Abstract
Currently, the clinical dynamics of glucagon need to be revised based on previous data obtained from conventional glucagon radioimmunoassays. In the present study, we evaluated plasma glucagon levels in type 1 diabetes patients using a newly‐developed sandwich enzyme‐linked immunosorbent assay, and its association with clinical parameters and markers of diabetes complications were statistically assessed. The plasma glucagon level in 77 Japanese type 1 diabetes patients was 28.1 ± 17.7 pg/mL, and comparable with that reported previously for type 2 diabetes patients. However, the values were widely spread and did not correlate with plasma glucose values. Additionally, the average glucagon levels in patients in a hypoglycemic state (glucose level <80 mg/dL) did not increase (21.7 ± 12.2 pg/mL). The average glucagon level of patients experiencing hypoglycemia unawareness was significantly lower. Plasma glucagon levels evaluated using the new enzyme‐linked immunosorbent assay were dysregulated in type 1 diabetes patients in respect to plasma glucose levels, suggesting dysregulation of secretion.
Keywords: Glucagon, Hypoglycemia, Type 1 diabetes
Introduction
The pathophysiological significance of inadequate glucagon secretion is widely appreciated in the clinical status of diabetes, in both hyperglycemia and hypoglycemia1, 2. However, the assessment of glucagon dynamics currently needs to be revised due to the inaccuracy of the previously used conventional radioimmunoassay for glucagon3. Recently, a dual‐antibody sandwich enzyme‐linked immunosorbent assay (ELISA) resolving previous issues of conventional radioimmunoassays has been developed4. Indeed, the higher accuracy of this ELISA method compared with conventional radioimmunoassay was confirmed by a comparison with the direct assessment of glucagon using liquid chromatography‐high resolution mass spectrometry with parallel reaction monitoring5. In that study, plasma glucagon levels in type 1 diabetes patients were re‐evaluated using the new ELISA to investigate the effect of glucagon on the pathophysiology of type 1 diabetes.
Methods
A total of 77 Japanese patients with type 1 diabetes were recruited and enrolled in the present study after having provided written informed consent. The study protocol was approved by the local ethics committee of Osaka University Hospital (no. 12372), and was carried out in accordance with the tenets of the Declaration of Helsinki. Details of the method are also presented in the Data S1. The clinical assessments included a medical history interview, physical examination, blood sampling and evaluation of micro‐/macrovascular complications, as previously reported6, 7, 8. Venous blood samples were taken at the time of the visit without any restrictions on food intake, insulin treatment or daily activities, and laboratory analyses were carried out at SRL Inc. (Tokyo, Japan). Plasma glucagon concentration was measured using the specific dual‐antibody sandwich ELISA (Mercodia AB, Uppsala, Sweden/Cosmic Corporation Co., Ltd., Tokyo, Japan), according to the manufacturers’ instructions. Statistical analyses were carried out using JMP Pro version 13.2.0 (SAS Institute, Cary, NC, USA). Data are expressed as mean ± standard deviation.
Results
The analyzed clinical characteristics of the participants are presented in Table1, indicating a group comprising young adults with relatively well‐controlled diabetes, but with exhausted endogenous insulin production. The plasma glucagon level was 28.1 ± 17.7 pg/mL, which was comparable with that obtained in a previous report of Japanese patients with type 2 diabetes9. However, the values were widely spread (5.7–95.2 pg/mL), and did not correlate with plasma glucose levels (33–401 mg/dL; Figure 1a). The glucagon levels did not show significant differences between patients with normoglycemia and those with slight hyperglycemia (Figure 1b). In addition, the mean glucagon level was not increased in a hypoglycemic state, and not suppressed in an apparent hyperglycemic state (Figure 1b). The average glucagon levels of patients experiencing hypoglycemia unawareness (lack of autonomic symptoms when plasma glucose level is <70 mg/dL) were significantly lower than for the other patients (Figure 1c). Glucagon levels did not show a significant correlation with various clinical parameters (Table1; Figures S1–S4) in the univariate analyses, except for the serum blood urea nitrogen (BUN) concentration. Glucagon levels correlated significantly with the serum BUN level (Figure 1d), but did not correlate with creatinine levels and estimated glomerular filtration rate (Figure S1), suggesting a link between glucagon function and amino acid metabolism10. In contrast, the serum BUN concentration did not correlate with plasma glucose levels.
