Abstract
The renin–angiotensin system (RAS), a crucial regulator of systemic blood pressure (circulatory RAS), plays distinct roles in pathological angiogenesis and inflammation in various organs (tissue RAS), such as diabetic microvascular complications. Using ocular clinical samples and animal disease models, we elucidated molecular mechanisms in which tissue RAS excites the expression of vascular endothelial growth factor (VEGF)‐A responsible for retinal inflammation and angiogenesis, the two major pathological events in diabetic retinopathy (DR). Furthermore, we showed the involvement of (pro)renin receptor [(P)RR] in retinal RAS activation and its concurrent intracellular signal transduction (e.g., extracellular signal‐regulated kinase); namely, the (P)RR‐induced dual pathogenic bioactivity referred to as the receptor‐associated prorenin system. Indeed, neovascular endothelial cells in the fibrovascular tissue collected from eyes with proliferative DR were immunoreactive for the receptor‐associated prorenin system components including prorenin, (P)RR, phosphorylated extracellular signal‐regulated kinase and VEGF‐A. Protein levels of soluble (P)RR increased with its positive correlations with prorenin, renin enzymatic activity and VEGF in the vitreous of proliferative DR eyes, suggesting a close link between (P)RR and VEGF‐A‐driven angiogenic activity. Furthermore, we revealed an unsuspected, PAPS‐independent role of (P)RR in glucose‐induced oxidative stress. Recently, we developed an innovative single‐strand ribonucleic acid interference molecule selectively targeting human and mouse (P)RR, and confirmed its efficacy in suppressing diabetes‐induced retinal inflammation in mice. Our data using clinical samples and animal models suggested the significant implication of (P)RR in the pathogenesis of DR, and the potential usefulness of the ribonucleic acid interference molecule as a therapeutic agent to attenuate ocular inflammation and angiogenesis.
Keywords: Diabetic retinopathy, (Pro)renin receptor, Receptor‐associated prorenin system
Introduction
Diabetic retinopathy (DR), the most common microvascular complication in patients with diabetes, is a leading cause of severe vision loss and blindness in developed countries. Proliferative DR (PDR) is the advanced stage of DR, characterized by proliferation of fibrovascular tissue formed by the extension of retinal angiogenesis into the vitreous cavity, and the fibrovascular tissue formation leads to serious complications including traction retinal detachment and vitreous hemorrhage. A growing body of evidence has accumulated to show that inflammation with leukocyte infiltration plays a crucial role in the pathogenesis of vision‐threatening retinal diseases, such as DR1, which is now considered as an inflammatory as well as angiogenic disease. In acute inflammation, leukocytes infiltrate to extravascular tissues to recognize and exclude the offending agent, mainly contributing to tissue repair. Conversely, leukocytes in chronic inflammation, as seen in diabetes, cause tissue damage as a result of sequential secretion of cytokines, chemical mediators and reactive oxygen species, thus developing a functional maladaptation and tissue remodeling. Inflammatory leakage from the dilated hyperpermeable vasculature leads to the entry of protein‐ and lipid‐containing fluid into the retinal parenchyma, causing frequently observed DR lesions including macular edema and hard exudates. Of numerous cytokines and growth factors contributing to the molecular pathogenesis of DR, vascular endothelial growth factor (VEGF)‐A has been proven to be the key inflammatory and angiogenic mediator in DR2, 3, 4. VEGF‐A is a secreted cytokine and the most potent angiogenic factor induced mainly by hypoxia‐ and redox‐sensitive transcription factors, and promotes numerous physiological events, such as embryonic development and wound healing. In contrast, VEGF‐A is also associated with several pathological events related to various diseases, such as cancer and diabetes. During tumor growth, VEGF‐A is predominantly required for new vessel formation, and thus is the major molecular target for anti‐angiogenic therapy in cancer5. Of several VEGF‐A isoforms, we elucidated the significant involvement of VEGF165 with angiogenic activity in PDR, showing that fibrovascular tissues expressing both VEGF receptor‐2 and neuropilin‐1, VEGF165‐specific receptor, were extremely vascularized1, 6, 7. VEGF‐A increases the expression of various molecules and subsequently facilitates the infiltration of VEGF‐A‐releasing leukocytes, which marks a positive feedback loop amplifying the development of retinal inflammation (macular edema) and angiogenesis (fibrovascular proliferation) seen in DR1, 6, 7. In the past decade, therapies targeting VEGF‐A have revolutionized the treatment of DR; however, there are several limitations to the therapeutic strategy to inhibit the single molecule in the downstream of a series of pathogenic cascades, and thus an alternative and additive treatment for suppressing some upstream key process at the earlier stages of disease would be desirable as the next‐generation management of DR.
The renin–angiotensin system (RAS) was originally considered as a central regulatory mechanism for sodium and fluid homeostasis in controlling systemic blood pressure (i.e., circulatory RAS). Recently, RAS components were also reported to be expressed in numerous tissues independently of circulatory RAS, which is hence called ‘tissue RAS’8. Tissue RAS works in a paracrine fashion, and controls several physiological and pathological processes including cell signaling and growth, angiogenesis, and tissue remodeling9, 10, 11. The (pro)renin receptor ([P]RR), which is located in the upstream of tissue RAS, binds with prorenin leading to not only the activation of tissue RAS, but also its intracellular signal transduction, and regulates the expression of various pathogenic molecules including VEGF‐A12. This dual activation of tissue RAS and RAS‐independent signaling pathways through (P)RR is now referred to as the receptor‐associated prorenin system (RAPS)12. In the present review, we discuss recent progress in the understanding of the significant contribution of (P)RR to the pathogenesis of DR, in combination with our effort to develop an innovative therapeutic agent against (P)RR.
Tissue RAS in Diabetic Retinopathy
The initial step of circulatory RAS necessitates the indispensable process of proteolytic activation of prorenin, whereby prorenin is changed to its mature active form; that is, renin by the processing enzymes (e.g., cathepsin B) exclusively in juxtaglomerular cells in the kidney to digest the prorenin prosegment that folds into an active‐site cleft of renin. Renin is a rate‐limiting enzyme in circulatory RAS for the shedding of angiotensinogen to angiotensin I (Ang I), which angiotensin‐converting enzyme changes to angiotensin II (Ang II), the effector molecule that binds to its cognate receptors, Ang II type 1 receptor (AT1R) and Ang II type 2 receptor. By contrast, tissue RAS is characterized by independence from the processing enzyme‐based proteolytic activation of prorenin to acquire renin activity, and requires an alternative triggering step caused by (P)RR, as discussed below. Tissue RAS plays several important roles in pathological vascular events, such as angiogenesis and inflammation, and various organ abnormalities are shown to be caused by tissue RAS activation. Regarding its relationship with the eye, multiple clinical trials, such as the EUCLID study, DIRECT‐Prevent 1, ‐Protect 1 and ‐Protect 2, and the RAS study, showed that inhibition of AT1R or angiotensin‐converting enzyme resulted in blood pressure‐unrelated beneficial effects on the incidence and progression of DR13, 14, 15, 16. Ang II levels were reported to significantly increase in the vitreous fluid of PDR eyes, with its significant correlation with VEGF levels17, 18. Furthermore, we have shown the significant involvement of the AngII/AT1R signaling pathway in inflammation‐related ocular angiogenesis, which causes upregulated expression of VEGF‐A, C‐C chemokine ligand (CCL)2/monocyte chemotactic protein (MCP)‐1 and intercellular adhesion molecule‐119, 20, all of which were verified to be responsible for the pathogenesis of DR. These several reports indicate that the activation of tissue RAS in the diabetic eye is the major key event predisposing to the development and deterioration of DR.
