hypothalamic endoplasmic reticulum stress in mice. Diabetes 2012; 61: 3084–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun Q, Spiegelman D, van Dam RM, et al White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 2010; 170: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Panlasigui LN, Thompson LU. Blood glucose lowering effects of brown rice in normal and diabetic subjects. Int J Food Sci Nutr 2006; 57: 151–158. [DOI] [PubMed] [Google Scholar]
- 16. Shimabukuro M, Higa M, Shiroma‐Kinjo R, et al Effects of brown rice diet on visceral obesity and endothelial function: the BRAVO study. British J Nutr 2014; 111: 310–320. [DOI] [PubMed] [Google Scholar]
- 17. Kozuka C, Yabiku K, Takayama C, et al Natural food science based novel approach toward prevention and treatment of obesity and type 2 diabetes: recent studies on brown rice and γ‐oryzanol. Obes Res Clin Pract 2013; 7: e165–e172. [DOI] [PubMed] [Google Scholar]
- 18. Kozuka C, Sunagawa S, Ueda R, et al γ‐Oryzanol protects pancreatic beta‐cells against endoplasmic reticulum stress in male mice. Endocrinology 2015; 156: 1242–1250. [DOI] [PubMed] [Google Scholar]
- 19. Kozuka C, Sunagawa S, Ueda R, et al A novel insulinotropic mechanism of whole grain‐derived gamma‐oryzanol via the suppression of local dopamine D2 receptor signalling in mouse islet. Br J Pharmacol 2015; 172: 4519–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simpson N, Maffei A, Freeby M, et al Dopamine‐mediated autocrine inhibitory circuit regulating human insulin secretion in vitro . Mol Endocrinol 2012; 26: 1757–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stice E, Spoor S, Bohon C, et al Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008; 322: 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noble EP. Addiction and its reward process through polymorphisms of the D2 dopamine receptor gene: a review. Eur Psychiatry 2000; 15: 79–89. [DOI] [PubMed] [Google Scholar]
- 23. Stice E, Yokum S, Blum K, et al Weight gain is associated with reduced striatal response to palatable food. J Neurosci 2010; 30: 13105–13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci 2011; 12: 638–651. [DOI] [PubMed] [Google Scholar]
- 25. Fujiki K, Kano F, Shiota K, et al Expression of the peroxisome proliferator activated receptor gamma gene is repressed by DNA methylation in visceral adipose tissue of mouse models of diabetes. BMC Biol 2009; 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kozuka C, Kaname T, Shimizu‐Okabe C, et al Impact of brown rice‐specific γ‐oryzanol on epigenetic modulation of dopamine D2 receptor in brain striatum of high fat diet‐induced obese mice. Diabetologia 2017; 60: 1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turnbaugh PJ, Ley RE, Mahowald MA, et al An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 28. Walters WA, Xu Z, Knight R. Meta‐analyses of human gut microbes associated with obesity and IBD. FEBS Lett 2014; 588: 4223–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]