Table 1.
Clinical characteristics of the study group
| Mean ± SD | Mean ± SD | ||
|---|---|---|---|
| Age (years) | 33.9 ± 6.9 | AST (U/L) | 19.3 ± 5.6 |
| Female n (%) | 54 (70.1) | ALT (U/L) | 15.4 ± 9.0 |
| Duration of diabetes (years) | 25.2 ± 7.6 | GGT (U/L) | 17.9 ± 11.2 |
| Hypoglycemia unawareness (%) | 21 (27.3) | ||
| History of smoking, n (%) | 16 (20.8) | BUN (mg/dL) | 13.1 ± 3.0 |
| BMI (kg/m2) | 23.7 ± 3.5 | UA (mg/dL) | 4.1 ± 1.2 |
| Waist circumference | 79.1 ± 10.9 | Cr (mg/dL) | 0.65 ± 0.11 |
| Systolic BP (mmHg) | 120 ± 17 | eGFR (mL/min/1.73 m2) | 94.3 ± 15.6 |
| Diastolic BP (mmHg) | 71 ± 11 | ||
| HbA1c (%) | 7.4 ± 1.0 | LDL‐C (mg/dL) | 106.3 ± 25.7 |
| GA (%) | 22.4 ± 4.1 | HDL‐C (mg/dL) | 70.6 ± 17.0 |
| Daily total insulin dose (U/day) | 44.1 ± 20.0 | TG (mg/dL) | 99.9 ± 101.8 |
| Insulin dose (U/kg bodyweight) | 0.69 ± 0.23 | TC (mg/dL) | 192.9 ± 29.2 |
| Urine CPR (μg/g・Cr) | 4.16 ± 3.32 | ||
| Proliferative retinopathy (%) | 7 (9) | max‐IMT (mm) | 0.77 ± 0.12 |
| CVR‐R (%) | 4.24 ± 2.31 | ABI | 1.03 ± 0.10 |
| PWV (cm/s) | 1,325 ± 207 | ||
| Sampling time after meal (h) | 2.6 ± 1.9 | hs‐CRP (mg/dL) | 830 ± 1,443 |
Total n = 77. Data are expressed as mean ± standard deviation or number (percentage). ABI, ankle‐brachial index; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CPR, C‐peptide immunoreactivity; Cr, creatinine; CVR‐R, coefficient of variation of R‐R intervals; eGFR, estimated glomerular filtration rate; GA, glycoalbumin; GGT, γ‐glutamyltransferase; HbA1c, glycated hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitive C‐reactive protein; IMT, intima media thickness; LDL‐C, low‐density lipoprotein cholesterol; PWV, pulse wave velocity; TC, total cholesterol; TG, triglyceride; UA, uric acid.
Figure 1.
