Abstract
The apoptotic effects of shikonin (5,8-dihydroxy-2-[(1R)-1-hydroxy-4-methylpent-3-enyl]naphthalene-1,4-dione) on the human colon cancer cell line SNU-407 were investigated in this study. Shikonin showed dose-dependent cytotoxic activity against SNU-407 cells, with an estimated IC50 value of 3 µM after 48 h of treatment. Shikonin induced apoptosis, as evidenced by apoptotic body formation, sub-G1 phase cells, and DNA fragmentation. Shikonin induced apoptotic cell death by activating mitogen-activated protein kinase family members, and the apoptotic process was mediated by the activation of endoplasmic reticulum (ER) stress, leading to activation of the PERK/elF2α/CHOP apoptotic pathway, and mitochondrial Ca2+ accumulation. Shikonin increased mitochondrial membrane depolarization and altered the levels of apoptosis-related proteins, with a decrease in B cell lymphoma (Bcl)-2 and an increase in Bcl-2-associated X protein, and subsequently, increased expression of cleaved forms of caspase-9 and -3. Taken together, we suggest that these mechanisms, including MAPK signaling and the ER-and mitochondria-mediated pathways, may underlie shikonin-induced apoptosis related to its anticancer effect.
Keywords: Shikonin, Human colon cancer, Apoptosis, Mitochondria, Endoplasmic reticulum
INTRODUCTION
Colon cancer occurs frequently and is one of the most important causes of cancer-related mortality in the elderly generation (Kim et al., 2015). Both genetic and environmental factors are considered to be associated with carcinogenesis of the colon (Slattery et al., 2016; Theodoratou et al., 2017). Currently, surgical resection is the main treatment and is highly effective for localized colorectal cancer (Osada et al., 2018). However, patients with late-stage disease have a poor prognosis. Of the patients diagnosed with colon cancer who undergo potentially curative surgical resection, approximately 25–40% will suffer tumor recurrence, and the overall mortality of the disease is approximately 40% (Zarour et al., 2017). To address this issue, chemotherapies have been improved to induce apoptosis to increase the survival rates.
In recent years, numerous natural compounds have been examined for their antitumor potential in vitro and in vivo. In particular, shikonin, a naphthoquinone derived from the root of Lithospermum erythrorhizon (Je et al., 2015), has anticancer activity against various types of cancer in vitro and in vivo (Hashimoto et al., 1999). This activity is associated with apoptosis induction (Gao et al., 2002) and regulation of the proliferation of cancer cells (Yingkun et al., 2010). Recently, Liang et al. (2017) reported that shikonin induces reactive oxygen species-based mitochondria-mediated apoptosis in colon cancer. Though the cytotoxic properties of shikonin against cancer cells have been extensively investigated, the details of the apoptotic effect of this compound in human colon cancer cells in terms of endoplasmic reticulum (ER) and mitochondrial involvement remain unclear.
Apoptosis is a form of programmed cell death necessary for physiological growth, immunity, and oncogenesis inhibition. Cancer progression is related to various critical processes, such as abnormal cell proliferation upon oncogene activation, which suppresses apoptosis (Rajasekar et al., 2012).
The mitogen-activated protein kinase (MAPK) family, which includes c-Jun-N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and p38 MAPK, is momentous mediators of signal pathways of cell growth, proliferation, differentiation, and apoptosis. Recent evidence proved that the activated MAPK signaling pathway can induce cancer cell death (Wakita et al., 2015). Apoptosis can be induced via intrinsic and extrinsic pathways; the intrinsic pathway is caused by intracellular events related to the ER and mitochondria. The ER is a multifunctional organelle, the primary functions of which are protein synthesis and folding. It also serves as an important location for Ca2+ storage and cell signaling. Perturbations of the functions of the ER result in ER stress, which can activate the expression of ER stress transducers and promotes apoptosis (Tsai et al., 2018). The B cell lymphoma (Bcl)-2 family, which comprises the anti-apoptotic proteins Bcl-2 and Bcl-xL, and the pro-apoptotic proteins Bcl-2-associated X (Bax), Bcl-2 homologous antagonist killer, and Bcl-2-associated death promoter, are the main mediators of mitochondria-mediated apoptosis (Chi, 2014; Gross, 2016; Radha and Raghavan, 2017). The crucial event in mitochondria-related apoptosis is permeability conversion and the subsequent activation of caspases (Fogarty and Bergmann, 2017; Xiong et al., 2014). The cleaved forms of caspase-9 and caspase-3 are important mediators of apoptosis.