(P)RR in Diabetic Retinopathy
Various organ impairments are reported to result from tissue RAS activation; however, the detailed molecular mechanism for activating tissue RAS still remains unknown. (P)RR, also known as ATP6AP2 (i.e., the gene name for [P]RR) and identified as a single transmembrane protein consisting of 350 amino acids, binds to prorenin to exert renin activity through changes in the three‐dimensional structure of prorenin prosegment (i.e., activated prorenin) to expose its enzymatic active‐site cleft without the conventional proteolysis of the prorenin prosegment (i.e., renin) achieved by the processing enzymes. This receptor‐based non‐proteolytic activation of prorenin plays an important role in tissue, but not circulatory, RAS activation, because the membrane‐bound (P)RR is shown to exist in various tissues, but not in the circulation21. Furthermore, (P)RR interacting with prorenin has been shown to trigger RAS‐independent signaling pathways through phosphorylation of extracellular signal‐regulated kinase (ERK)1/221, 22. Indeed, neovascular endothelial cells of the fibrovascular tissue excised from eyes with PDR were immunopositive for prorenin, (P)RR, phosphorylated ERK1/2 and VEGF‐A, in accordance with our in vitro data showing that the prorenin–(P)RR–ERK axis led to an increase in VEGFA expression in human retinal microvascular endothelial cells23. Thus, we proposed the nomenclature, ‘RAPS,’ for the (P)RR‐induced dual activation of tissue RAS and RAS‐independent intracellular signals. (P)RR can interact with both renin and prorenin; however, the binding affinity of renin is much lower than that of prorenin24, which marks the background of this notation as the ‘(pro)renin,’ but not renin, receptor. RAPS was shown to contribute to the molecular pathogenesis of various ocular disease animal models, such as diabetes‐induced retinal inflammation, laser‐induced choroidal neovascularization, endotoxin‐induced uveitis and oxygen‐induced retinopathy25, 26, 27, 28.
Vitreous RAS and Retinal Receptor‐Associated Prorenin System
Notably, (P)RR was shown to be digested by proteases to turn into a soluble form of (P)RR [s(P)RR]; however, in vitro, it still possesses an ability for non‐proteolytic activation of prorenin, initiating the conversion of angiotensinogen to Ang I29. We have shown that the protein levels of s(P)RR, prorenin, activated prorenin and VEGF‐A, in combination with renin activity levels, were remarkably higher in the vitreous fluid samples of PDR eyes than in those of non‐diabetic control eyes23, 30. The vascular density of the fibrovascular tissue and the vitreous protein levels of prorenin, activated prorenin and VEGF‐A in PDR eyes were all correlated with elevated levels of s(P)RR generated through shedding of membrane‐bound (P)RR from neovascular endothelial cells of the fibrovascular tissue into the vitreous fluid23. Importantly, elevated renin activity levels showed positive correlations with the vitreous s(P)RR, prorenin, activated prorenin and VEGF‐A protein levels30. Our data suggest that the vitreous renin activity stems from s(P)RR‐mediated non‐proteolytic activation of prorenin, suggesting the important role of (P)RR in the pathogenesis of PDR. RAS components including (P)RR and prorenin were actually detected in human PDR fibrovascular tissues, normal ocular tissues and various human retinal cell lines, such as the retinal pigment epithelium (RPE)23, 31, 32, and the vitreous prorenin and Ang II levels were reported to increase in PDR eyes17, 18, 23, 33. Furthermore, a close relationship between the vitreous VEGF‐A protein and renin activity levels confirmed our concept of ‘vitreous RAS’ that is involved in the angiogenic activity of DR. Consequently, in concert with retinal RAPS caused by membrane‐type (i.e., full‐length) (P)RR (Figure 1a)30, vitreous RAS as a result of s(P)RR23 (Figure 1b) is presumed to control VEGF‐A expression in diabetic eyes.
Figure 1.
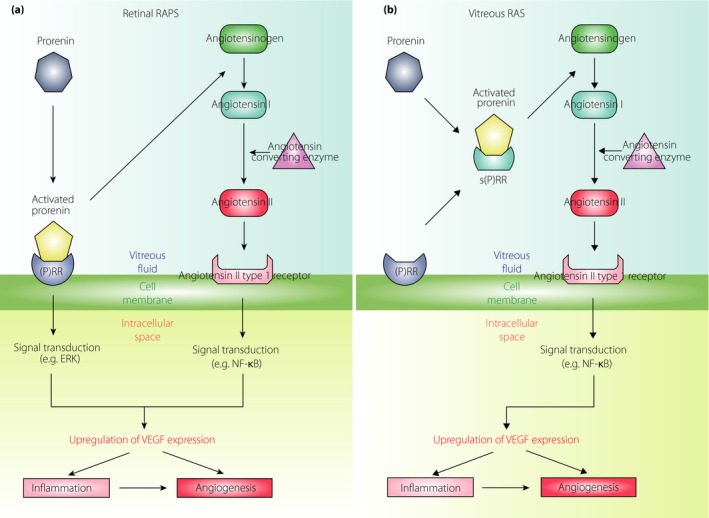
Molecular mechanisms in retinal (a) receptor‐associated prorenin system (RAPS) and (b) vitreous renin–angiotensin system (RAS) leading to vascular endothelial growth factor (VEGF)‐driven pathogenesis of diabetic retinopathy. Retinal RAPS is required for membrane‐type (pro)renin receptor ([P]RR), whereas vitreous RAS is caused by a soluble form of (P)RR (s[P]RR). Even when membrane‐bound (P)RR is truncated into its soluble form, s(P)RR, it continues to affect the pathogenesis driven by angiotensin II signaling. ERK, extracellular signal‐regulated kinase; NF‐κB, Nuclear factor‐κB. Reproduced from Kanda et al.30 with permission.
We have shown that (P)RR signaling through ERK1/223, 26 and AT1R signaling through nuclear factor (NF)‐κB19 play significant roles in upregulated VEGF‐A expression; however, it is challenging to define the ratio of involvement with the angiogenic activity in human PDR. The proprotein convertase, a disintegrin and metalloprotease domain 1934 and furin35, processes membrane‐bound (P)RR to s(P)RR, both of which were expressed in the neovascular endothelial cells of human PDR fibrovascular tissues23. Enzymatic activity and gene expression of these proteases in endothelial cells would likely determine the pathogenic balance between vitreous RAS and retinal RAPS. In the future, research into the physiological and pathological regulation of disintegrin and metalloprotease domain 19 and furin is necessary to reveal RAPS‐mediated molecular pathogenesis of DR.
Clinical Significance of Vitreous RAS
The importance of the pathogenic system ‘vitreous RAS’ might lead in part to a possibility of revising the recent surgical indication and concept of vitrectomy for DR. From a clinical point of view, vitrectomy is carried out in PDR eyes for the following reasons: (i) neovessel‐derived vitreous hemorrhage that interferes with the visual axis; and (ii) traction retinal detachment in which the retina is elevated by the vitreous that works as the scaffold of the contractile fibrovascular tissue arising from the retina. These two key classic indications to the advanced stage of PDR have long been applied in terms of a mechanical or physical cue. In contrast to this, our findings on vitreous renin activity imply the possibility that the vitreous works as the amplifier of the molecular pathogenesis of DR. Retinal surgeons often see surgical cases, in which diabetic macular edema, caused by VEGF‐A‐induced vascular hyperpermeability, is reduced soon after vitrectomy. This can be explicated at least in part by the concept of s(P)RR‐activated vitreous RAS, the activation of the downstream Ang II/AT1R/NF‐κB/VEGF‐A pathway causative for the pathogenesis of PDR (Figure 1b). Theoretically, the vitreous might not be just the pool of harmful cytokines, but the generator of pathogenic RAS components. Accordingly, the vitrectomy procedure has a biological sense, which might shift the recent surgical strategy to earlier intervention for broader indications to decrease the vitreous RAS‐mediated capability of generating VEGF‐A and various other pro‐inflammatory and angiogenic cytokines.