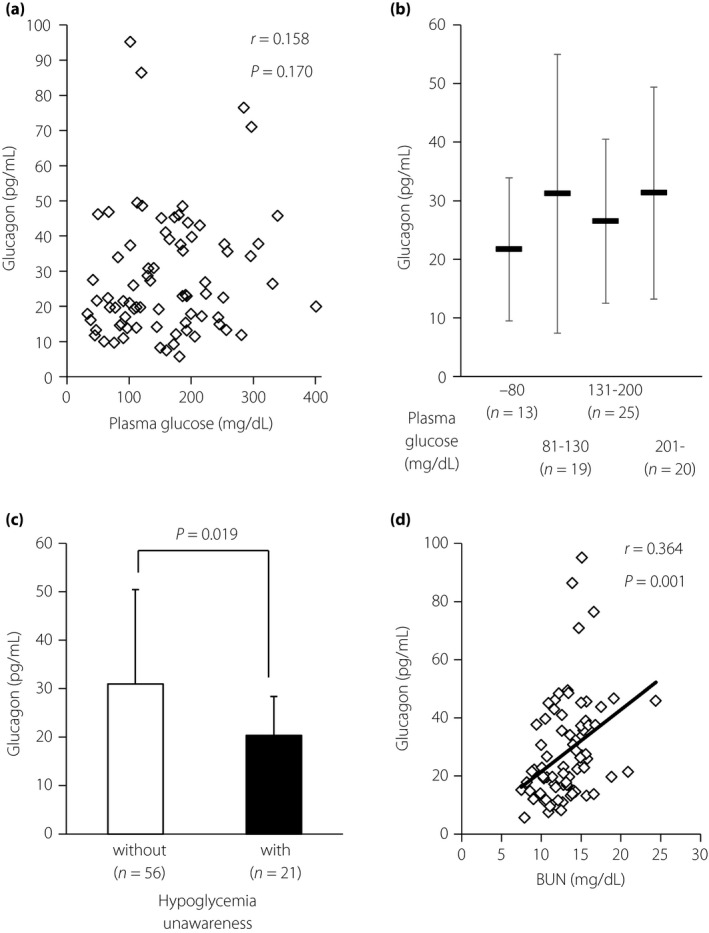
(a) Values of plasma glucose and glucagon in participants with type 1 diabetes (n = 77). Squares indicate individual samples. (b) Levels of plasma glucagon in participants with type 1 diabetes. Data are expressed as mean ± standard deviation. (c) Plasma glucagon levels in patients without (white, n = 56) and with (filled, n = 21) a history of hypoglycemia unawareness. Statistical analysis for comparison was carried out using the unpaired Student's t‐test. Data are expressed as mean ± standard deviation. (d) Association between serum blood urea nitrogen (BUN) and glucagon concentrations (n = 77). Squares indicate individual samples, and the line indicates the approximate straight line. Statistical analysis for correlation was carried out using Pearson's univariate test.
Discussion
Here, we report an evaluation of the plasma glucagon levels in type 1 diabetes patients with the new sandwich ELISA. The measured levels of glucagon were comparable with those reported in type 2 diabetes patients during oral glucose tolerance tests (estimated 28–46 pg/mL)9. These values were significantly higher compared with those observed in healthy participants (estimated 8–25 pg/mL), suggesting an implication of inadequately elevated glucagon in the induction of hyperglycemia9. Thus, the glucagon levels evaluated in the present study could also be higher than normal levels, especially in hyperglycemic states. In addition, the glucagon levels did not correlate with plasma glucose values, suggesting dysregulation of secretion, at least in terms of the systemic glycemic states, despite the patients having clinically well‐controlled diabetes. Importantly, the glucagon levels were not elevated in patients in a hypoglycemic state, indicating the involvement of unresponsive glucagon in hypoglycemia. Indeed, the glucagon levels in patients with a history of hypoglycemia unawareness were significantly lower, although they were assessed under various plasma glucose levels, further supporting the significance of glucagon incompetence in response to hypoglycemia.
The physiological secretion of glucagon is regulated through wide‐ranged mechanisms that involve nutrients and the neural and endocrine systems11. In addition to these, we have reported that insulin signaling in α‐cells is an intrinsic cellular mechanism regulating glucagon secretion12, 13, and that high glucose concentration directly induces excessive glucagon secretion through impaired insulin signaling14, indicating its pathophysiological significance in the disease. To date, the mechanism underlying dysregulation of glucagon secretion in type 1 diabetes patients remains unclear, but it is possibly due to insufficient insulin action on α‐cells, especially the deficient intra‐islet insulin from neighboring β‐cells. Another possibility is inadequate control of glucagon secretion by incretins. Incretins modify glucagon secretion; glucose‐dependent insulinotropic polypeptide enhances glucagon responses in hypoglycemic states, whereas glucagon‐like peptide‐1 suppresses glucagon secretion in hyperglycemic states. As the serum enzymatic activity of incretin‐degrading dipeptidyl peptidase‐4 was significantly higher in type 1 diabetes patients than in the healthy controls8, it is predicted that the effect of incretin on glucagon secretion is also impaired both in stimulation and suppression. Unchanged glucagon levels between hypoglycemic and hyperglycemic states in the current study further support this possibility. As the participants of the present study were relatively young patients with well‐controlled diabetes and fewer complications, including autonomic neuropathy, it is less likely that the dysregulated glucagon secretion was due to a defect in neural control.