The current study aimed to explore the apoptosis-inducing mechanisms of shikonin in SNU-407 human colon cancer cells.
MATERIALS AND METHODS
Reagents and chemicals
Shikonin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and was dissolved in dimethylsulfoxide (DMSO). The final concentration of DMSO did not exceed 0.02%. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bro mide (MTT), Hoechst 33342, propidium iodide (PI), and N-acetyl cysteine (NAC) were obtained from Sigma-Aldrich (St. Louis, MO, USA). 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbo cyanine chloride (JC-1), ER-Tracker Blue-White DPX and Rhod 2-acetoxymethyl ester (Rhod2-AM) were provided by Molecular Probes (Eugene, OR, USA). SP600125, SB203580, and U0126 were obtained from Calbiochem (San Diego, CA, USA). The other reagents and chemicals were all of analytical grade.
Cell culture
The human colon cancer cell line SNU-407 was purchased from the Korean Cell Line Bank (Seoul, Korea). The cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum, streptomycin (100 µg/ml), and penicillin (100 units/ml) in an incubator (37°C) with a humidified atmosphere of 5% CO2.
Cell viability assay
Cells were seeded into a 24-well plate at a density of 1.0×105 cells/ml, treated with 0, 0.625, 1.25, 2.5, 5, or 10 µM shikonin, and maintained at 37°C for 48 h. MTT solution was added to each well and after further incubation for 4 h, the absorbance at 540 nm was determined (Salimi et al., 2017).
Colony formation assay
Cells were seeded into 60-mm dishes to produce approximately 500 colonies per dish and were treated with shikonin (3 µM). The dishes were incubated for 14 days. Then, the colonies were stained with a Diff-Quik kit (Sysmex, Kobe, Japan) according to the manufacturer’s instructions. The colony forming cells (CFCs) were considered visible.
Nuclear staining with Hoechst 33342
Cells were treated with 3 µM shikonin and incubated at 37°C for 48 h. Then, 20 µM Hoechst 33342 was added to each well, and the cells were incubated at 37°C for 10 min. Stained cells were observed under a fluorescence microscope. The extent of nuclear condensation was evaluated, and apoptotic cells were counted.
Detection of sub-G1 hypodiploid cells
Cells were harvested and fixed in 70% ethanol at 4°C for 30 min. Then, the cells were incubated in a solution containing 50 mg/ml PI and 50 µg/ml RNase A in the dark at 37°C for 30 min. The cells were then examined in a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) (Lee, 2016).
Terminal deoxynucleotidyl transferase-mediated digoxigenin dUTP nick-end labeling (TUNEL) assay
The TUNEL assay was performed using an in-situ cell death detection kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Cells were seeded onto chamber slides at a density of 1.0×105 cells/well and after 20 h, they were treated with shikonin (3 µM) for 48 h. Then, the chamber slides were fixed, and the cells were permeabilized. After washing in PBS, the stained cells were visualized with a fluorescence microscope (Olympus IX 70, Olympus Optical Co., Tokyo, Japan) and quantified (Jeon et al., 2017).
Measurement of mitochondrial Ca2+
Cells were suspended in PBS containing Rhod2-AM (1 µM), incubated at 37°C for 15 min, and assessed by flow cytometry. In addition, cells were seeded in 4-well chambers and treated with Rhod2-AM at 37°C for 30 min. Images were captured on a confocal microscope using the Laser Scanning Microscope 5 PASCAL software (Carl Zeiss, Jena, Germany).
Detection of ER stress by ER staining
Cells were suspended in PBS containing ER-tracker Blue-White DPX probe (1 µM), incubated at 37°C for 15 min, and detected by flow cytometry. In addition, cells stained with the Blue-White DPX probe were evaluated by confocal microscopy using the Laser Scanning Microscope 5 PASCAL software.