Soluble (Pro)renin Receptor in Plasma of Patients with Diabetic Retinopathy
Besides intraocular environments, s(P)RR levels were elevated in the serum or plasma of patients with several disorders, such as hypertension, obesity, preeclampsia, and kidney and heart failure36, 37, 38, 39, 40, 41, 42. In the plasma of patients with PDR as well, we showed elevated s(P)RR, renin and activated prorenin protein levels, so as to investigate systemic factors related to plasma levels of these RAS‐initiators in patients with PDR43. Of several systemic parameters examined, random blood sugar and serum creatinine levels in the PDR patients were significantly higher than those in non‐diabetic controls. Plasma s(P)RR levels showed significant correlations with the estimated glomerular filtration rate and serum creatinine, but not prorenin or activated prorenin. Protein levels of tumor necrosis factor (TNF)‐α, complement factor D, and leucine‐rich α‐2‐glycoprotein 1 were notably elevated in the plasma of PDR patients as compared with controls. Furthermore, positive correlations were observed between s(P)RR and those inflammation‐related molecules, but not prorenin. Excessive glucose during long‐standing hyperglycemia in diabetes causes the stimulation of various pathways including advanced glycation end‐products formation, the polyol pathway, protein kinase C and p38α mitogen‐activated protein kinase activations, and the superoxide pathway44. Stimulation of these pathways causes chronic inflammation in numerous tissues, with an increase of inflammation‐related molecules including CCL2/MCP‐1, intercellular adhesion molecule‐1 and TNF‐α, all of which were shown to be elevated in the plasma of diabetes patients44, 45, 46, 47. Complement factor D, a family of serine protease, plays a crucial role in the alternative pathway of complement activation, and mediates chronic inflammation that contributes to diabetic microvascular complications48, 49, 50. Leucine‐rich α‐2‐glycoprotein 1, a biological marker for several inflammatory disorders, such as asthma, rheumatoid arthritis and ulcerative colitis51, 52, 53, contributes to the formation of pathological neovessels in the retina and tumors54, 55. Of the inflammation‐related molecules correlated with s(P)RR in the PDR plasma, TNF‐α, but not complement factor D or leucine‐rich α‐2‐glycoprotein 1, stimulation to human retinal microvascular endothelial cells enhanced the gene expression of (P)RR, but not prorenin, whereas treatment with high glucose upregulated both the messenger ribonucleic acid (RNA) expression of prorenin and (P)RR43. Our results showed close links between plasma s(P)RR and diabetes‐induced factors, such as chronic inflammation, renal impairment and hyperglycemia in the PDR patients (Figure 2).
Figure 2.
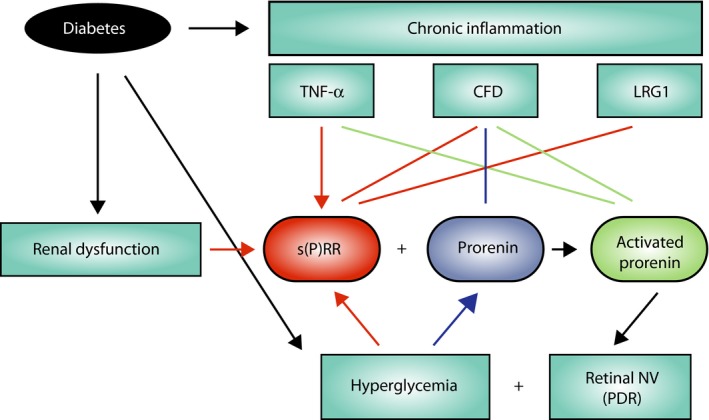
Association of plasma a soluble form of (pro)renin receptor (s[P]RR) with chronic inflammation, renal dysfunction and hyperglycemia in patients with proliferative diabetic retinopathy (PDR). A schema showing diabetes‐induced factors, such as chronic inflammation, renal dysfunction and hyperglycemia, as the potential regulators of plasma s(P)RR and prorenin levels, thus initiating the renin–angiotensin system activation to enhance retinal neovascularization (NV), the hallmark of PDR. Retinal NV, in turn, functions as a cellular source of these RAS initiators under hyperglycemia, generating the vicious cycle of the renin–angiotensin system and PDR. Arrows indicate cause–effect relationships, and lines represent correlations. CFD, complement factor D; LRG1, rich α‐2‐glycoprotein 1; PDH, pyruvate dehydrogenase; PDHB, pyruvate dehydrogenase E1 β subunit; TNF‐α, tumor necrosis factor‐α. Reproduced from Hase et al.43 with permission.
(Pro)renin Receptor in Glucose‐Induced Oxidative Stress
Recently, we showed an unsuspected function of (P)RR/ATP6AP2 in glucose metabolism31. In combination with immunoprecipitation using anti‐Atp6ap2 antibody and mass spectrometry analyses, we identified pyruvate dehydrogenase (PDH) complex as Atp6ap2‐binding proteins in the mature adult mouse retina. Additionally, yeast two‐hybrid assays showed direct molecular interaction between ATP6AP2 and the PDH E1 β subunit (PDHB). PDHB is one of the subunits composing PDH complex that changes pyruvate into acetyl‐CoA, connecting glycolysis to the tricarboxylic acid cycle and subsequent oxidative phosphorylation. Double labeling experiments showed co‐localization of Atp6ap2 with Pdhb in several retinal layers, such as the RPE layer. Depletion of ATP6AP2 decreased PDH activity, showing a predilection to anaerobic glycolysis in RPE cells. ATP6AP2 was suggested to prevent PDHB being phosphorylated, consequently regulating its protein stability. Decreased PDH activity owing to ATP6AP2 knockdown repressed glucose‐induced oxidative stress in RPE cells. These results revealed the novel biological function of ATP6AP2/(P)RR as a PDHB stabilizer associated with aerobic glucose metabolism and glucose‐induced reactive oxygen species production (Figure 3).
Figure 3.
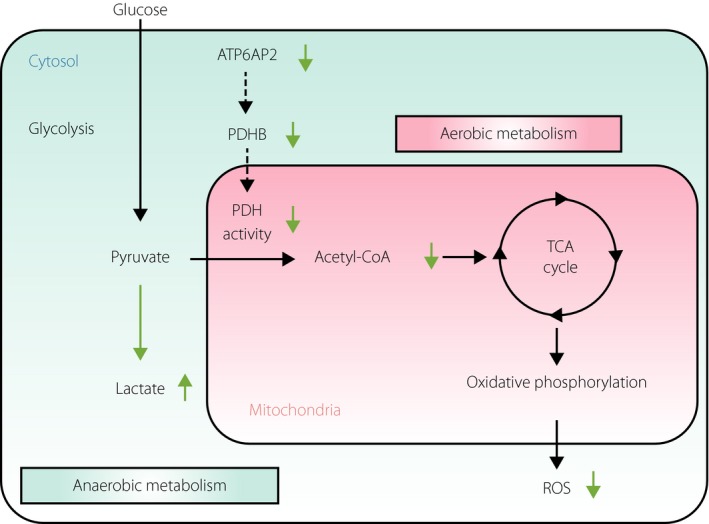
ATP6AP2/(pro)renin receptor involvement in pyruvate dehydrogenase (PDH)‐mediated aerobic glucose metabolism and oxidative stress. ATP6AP2/(pro)renin receptor blockade causes a metabolic shift from aerobic cellular respiration to anaerobic glycolysis (green arrows), leading consequently to a decrease in mitochondrial reactive oxygen species (ROS). Reproduced from Kanda et al.31 with permission. PDHB, pyruvate dehydrogenase E1 β subunit; TCA, tricarboxylic acid.