The glucagon levels were significantly correlated with the serum BUN concentration (Figure 1d), but not with renal and liver function (Figures S1 and S2). Recently, the role of glucagon in the acceleration of hepatic amino acid metabolism has been acknowledged as one of the chief physiological functions15. BUN concentration represents the states of renal function, as well as the turnover of proteins and amino acids, which are the main sources of nitrogen in humans. The positive correlation of plasma glucagon level with BUN concentration independent of renal function (Figure S1) provides strong clinical evidence of the link between glucagon secretion and amino acid metabolism. Detailed analyses of amino acids will clarify further roles for glucagon in the comprehensive metabolic control in the disease.
The current study is not without limitations. As the participants were all previously diagnosed with type 1 diabetes, fasting or restriction of insulin use to standardize glucose levels and insulin effect were not carried out from medical and ethical points of view to avoid any harmful effects, such as unnecessary hypo‐ and hyperglycemic episodes. Among the participants of this study, the inclusion of fulminant type 1 diabetes patients, in which reduced α‐cell mass is reported, cannot be completely excluded, as many of them are diagnosed before the establishment of the concept and diagnostic criteria of fulminant type 1 diabetes. Single samples were randomly obtained in relation to patient food intake (2.6 ± 1.9 h after a meal); therefore, plasma glucose levels were not constant and were widely spread. There was no significant correlation between time after a meal and plasma glucose or glucagon levels (Figure S3). While insulin can be considered as a dominant regulator of glucagon secretion, especially through the intra‐islet effect, serum insulin levels of the participants could not be assessed, because they are treated with various kinds of insulin analogs. Thus, the possible influences of injected insulin on plasma glucagon levels cannot be excluded in the present study. The plasma glucagon levels were correlated with neither urine C‐peptide levels representing endogenous insulin secretion capacity nor daily total insulin dose used for treatment (Figure S4). The number of participants was limited, and appropriate healthy and type 2 diabetes controls were lacking. It should be noted that the plasma glucose levels in healthy controls should be tightly controlled within a narrow range, regardless of food intake; therefore, it was challenging to obtain the samples under hypo‐ and extreme hyperglycemic states, as presented in this study. These clinical and technical limitations raised in the current study should be taken into account in further analyses and studies. Their resolutions will add more useful insights in understanding the mechanism(s) underlying the dysregulation of glucagon.
To date, there have been no clinical reports that have evaluated plasma glucagon levels in type 1 diabetes patients using this sandwich ELISA. The current data emphasize the pathophysiological consequences of glucagon in the disease, particularly in hypoglycemia, and suggest important clues for the future therapeutic approach to target glucagon.
Disclosure
The authors declare no conflict of interest.
Supporting information
Data S1 | Supplemental Methods; Study population, Preparation of plasma samples for glucagon assay, and Acknowledgments.
Figure S1 | (a) Values for serum creatinine and plasma glucagon in the participants with type 1 diabetes. (b) Values for estimated glomerular filtration rate and plasma glucagon in the participants with type 1 diabetes.
Figure S2 | Values for parameters representing liver function and plasma glucagon in the participants with type 1 diabetes.
Figure S3 | (a) Values for time after a meal and plasma glucose in the participants with type 1 diabetes. (b) Values for time after a meal and plasma glucagon in the participants with type 1 diabetes.
Figure S4 | (a) Values for urine C‐peptide and plasma glucagon in the participants with type 1 diabetes. (b) Values for the treatment insulin dose and plasma glucagon in the participants with type 1 diabetes.
Acknowledgments
The authors thank the staff of Cosmic Corporation Co., Ltd. (Tokyo, Japan) for their assistance in the glucagon ELISA assays. This study is partly supported by a research grant from the Japan Diabetes Foundation (to DK).