Detection of the mitochondrial membrane potential (Δψm)
Cells were stained with JC-1 (5 µM) and microscopic images were collected using a confocal microscope and Laser Scanning Microscope 5 PASCAL. In addition, stained cells were examined by flow cytometry (Becton Dickinson).
Western blot analysis
Cell lysates (40 µg of protein) were electrophoresed on a 12% sodium dodecyl sulfate-polyacrylamide gel. The proteins were then transferred to nitrocellulose membranes, which were subsequently incubated with primary antibodies followed by horseradish peroxidase-conjugated goat anti-mouse and goat anti-rabbit IgG secondary antibodies (Pierce, Rockford, IL, USA). Protein bands were visualized using an enhanced chemiluminescence western blotting detection kit (Amersham, Little Chalfont, Buckinghamshire, UK). Antibodies targeting the following proteins were used: phospho-PERK, cleaved caspase-12, Bcl-2, Bax, caspase-9, caspase-3, phospho-ERK1/2, ERK2, phospho-JNK1/2, JNK1/2, phospho-p38, and p38 (all from Cell Signaling Technology, Beverly, MA, USA), and phospho-IRE1α, C/EBP-homologous protein (CHOP), phospho-eIF2α, and GRP78 (all from Santa Cruz Biotechnology).
Reverse transcription-polymerase chain reaction (RT-PCR)
PCR conditions were as follows: initial denaturation for 5 min at 94°C; 37 cycles of 94°C for 20 sec, 48°C for 10 sec, and 72°C for 40 sec; and a final elongation step at 72°C for 10 min. The primers used to amplify human XBP1 and human GAPDH cDNA were as follows: XBP1-s, 5′-CCTTGTAGTTGAGAACCAGG-3′ (F) and 5′-GGGGCTTGGTATATATGTGG-3′ (R); GADPH, 5′-TCAAGTGGGGCGATGCTGGC-3′ (F-648 bp) and 5′-TGCCAGCCCCAGCGTCAAAG-3′ (R-648 bp).
Statistical analysis
All values are expressed as the mean ± the standard error of the mean. Data were analyzed using analysis of variance and Tukey’s test to determine pairwise differences. A p-value < 0.05 was considered to be statistically significant.
RESULTS
Shikonin restrains the growth of SNU-407 human colon cancer cells
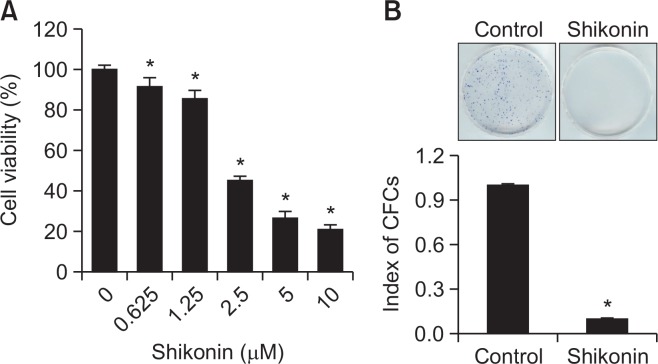
The cytotoxic effects of shikonin were assessed in SNU-407 human cancer cells. After treating SNU-407 cells with different concentrations of shikonin, the IC50 of shikonin in SNU-407 cells was calculated to be 3 µM (Fig. 1A). Therefore, the cells were treated with 3 µM shikonin for 48 h in subsequent experiments. To investigate whether shikonin can induce prolonged inhibition of cell growth, a colony formation assay was performed. The CFCs were considered visible. Compared with the control group, shikonin significantly suppressed colony formation by SNU-407 cells (Fig. 1B).
Fig. 1.
Cytotoxic effect of shikonin in human colon cancer SNU-407 cells. Cells were treated with (A) the indicated concentrations of shikonin for 48 h. Viability was assessed by the MTT assay. *p<0.05 vs. control cells. (B) Long-term cytotoxic effects of shikonin were detected by a colony formation assay. Approximately 500 colonies were seeded into each 60-mm dish, treated with shikonin, and incubated for 14 days. The resultant colonies were stained using a Diff-Quik kit. *p<0.05 vs. control cells.