Acute and chronic surplus glucose causes devastating alterations in energy metabolism. Under the aerobic condition, elevation of pyruvate, a product of cytosolic glycolysis, facilitates the PDH‐mediated conversion of pyruvate to acetyl‐CoA followed by mitochondrial nicotinamide adenine dinucleotide‐dependent adenosine triphosphate (ATP) synthesis combined with leakage of excess superoxide, causing oxidative stress responsible for the pathogenesis of diabetes56, 57. Rationally, ATP6AP2 inhibition causes decreased PDH activity, leading to a metabolic shift from aerobic cellular respiration to anaerobic glycolysis together with reduction in mitochondrial oxidative stress. This molecular mechanism is also explained by earlier results showing that PDH was involved in the production of mitochondrial reactive oxygen species and that PDH activity suppression in turn abated oxidative stress58, 59, 60.
The respiratory and circulatory systems, together with efficient energy generation, have been acquired and modified to adapt properly to several environmental changes linked to oxygen concentration during evolution. Energy metabolism is especially active in the vertebrate retina, particularly photoreceptor and RPE cells obtaining ample oxygen supply from the well‐developed vasculature of the choroid, which has never been provided for the invertebrate retina with photoreceptors arrayed in front (i.e., back‐to‐front in comparison with the vertebrate retina). Intriguingly, the amino acid sequence of ATP6AP2 at the specific region binding to PDHB is highly conserved in mammals and fish, but not in Drosophila 61, showing that the highly conserved sequence of Atp6ap2 makes it possible to consume high oxygen in the retina by ensuring Pdh enzymatic activity from Pdhb protein degradation. Ironically, as the price for the ATP6AP2‐dependent efficient energy generation acquired in human eyes, glucose‐induced oxidative stress has emerged as the underlying pathogenesis of DR in the current era of excessive eating.
ATP6AP2/(P)RR plays a crucial role in RAPS activation, which is associated with the molecular pathogenesis of end‐organ damage, such as angiogenesis and inflammation seen in DR12, 23, 26, 30. In concert with our results on its contribution to oxidative stress, ATP6AP2/(P)RR is thought to be pathogenic, with its domain interacting with prorenin and PDHB alike, initiating the distinctly different pathways linked to the molecular pathogenesis of DR (i.e., the activation of retinal RAPS and the generation of mitochondrial reactive oxygen species, respectively). Our new findings on ATP6AP2/(P)RR‐PDHB interaction, were also reproduced in a more recent report on hepatic energy metabolism62.
(Pro)renin Receptor in Uveitis
In addition to DR, uveitis is also characterized by intraocular inflammation that causes vision loss and blindness after occlusive retinal vasculitis, serous retinal detachment, secondary epiretinal membrane, macular edema and angle‐closure glaucoma in some severe cases. To investigate prorenin and s(P)RR involvement in uveitis, we carried out enzyme‐linked immunosorbent assay experiments using vitreous aspirates from eyes of patients with uveitis and non‐uveitic control eyes with idiopathic epiretinal membrane and macular hole63. Notably, RAPS components including prorenin, s(P)RR and activated prorenin significantly increased in the vitreous fluid of uveitic eyes compared with controls. Furthermore, elevated prorenin and s(P)RR levels were significantly correlated with each other. The vitreous levels of cytokines, such as CCL2/MCP‐1, interleukin‐6, platelet‐derived growth factor‐BB and VEGF‐A, key molecules responsible for neovascularization and inflammation, are known to increase in the eyes of sarcoid uveitis patients64. To investigate the relationship between RAPS activation and ocular inflammation, we examined the protein levels of several cytokines in vitreous samples from our uveitic case series. As compared with control eyes, protein levels of CCL2/MCP‐1, interleukin‐6, platelet‐derived growth factor‐BB and VEGF‐A, but not TNF‐α, increased in uveitic eyes. Furthermore, elevated CCL2/MCP‐1 levels were significantly correlated with increased RAPS parameters, including (s)PRR, prorenin and activated prorenin. These results show that the close link between the RAPS components and CCL2/MCP‐1 levels would validate the pathogenic role of (P)RR that contributes to inflammation in human uveitis. Furthermore, we have shown that RAPS activation contributes to the molecular pathogenesis, including inflammation, angiogenesis and fibrosis, of other ocular disorders, such as conjunctival lymphoma65 and idiopathic epiretinal membrane66.
(Pro)renin Receptor in Retinal Development
(P)RR was initially identified as a cleaved form of (P)RR working as a subunit of vacuolar H+‐ATPase (v‐ATPase), an ATP‐dependent multi‐subunit proton pump, and termed ATP6 accessory protein 2 (Atp6ap2)21, 67. The v‐ATPase plays important roles in numerous physiological and fundamental cellular activities, such as endocytosis, processing of proteins, and the activation of lysosomal and autophagosomal enzymes68. Atp6ap2/(P)RR and v‐ATPase‐mediated acidification were recently reported to be important for both of the Wnt/β‐catenin and Wnt/planar cell polarity signaling pathways in Drosophila and Xenopus 69, 70, 71. Cardiomyocyte‐ or podocyte‐specific gene deletion in mice showed that Atp6ap2 is necessary for the maintenance of cell structure and survival72, 73, 74. Consequently, Atp6ap2/(P)RR is proposed to be associated not only with tissue RAS activation, but with various physiological processes through the v‐ATPase function.
Although our data using clinical samples showed that ATP6AP2/(P)RR contributes to angiogenic activity in human PDR eyes23, 30, there was no report showing functional analysis or the physiological role of ATP6AP2/(P)RR in the mammalian retina. Furthermore, to avoid unexpected adverse effects in clinical trials, it is necessary to understand the physiological function of a target molecule. Given that (P)RR is one of embryonic‐essential genes75, as well as VEGF‐A76, we generated photoreceptor (i.e., rod and cone)‐specific conditional knock‐out mice using the Cre‐LoxP system, so as to show the essential role of Atp6ap2/(P)RR in photoreceptor development32. The absence of photoreceptor Atp6ap2/(P)RR did not cause significant changes in retinal cell differentiation, but in disorganization of laminar formation in the outer nuclear layer combined with critically impaired photoreceptor cell alignment. Cell polarity and adhesion proteins co‐localized with Atp6ap2/(P)RR at the apical edge of the normally developing retina; however, these molecules were markedly interspersed and retinal progenitor cells mis‐localized from the apical surface in Atp6ap2/(P)RR‐conditional knock‐out mice. Of cell polarity and adhesion proteins, we identified that Atp6ap2/(P)RR bound to partitioning defective 3 homolog (PAR3) protein with co‐immunoprecipitation using mouse retinal homogenates and ATP6AP2/(P)RR‐transfected human embryonic kidney 293T cells. The Par family proteins contribute to forming cell polarity, controlling cellular asymmetry through distribution and formation of molecular complexes77. Par3 makes a complex with atypical protein kinase C (aPKC)λ and Par6 (i.e., the Par–atypical protein kinase C system), specifically located on cellular junctions, and is involved in the cell polarity regulation in various cells78. Furthermore, direct molecular binding between ATP6AP2/(P)RR and PAR3 was observed in yeast two‐hybrid assays. Our findings showed the new physiological function of Atp6ap2/(P)RR during retinal development involved in laminar formation (Figure 4).