J Diabetes Investig 2019; 10: 62–66
References
- 1. Lund A, Bagger JI, Christensen M, et al Glucagon and type 2 diabetes: the return of the alpha cell. Curr Diab Rep 2014; 14: 555. [DOI] [PubMed] [Google Scholar]
- 2. Cryer PE. Minireview: glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology 2012; 153: 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bak MJ, Albrechtsen NW, Pedersen J, et al Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur J Endocrinol 2014; 170: 529–538. [DOI] [PubMed] [Google Scholar]
- 4. Wewer Albrechtsen NJ, Hartmann B, Veedfald S, et al Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia 2014; 57: 1919–1926. [DOI] [PubMed] [Google Scholar]
- 5. Miyachi A, Kobayashi M, Mieno E, et al Accurate analytical method for human plasma glucagon levels using liquid chromatography‐high resolution mass spectrometry: comparison with commercially available immunoassays. Anal Bioanal Chem 2017; 409: 5911–5918. [DOI] [PubMed] [Google Scholar]
- 6. Kawamori R, Yamasaki Y, Matsushima H, et al Prevalence of carotid atherosclerosis in diabetic patients. Ultrasound high‐resolution B‐mode imaging on carotid arteries. Diabetes Care 1992; 15: 1290–1294. [DOI] [PubMed] [Google Scholar]
- 7. Yamashina A, Tomiyama H, Takeda K, et al Validity, reproducibility, and clinical significance of noninvasive brachial‐ankle pulse wave velocity measurement. Hypertens Res 2002; 25: 359–364. [DOI] [PubMed] [Google Scholar]
- 8. Osawa S, Kawamori D, Katakami N, et al Significant elevation of serum dipeptidyl peptidase‐4 activity in young‐adult type 1 diabetes. Diabetes Res Clin Pract 2016; 113: 135–142. [DOI] [PubMed] [Google Scholar]
- 9. Matsuo T, Miyagawa J, Kusunoki Y, et al Postabsorptive hyperglucagonemia in patients with type 2 diabetes mellitus analyzed with a novel enzyme‐linked immunosorbent assay. J Diabetes Investig 2016; 7: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wewer Albrechtsen NJ, Kuhre RE, Pedersen J, et al The biology of glucagon and the consequences of hyperglucagonemia. Biomark Med 2016; 10: 1141–1151. [DOI] [PubMed] [Google Scholar]
- 11. Kawamori D, Welters HJ, Kulkarni RN. Molecular pathways underlying the pathogenesis of pancreatic alpha‐cell dysfunction. Adv Exp Med Biol 2010; 654: 421–445. [DOI] [PubMed] [Google Scholar]
- 12. Kawamori D, Kurpad AJ, Hu J, et al Insulin signaling in alpha cells modulates glucagon secretion in vivo . Cell Metab 2009; 9: 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawamori D, Akiyama M, Hu J, et al Growth factor signalling in the regulation of alpha‐cell fate. Diabetes Obes Metab 2011; 13(Suppl 1): 21–30. [DOI] [PubMed] [Google Scholar]
- 14. Katsura T, Kawamori D, Aida E, et al Glucotoxicity induces abnormal glucagon secretion through impaired insulin signaling in InR1G cells. PLoS ONE 2017; 12: e0176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holst JJ, Wewer Albrechtsen NJ, Pedersen J, et al Glucagon and amino acids are linked in a mutual feedback cycle: the liver‐alpha‐cell axis. Diabetes 2017; 66: 235–240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 | Supplemental Methods; Study population, Preparation of plasma samples for glucagon assay, and Acknowledgments.
Figure S1 | (a) Values for serum creatinine and plasma glucagon in the participants with type 1 diabetes. (b) Values for estimated glomerular filtration rate and plasma glucagon in the participants with type 1 diabetes.
Figure S2 | Values for parameters representing liver function and plasma glucagon in the participants with type 1 diabetes.
Figure S3 | (a) Values for time after a meal and plasma glucose in the participants with type 1 diabetes. (b) Values for time after a meal and plasma glucagon in the participants with type 1 diabetes.
Figure S4 | (a) Values for urine C‐peptide and plasma glucagon in the participants with type 1 diabetes. (b) Values for the treatment insulin dose and plasma glucagon in the participants with type 1 diabetes.