Shikonin induces apoptosis in SNU-407 human colon cancer cells
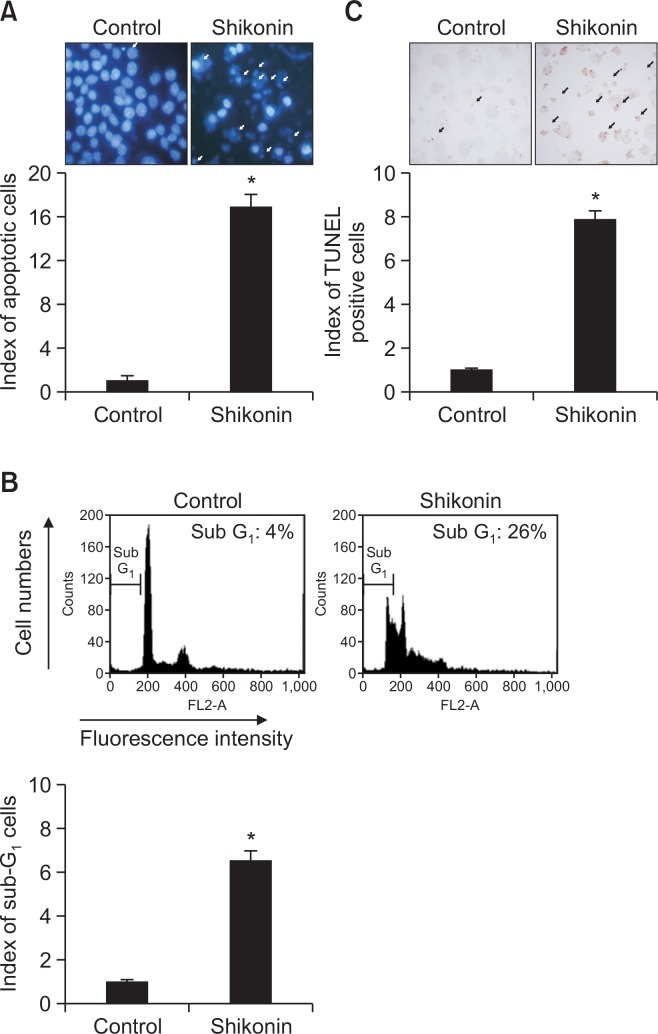
To examine whether the cytotoxic effects of shikonin occurred via apoptosis, we assessed apoptotic body formation, the number of cells in the sub-G1 phase of the cell cycle, and DNA fragmentation. Apoptotic body formation as assessed by using Hoechst 33342 staining was increased in shikonin-treated cells compared with control cells (Fig. 2A). Moreover, the number of cells in sub-G1 phase was significantly increased in shikonin-treated cells compared with control cells (26% vs. 4%, respectively; Fig. 2B). The number of TUNEL-positive cells was increased in shikonin-treated cells compared with control cells (Fig. 2C).
Fig. 2.
Shikonin induces apoptosis in SNU-407 cells. (A) Apoptotic body formation (arrows) was observed by fluorescence microscopy after Hoechst 33342 staining. *p<0.05 vs. control cells. (B) Sub-G1 cells were detected by flow cytometry after PI staining. *p<0.05 vs. control cells. (C) DNA fragmentation indicative of apoptosis was assessed by the TUNEL assay. *p<0.05 vs. control cells.