Figure 4.
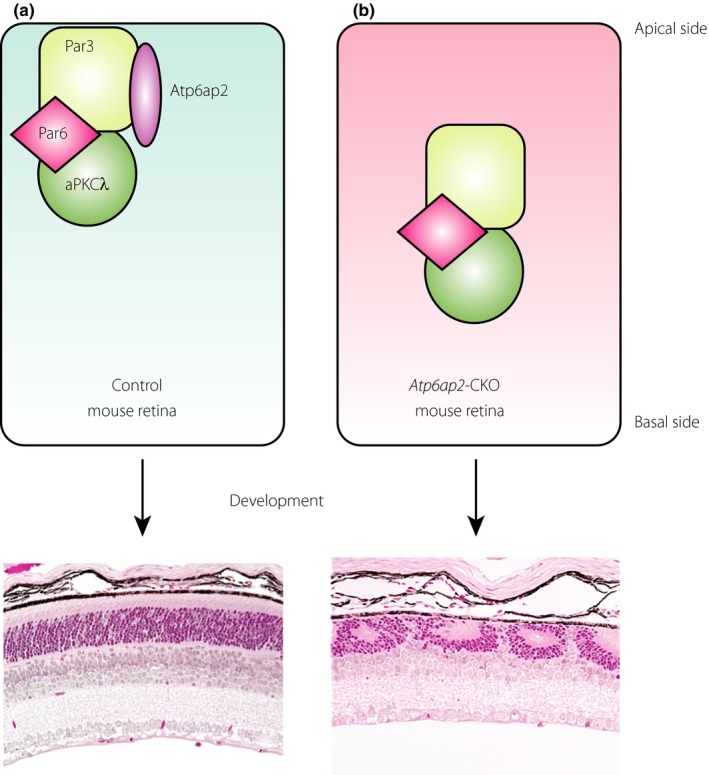
Impaired retinal development in photoreceptor‐specific Atp6ap2‐deficient mouse. (a) Normal and (b) photoreceptor‐specific Atp6ap2 deficient mouse retina. Atp6ap2/(pro)renin receptor binds to partitioning defective 3 homolog (Par3) as a cell polarity determinant required for retinal laminar organization during physiological development. Modified from Kanda et al.32 with permission. aPKCλ, atypical protein kinase Cλ.
We suggest that this novel cellular activity of ATP6AP2/(P)RR contributing to the Par–atypical protein kinase C system, besides the v‐ATPase function, the activation of tissue RAS and the stabilization of PDHB protein, is the fourth biological role of Atp6ap2/(P)RR in retinal laminar formation (Figure 5). However, this physiological role of (P)RR during development would not be a hurdle for our inhibition of (P)RR for the purpose of treating DR and other inflammatory diseases, such as uveitis, because the pathological role of (P)RR as the RAPS activator is basically seen in the adult stage.
Figure 5.
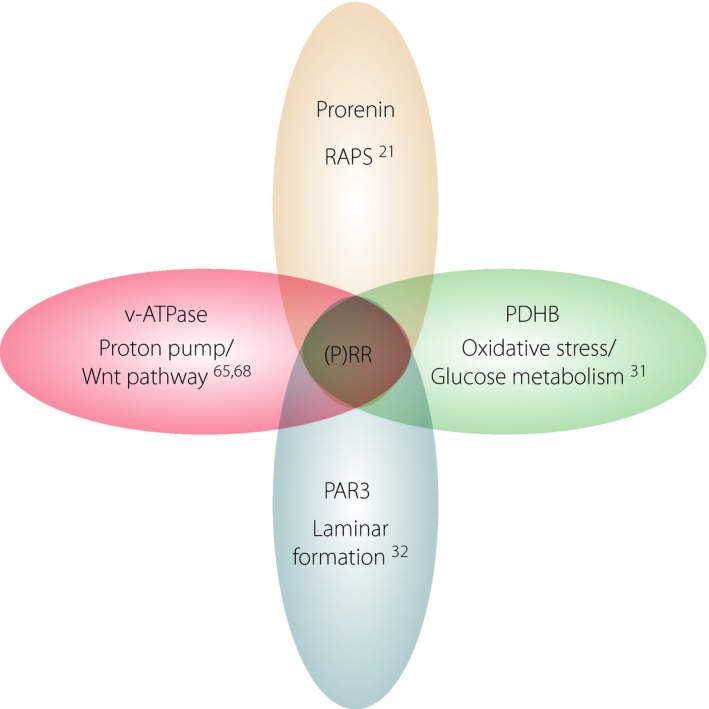
Binding partners and biological functions of (pro)renin receptor ([P]RR)/ATP6AP2. (P)RR/ATP6AP2 interacts with various molecules to exert distinctly different functions. PAR3, partitioning defective 3 homolog; PDHB, pyruvate dehydrogenase E1 β subunit; RAPS, receptor‐associated prorenin system; v‐ATPase, vacuolar‐type H+‐adenosine triphosphatase.
Development of a Novel Single‐Strand RNA Interference Therapeutic Agent Targeting (Pro)renin Receptor
Based on our findings23, 25, 26, 27, 28, 30, 43, 65, 66, a blockade of (P)RR is theorized to inhibit the cascade of events crucial in various vascular abnormalities represented by inflammation and angiogenesis. Aliskiren, a direct renin inhibitor, competitively inhibited the renin enzymatic activity of both renin and activated prorenin through interaction with (P)RR in vitro; however, RAS inhibitors including aliskiren have no efficacy of blocking (P)RR's own downstream signals29. (P)RR blocker, also known as handle‐region peptide, is a peptide with the structure of the handle region of the prorenin prosegment working as a decoy for (P)RR. At present, (P)RR blocker is the only available agent to inhibit prorenin‐(P)RR interaction (i.e., acquisition of renin enzymatic activity and transduction of receptor signaling) subsequently leading to RAPS activation79. We and others have shown that (P)RR blocker potently suppressed the pathogenesis of various disease models in target organs (e.g., DR, diabetic nephropathy, neovascular age‐related macular degeneration, uveitis and retinopathy of prematurity)22, 23, 25, 26, 27. However, a blockade of ligand‐receptor protein interaction using decoy peptides has several limitations: (i) requirement of an excessive amount of peptides; (ii) induction of immune response and autoantibodies; (iii) protease resistance; and (iv) high molecular weight. These inherent and serous issues with decoy peptides are likely to preclude future clinical application.
To block the pathological function of (P)RR, we designed a new class of RNA interference (RNAi) agent, proline‐modified short hairpin RNA (PshRNA), to knockdown human and mouse (P)RR mRNA (Figure 6). RNAi is basically considered to have minimal cellular toxicity due to its endogenous cellular function for controlling gene expression, and thus contains attractive and promising aspects for the development of new therapies. With regard to clinical application, however, there are some obstacles that need to be overcome. In general, canonical double‐strand small interfering RNAs (siRNAs) are recognized by family members of the toll‐like receptor (TLR) and retinoic acid‐inducible gene‐I‐like receptor, causing the activation of intracellular signaling pathways to initiate innate immunity80. Recently, a single‐strand RNAi has proved to be capable of sequence‐specific gene silencing through the RNAi system without off‐target expression of inflammatory cytokines via TLR‐mediated signal transduction in rodent eyes81. Further, although an annealing process is required for the generation of a double‐strand siRNA, it is not necessary for a single‐strand RNAi because the linker is replaced with proline derivatives and can therefore self‐anneal. Previous studies showed that PshRNAs were more stable against nucleases than canonical double‐strand siRNAs82. Taken together, the single‐strand RNAi strategy appears to overcome some of the drawbacks faced by canonical double‐strand siRNAs.
Figure 6.
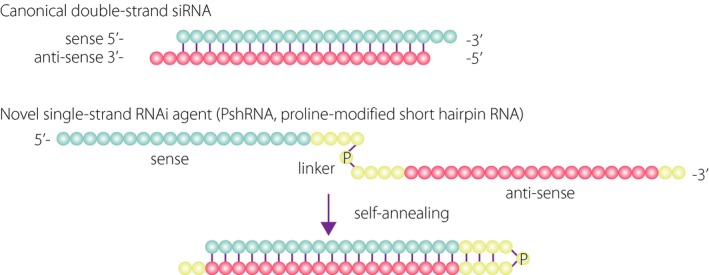
Structure of proline‐modified short hairpin ribonucleic acid (PshRNA). Structure of canonical double‐strand small interfering RNA (siRNA) and novel single‐strand RNA interference (PshRNA) agents. Blue circles indicate the sense strand of a target gene, red circles are the antisense strand and yellow circles are the linker region. P indicates a proline derivative. RNAi, ribonucleic acid interference.
To design PshRNA against (P)RR ([P]RR‐PshRNA), we first carried out in silico analysis regarding various parameters, such as length, structure, sequence, and chemical and nucleotide compositions, all of which mediate efficient RNAi, and generated several candidate RNAi agents targeting a different nucleotide sequence of (P)RR gene common to both species. We then tested their knockdown efficiency in preliminary experiments using human and mouse cell lines, so as to select one candidate as (P)RR‐PshRNA in terms of both potency and persistency of (P)RR knockdown. Real‐time reverse transcription polymerase chain reaction and immunoblot analyses showed that the levels of (P)RR/ATP6AP2 transcript and product significantly decreased after exposure to human RPE and mouse endothelial cells with (P)RR‐PshRNA as well as (P)RR‐siRNA in a dose‐dependent manner. To determine tissue distribution of (P)RR‐PshRNA injected into the vitreous cavity of murine eyes, we used tetramethylrhodamine‐labeled (P)RR‐PshRNA. The labeled (P)RR‐PshRNA signals were deeply penetrated and widely distributed to the ganglion cell layer, inner and outer nuclear layers, and RPE in the posterior segment of the eye, and to the corneal epithelium and stroma in the anterior segment of the eye. Importantly, the newly designed (P)RR‐PshRNA showed more robust nuclease resistance than the conventional double‐strand (P)RR‐siRNA, and did not affect retinal function and structure.
The endotoxin‐induced uveitis model is frequently used as an acute inflammation model in various organs including the eye, and streptozotocin‐induced diabetes is a type 1 diabetes model due to impaired insulin secretion from pancreatic β‐cells injured by streptozotocin toxicity. Given that DR has recently been regarded as an inflammatory disorder, we used these models to examine the effect of (P)RR‐PshRNA on acute and chronic inflammation. Previously, we reported the significant suppression of intraocular inflammation in this model by blocking AT1R and (P)RR to inhibit tissue RAS and RAPS, respectively19, 26. (P)RR‐PshRNA application to mice caused significant amelioration of acute (uveitic) and chronic (diabetic) models of ocular inflammation; that is, the total number of retinal adherent leukocytes, and upregulated gene expression levels of Il‐6, Ccl2/Mcp‐1, Icam‐1, Tnf‐a and (P)RR/Atp6ap2, as seen in endotoxin‐induced uveitis and streptozotocin‐induced diabetes mice treated with phosphate‐buffered saline or control‐PshRNA, were significantly suppressed with administration of (P)RR‐PshRNA63. As we described above, using conditional knockout mice, we have revealed that (P)RR/Atp6ap2 contributes to physiologically essential cellular functions that are independent of RAPS; however, we did not observe any adverse events after application with (P)RR‐PshRNA in vivo and in vitro. It is possible that the in vivo knockdown at the dose is too weak to cause any side‐effects, despite its significant efficacy in suppressing ocular inflammation. Importantly, the currently designed sequence for RNAi targeting (P)RR is common to human and mouse genes, indicating that the multimodal animal testing with (P)RR‐PshRNA would also serve as a useful reference for human clinical trials.
Conclusion
Our results might lead to a novel understanding of the molecular mechanism in pathological events including glucose‐induced oxidative stress, vascular inflammation and retinal angiogenesis, all of which are regulated by (P)RR. VEGF, the key modulator of DR, is dually governed by retinal RAPS and vitreous RAS, either of which is triggered by (P)RR and s(P)RR, respectively. Our ongoing development of (P)RR‐PshRNA, an innovative single‐strand RNAi agent targeting (P)RR, will soon promote clinical research on several eye diseases, especially DR, thus aiming at further improvement of visual prognosis in DR patients.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was supported in part by the Bayer Japan Retina Award (to AK), the Institute of Science of Blood Pressure and Hormone (to AK), the Takeda Science Foundation (to AK), the New Energy and Industrial Technology Development Organization (NEDO), and MEXT KAKENHI Grant Number 16H05484 (to SI) and 16K11279 (to AK).