Involvement of the MAPK pathway in shikonin-induced apoptosis
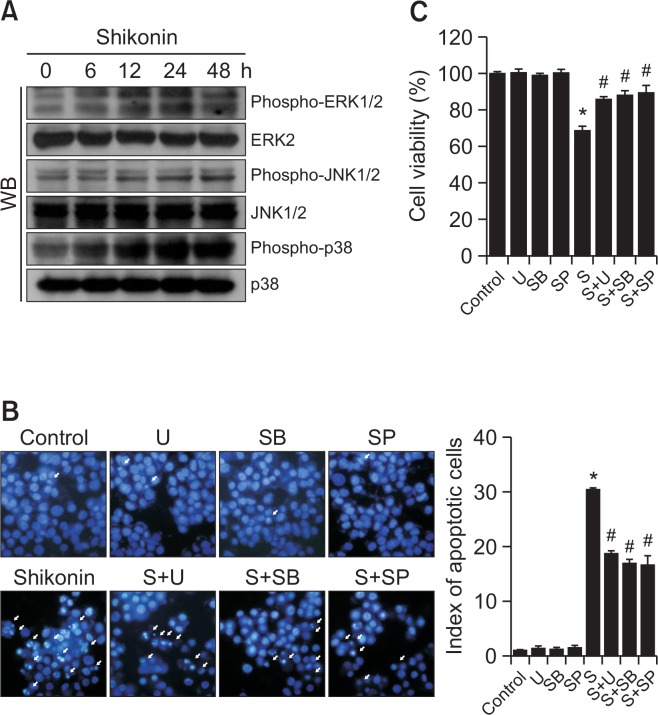
Shikonin treatment activated ERK, JNK, and p38 MAPK in a time-dependent manner (Fig. 3A). We treated cells with specific inhibitors of ERK, JNK, and p38 MAPK to determine whether this would attenuate shikonin-induced cell death. U0126 (an inhibitor of ERK), SP600125 (an inhibitor of JNK), and SB203580 (an inhibitor of p38 MAPK) attenuated the apoptotic effect of shikonin as indicated by Hoechst 33342 staining analysis (Fig. 3B). These results were confirmed by an MTT assay (Fig. 3C). Together, these findings suggested that shikonin-induced apoptotic cell death is related with activation of MAPKs.
Fig. 3.
Shikonin induces apoptosis via the MAPK pathway. (A) Western blot analysis of the phosphorylation state of MAPK family members. After treatment with MAPK inhibitors and/or shikonin. (B) apoptotic body formation (arrows) was observed by fluorescence microscopy after Hoechst 33342 staining, and (C) cell viability was assessed using the MTT assay. *p<0.05 vs. control cells, #p<0.05 vs. shikonin-treated cells. U, U0126; SB, SB203580; SP, SP600125; S, shikonin.
Shikonin-induced apoptosis is mediated by an increased ER stress response
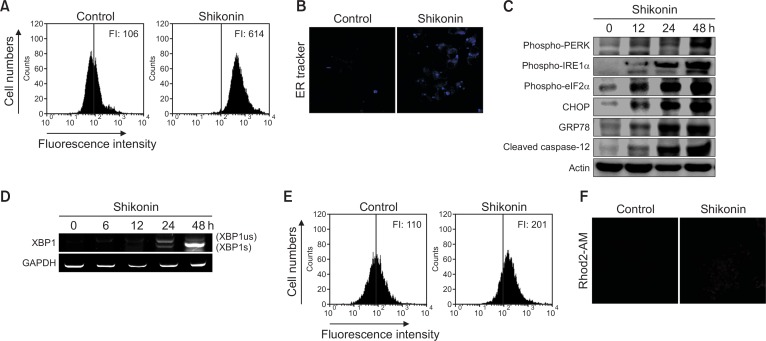
We next investigated the relationship between shikonin-induced apoptosis and ER stress in SNU-407 cells. When cells are under stress, the accumulation of unfolded and misfolded proteins in the ER lumen causes ER stress. The induction of ER stress by shikonin significantly increased the fluorescence intensity of the ER-specific dye ER-tracker Blue-White DPX as indicated by flow cytometry and image analysis (Fig. 4A, 4B). If the ER stress persists, it may activate downstream proteins, subsequently causing apoptosis through pathways mediated by PKR-like ER-associated kinase (PERK) and inositol-requiring enzyme-1α (IRE1α). By measuring the expression levels of proteins associated with these pathways, we found that the expression of phosphorylated PERK (phospho-PERK), phosphorylated IRE1α (phospho-IRE1α), phosphorylated eIF2α (phospho-eIF2α), CHOP, GRP78, cleaved caspase-12, and spliced mRNA of XBP1 was increased (Fig. 4C, 4D). These results suggested that the mechanism of shikonin-induced apoptosis is mediated by the PERK/eIF2α/CHOP stress response pathway in the ER. To evaluate whether shikonin stimulates Ca2+ release from ER stores and induces mitochondrial Ca2+ accumulation, which can trigger apoptosis, we detected mitochondrial Ca2+ levels using flow cytometry and image analysis after staining the cells with Rhod2-AM. Shikonin significantly increased the Rhod2-AM fluorescence intensity as compared to that in control cells (Fig. 4E, 4F).