J Diabetes Investig 2019; 10: 6–17
References
- 1. Ishida S, Usui T, Yamashiro K, et al VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci 2003; 44: 2155–2162. [DOI] [PubMed] [Google Scholar]
- 2. Adamis AP, Miller JW, Bernal MT, et al Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994; 118: 445–450. [DOI] [PubMed] [Google Scholar]
- 3. Aiello LP, Avery RL, Arrigg PG, et al Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994; 331: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 4. Malecaze F, Clamens S, Simorre‐Pinatel V, et al Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor‐like activity in proliferative diabetic retinopathy. Arch Ophthalmol 1994; 112: 1476–1482. [DOI] [PubMed] [Google Scholar]
- 5. Hurwitz H, Fehrenbacher L, Novotny W, et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–2342. [DOI] [PubMed] [Google Scholar]
- 6. Ishida S, Usui T, Yamashiro K, et al VEGF164‐mediated inflammation is required for pathological, but not physiological, ischemia‐induced retinal neovascularization. J Exp Med 2003; 198: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Usui T, Ishida S, Yamashiro K, et al VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest Ophthalmol Vis Sci 2004; 45: 368–374. [DOI] [PubMed] [Google Scholar]
- 8. Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin‐angiotensin systems. Physiol Rev 2006; 86: 747–803. [DOI] [PubMed] [Google Scholar]
- 9. Ager EI, Neo J, Christophi C. The renin‐angiotensin system and malignancy. Carcinogenesis 2008; 29: 1675–1684. [DOI] [PubMed] [Google Scholar]
- 10. Ramalho FS, Ramalho LN, Castro‐e‐Silva Junior O, et al Effect of angiotensin‐converting enzyme inhibitors on liver regeneration in rats. Hepatogastroenterology 2002; 49: 1347–1351. [PubMed] [Google Scholar]
- 11. Yayama K, Miyagi R, Sugiyama K, et al Angiotensin II regulates liver regeneration via type 1 receptor following partial hepatectomy in mice. Biol Pharm Bull 2008; 31: 1356–1361. [DOI] [PubMed] [Google Scholar]
- 12. Satofuka S, Kanda A, Ishida S. Receptor‐associated prorenin system in the pathogenesis of retinal diseases. Front Biosci 2012; 4: 1449–1460. [DOI] [PubMed] [Google Scholar]
- 13. Chaturvedi N, Porta M, Klein R, et al Effect of candesartan on prevention (DIRECT‐Prevent 1) and progression (DIRECT‐Protect 1) of retinopathy in type 1 diabetes: randomised, placebo‐controlled trials. Lancet 2008; 372: 1394–1402. [DOI] [PubMed] [Google Scholar]
- 14. Chaturvedi N, Sjolie AK, Stephenson JM, et al Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin‐Dependent Diabetes Mellitus. Lancet 1998; 351: 28–31. [DOI] [PubMed] [Google Scholar]
- 15. Mauer M, Zinman B, Gardiner R, et al Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009; 361: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sjolie AK, Klein R, Porta M, et al Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT‐Protect 2): a randomised placebo‐controlled trial. Lancet 2008; 372: 1385–1393. [DOI] [PubMed] [Google Scholar]
- 17. Funatsu H, Yamashita H, Nakanishi Y, et al Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Br J Ophthalmol 2002; 86: 311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao BB, Chen X, Timothy N, et al Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J Proteome Res 2008; 7: 2516–2525. [DOI] [PubMed] [Google Scholar]
- 19. Nagai N, Izumi‐Nagai K, Oike Y, et al Suppression of diabetes‐induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor‐kappaB pathway. Invest Ophthalmol Vis Sci 2007; 48: 4342–4350. [DOI] [PubMed] [Google Scholar]
- 20. Nagai N, Noda K, Urano T, et al Selective suppression of pathologic, but not physiologic, retinal neovascularization by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci 2005; 46: 1078–1084. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen G, Delarue F, Burckle C, et al Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 2002; 109: 1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ichihara A, Hayashi M, Kaneshiro Y, et al Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest 2004; 114: 1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanda A, Noda K, Saito W, et al (Pro)renin receptor is associated with angiogenic activity in proliferative diabetic retinopathy. Diabetologia 2012; 55: 3104–3113. [DOI] [PubMed] [Google Scholar]
- 24. Nabi AH, Kageshima A, Uddin MN, et al Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med 2006; 18: 483–488. [PubMed] [Google Scholar]
- 25. Satofuka S, Ichihara A, Nagai N, et al (Pro)renin receptor promotes choroidal neovascularization by activating its signal transduction and tissue renin‐angiotensin system. Am J Pathol 2008; 173: 1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Satofuka S, Ichihara A, Nagai N, et al (Pro)renin receptor‐mediated signal transduction and tissue renin‐angiotensin system contribute to diabetes‐induced retinal inflammation. Diabetes 2009; 58: 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Satofuka S, Ichihara A, Nagai N, et al Suppression of ocular inflammation in endotoxin‐induced uveitis by inhibiting nonproteolytic activation of prorenin. Invest Ophthalmol Vis Sci 2006; 47: 2686–2692. [DOI] [PubMed] [Google Scholar]
- 28. Satofuka S, Ichihara A, Nagai N, et al Role of nonproteolytically activated prorenin in pathologic, but not physiologic, retinal neovascularization. Invest Ophthalmol Vis Sci 2007; 48: 422–429. [DOI] [PubMed] [Google Scholar]
- 29. Biswas KB, Nabi AN, Arai Y, et al Qualitative and quantitative analyses of (pro)renin receptor in the medium of cultured human umbilical vein endothelial cells. Hypertens Res 2010; 34: 735–739. [DOI] [PubMed] [Google Scholar]
- 30. Kanda A, Noda K, Saito W, et al Vitreous renin activity correlates with vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol 2013; 97: 666–668. [DOI] [PubMed] [Google Scholar]
- 31. Kanda A, Noda K, Ishida S. ATP6AP2/(pro)renin receptor contributes to glucose metabolism via stabilizing the pyruvate dehydrogenase E1 beta subunit. J Biol Chem 2015; 290: 9690–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kanda A, Noda K, Yuki K, et al Atp6ap2/(pro)renin receptor interacts with Par3 as a cell polarity determinant required for laminar formation during retinal development in mice. J Neurosci 2013; 33: 19341–19351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Danser AH, van den Dorpel MA, Deinum J, et al Renin, prorenin, and immunoreactive renin in vitreous fluid from eyes with and without diabetic retinopathy. J Clin Endocrinol Metab 1989; 68: 160–167. [DOI] [PubMed] [Google Scholar]
- 34. Yoshikawa A, Aizaki Y, Kusano K, et al The (pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertens Res 2011; 34: 599–605. [DOI] [PubMed] [Google Scholar]
- 35. Cousin C, Bracquart D, Contrepas A, et al Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 2009; 53: 1077–1082. [DOI] [PubMed] [Google Scholar]
- 36. Amari Y, Morimoto S, Nakajima F, et al Serum soluble (pro)renin receptor levels in maintenance hemodialysis patients. PLoS One 2016; 11: e0158068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fukushima A, Kinugawa S, Homma T, et al Increased plasma soluble (pro)renin receptor levels are correlated with renal dysfunction in patients with heart failure. Int J Cardiol 2013; 168: 4313–4314. [DOI] [PubMed] [Google Scholar]
- 38. Hamada K, Taniguchi Y, Shimamura Y, et al Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol 2013; 17: 848–856. [DOI] [PubMed] [Google Scholar]
- 39. Morimoto S, Ando T, Niiyama M, et al Serum soluble (pro)renin receptor levels in patients with essential hypertension. Hypertens Res 2014; 37: 642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan P, Shamansurova Z, Bisotto S, et al Impact of the prorenin/renin receptor on the development of obesity and associated cardiometabolic risk factors. Obesity (Silver Spring) 2014; 22: 2201–2209. [DOI] [PubMed] [Google Scholar]
- 41. Thomason J, Reyes M, Allen SR, et al Elevation of (pro)renin and (pro)renin receptor in preeclampsia. Am J Hypertens 2015; 28: 1277–1284. [DOI] [PubMed] [Google Scholar]
- 42. Watanabe N, Bokuda K, Fujiwara T, et al Soluble (pro)renin receptor and blood pressure during pregnancy: a prospective cohort study. Hypertension 2012; 60: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 43. Hase K, Kanda A, Hirose I, et al Systemic factors related to soluble (pro)renin receptor in plasma of patients with proliferative diabetic retinopathy. PLoS One 2017; 12: e0189696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Graves DT, Kayal RA. Diabetic complications and dysregulated innate immunity. Front Biosci 2008; 13: 1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doganay S, Evereklioglu C, Er H, et al Comparison of serum NO, TNF‐alpha, IL‐1beta, sIL‐2R, IL‐6 and IL‐8 levels with grades of retinopathy in patients with diabetes mellitus. Eye (Lond) 2002; 16: 163–170. [DOI] [PubMed] [Google Scholar]
- 46. Guha M, Bai W, Nadler JL, et al Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia‐induced oxidant stress‐dependent and ‐independent pathways. J Biol Chem 2000; 275: 17728–17739. [DOI] [PubMed] [Google Scholar]
- 47. Shanmugam N, Reddy MA, Guha M, et al High glucose‐induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 2003; 52: 1256–1264. [DOI] [PubMed] [Google Scholar]
- 48. Flyvbjerg A. Diabetic angiopathy, the complement system and the tumor necrosis factor superfamily. Nat Rev Endocrinol 2010; 6: 94–101. [DOI] [PubMed] [Google Scholar]
- 49. Fujita T, Hemmi S, Kajiwara M, et al Complement‐mediated chronic inflammation is associated with diabetic microvascular complication. Diabetes Metab Res Rev 2013; 29: 220–226. [DOI] [PubMed] [Google Scholar]
- 50. Ostergaard J, Hansen TK, Thiel S, et al Complement activation and diabetic vascular complications. Clin Chim Acta 2005; 361: 10–19. [DOI] [PubMed] [Google Scholar]
- 51. Fujimoto M, Serada S, Suzuki K, et al Leucine‐rich alpha2 ‐glycoprotein as a potential biomarker for joint inflammation during anti‐interleukin‐6 biologic therapy in rheumatoid arthritis. Arthritis Rheumatol 2015; 67: 2056–2060. [DOI] [PubMed] [Google Scholar]
- 52. Honda H, Fujimoto M, Miyamoto S, et al Sputum leucine‐rich alpha‐2 glycoprotein as a marker of airway inflammation in asthma. PLoS One 2016; 11: e0162672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shinzaki S, Matsuoka K, Iijima H, et al Leucine‐rich alpha‐2 glycoprotein is a serum biomarker of mucosal healing in ulcerative colitis. J Crohns Colitis 2017; 11: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang X, Abraham S, McKenzie JA, et al LRG1 promotes angiogenesis by modulating endothelial TGF‐beta signalling. Nature 2013; 499: 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang J, Zhu L, Fang J, et al LRG1 modulates epithelial‐mesenchymal transition and angiogenesis in colorectal cancer via HIF‐1alpha activation. J Exp Clin Cancer Res 2016; 35: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Du X, Matsumura T, Edelstein D, et al Inhibition of GAPDH activity by poly(ADP‐ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 2003; 112: 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rahimi R, Nikfar S, Larijani B, et al A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 2005; 59: 365–373. [DOI] [PubMed] [Google Scholar]
- 58. Kumar V, Kota V, Shivaji S. Hamster sperm capacitation: role of pyruvate dehydrogenase A and dihydrolipoamide dehydrogenase. Biol Reprod 2008; 79: 190–199. [DOI] [PubMed] [Google Scholar]
- 59. Starkov AA, Fiskum G, Chinopoulos C, et al Mitochondrial alpha‐ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci 2004; 24: 7779–7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tretter L, Adam‐Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha‐ketoglutarate dehydrogenase. J Neurosci 2004; 24: 7771–7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Burckle C, Bader M. Prorenin and its ancient receptor. Hypertension 2006; 48: 549–551. [DOI] [PubMed] [Google Scholar]
- 62. Ren L, Sun Y, Lu H, et al (Pro)renin receptor inhibition reprograms hepatic lipid metabolism and protects mice from diet‐induced obesity and hepatosteatosis. Circ Res 2018; 122: 730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kanda A, Ishizuka ET, Shibata A, et al A novel single‐strand RNAi therapeutic agent targeting the (pro)renin receptor suppresses ocular inflammation. Mol Ther Nucleic Acids 2017; 7: 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nagata K, Maruyama K, Uno K, et al Simultaneous analysis of multiple cytokines in the vitreous of patients with sarcoid uveitis. Invest Ophthalmol Vis Sci 2012; 53: 3827–3833. [DOI] [PubMed] [Google Scholar]
- 65. Ishizuka ET, Kanda A, Kase S, et al Involvement of the receptor‐associated prorenin system in the pathogenesis of human conjunctival lymphoma. Invest Ophthalmol Vis Sci 2015; 56: 74–80. [DOI] [PubMed] [Google Scholar]
- 66. Dong Y, Kanda A, Noda K, et al Pathologic roles of receptor‐associated prorenin system in idiopathic epiretinal membrane. Sci Rep 2017; 7: 44266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ludwig J, Kerscher S, Brandt U, et al Identification and characterization of a novel 9.2‐kDa membrane sector‐associated protein of vacuolar proton‐ATPase from chromaffin granules. J Biol Chem 1998; 273: 10939–10947. [DOI] [PubMed] [Google Scholar]
- 68. Nishi T, Forgac M. The vacuolar (H+)‐ATPases – nature's most versatile proton pumps. Nat Rev Mol Cell Biol 2002; 3: 94–103. [DOI] [PubMed] [Google Scholar]
- 69. Buechling T, Bartscherer K, Ohkawara B, et al Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr Biol 2010; 20: 1263–1268. [DOI] [PubMed] [Google Scholar]
- 70. Cruciat CM, Ohkawara B, Acebron SP, et al Requirement of prorenin receptor and vacuolar H+‐ATPase‐mediated acidification for Wnt signaling. Science 2010; 327: 459–463. [DOI] [PubMed] [Google Scholar]
- 71. Hermle T, Saltukoglu D, Grunewald J, et al Regulation of Frizzled‐dependent planar polarity signaling by a V‐ATPase subunit. Curr Biol 2010; 20: 1269–1276. [DOI] [PubMed] [Google Scholar]
- 72. Kinouchi K, Ichihara A, Sano M, et al The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+‐ATPase assembly in murine cardiomyocytes. Circ Res 2010; 107: 30–34. [DOI] [PubMed] [Google Scholar]
- 73. Oshima Y, Kinouchi K, Ichihara A, et al Prorenin receptor is essential for normal podocyte structure and function. J Am Soc Nephrol 2011; 22: 2203–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Riediger F, Quack I, Qadri F, et al Prorenin receptor is essential for podocyte autophagy and survival. J Am Soc Nephrol 2011; 22: 2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Amsterdam A, Nissen RM, Sun Z, et al Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci USA 2004; 101: 12792–12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Carmeliet P, Ferreira V, Breier G, et al Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996; 380: 435–439. [DOI] [PubMed] [Google Scholar]
- 77. Guo S, Kemphues KJ. Molecular genetics of asymmetric cleavage in the early Caenorhabditis elegans embryo. Curr Opin Genet Dev 1996; 6: 408–415. [DOI] [PubMed] [Google Scholar]
- 78. Suzuki A, Ohno S. The PAR‐aPKC system: lessons in polarity. J Cell Sci 2006; 119: 979–987. [DOI] [PubMed] [Google Scholar]
- 79. Suzuki F, Hayakawa M, Nakagawa T, et al Human prorenin has “gate and handle” regions for its non‐proteolytic activation. J Biol Chem 2003; 278: 22217–22222. [DOI] [PubMed] [Google Scholar]
- 80. Kleinman ME, Yamada K, Takeda A, et al Sequence‐ and target‐independent angiogenesis suppression by siRNA via TLR3. Nature 2008; 452: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Takahashi H, Ichihara A, Kaneshiro Y, et al Regression of nephropathy developed in diabetes by (Pro)renin receptor blockade. J Am Soc Nephrol 2007; 18: 2054–2061. [DOI] [PubMed] [Google Scholar]
- 82. Hamasaki T, Suzuki H, Shirohzu H, et al Efficacy of a novel class of RNA interference therapeutic agents. PLoS One 2012; 7: e42655. [DOI] [PMC free article] [PubMed] [Google Scholar]