Fig. 4.
Shikonin induces ER stress in SNU-407 cells. Cells were exposed to 3 µM shikonin for 24 h, harvested, and loaded with the ER-tracker Blue-White DPX probe (1 µM). ER stress was measured by (A) flow cytometry and (B) confocal microscopy. FI: fluorescence intensity. Representative confocal microscopic images show elevated blue fluorescence intensity of Blue-White DPX probe in the shikonin-treated cells. (C) Cell lysates were subjected to electrophoresis, and phospho-PERK, phospho-eIF2α, phospho-IRE1α, CHOP, GRP78, and cleaved caspase-12 were detected by western blotting. (D) The level of spliced XBP1 mRNA (XBP1s) was measured by RT-PCR. us, unspliced; s, spliced. Mitochondrial Ca2+ level was measured by (E) flow cytometry and (F) confocal microscopy after staining the cells with Rhod2-AM.
Involvement of the mitochondrial pathway in shikonin-induced apoptosis
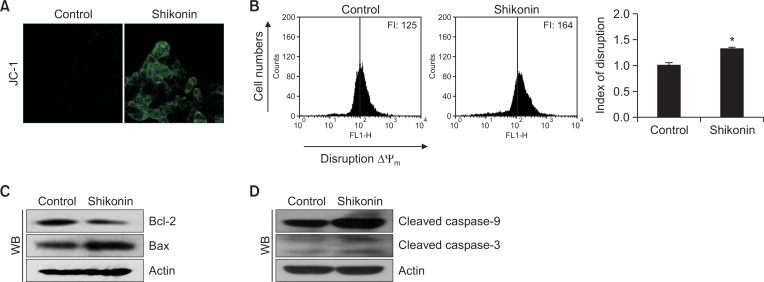
Apoptosis occurs via mitochondrial membrane permeabilization/depolarization (Jeong and Seol, 2008). Confocal microscopic images revealed that the mitochondria of control cells barely exhibited green JC-1 fluorescence, which is an indicative of Δψm depolarization, while shikonin-treated cells exhibited increased green mitochondrial fluorescence by JC-1 staining (Fig. 5A). Shikonin-induced loss in Δψm was confirmed by flow-cytometric analysis (Fig. 5B), demonstrating that shikonin disrupted the Δψm. We then investigated the levels of proteins associated with mitochondria-mediated apoptosis. Shikonin downregulated the level of the pro-survival/anti-apoptotic protein Bcl-2 and upregulated that of the pro-apoptotic protein Bax (Fig. 5C). Additionally, shikonin treatment induced significantly elevated levels of activated caspase-9 and caspase-3 (Fig. 5D).
Fig. 5.
Shikonin induces apoptosis via the mitochondrial and caspase pathways. Cells were stained with JC-1, and Δψm was assessed by (A) confocal microscopy and (B) flow cytometry.*p<0.05 vs. control cells. Western blot analysis of (C) Bcl-2 and Bax, and (D) cleaved caspase-9 and cleaved caspase-3, using the appropriate primary antibodies.
DISCUSSION
The ability of cancer chemotherapeutic agents to induce cell-cycle arrest and apoptosis is a dominating factor in defining their therapeutic relevance (Mills et al., 2018). In the present study, shikonin potently suppressed the growth of SNU-407 cells (Fig. 1). Accordingly, the levels of chromatin condensation in the nuclei, DNA fragmentation, and sub-G1 phase cells increased following shikonin treatment, demonstrating that the cytotoxic effect of shikonin involves the apoptotic pathway (Fig. 2). Additionally, the MAPK signaling pathway is suggested to be related with anticancer effects (Zhang et al., 2015). Shikonin treatment markedly induced the phosphorylation of three MAPKs, which indicated that the anticancer effect of shikonin in the colon cancer cell line SNU-407 is mediated by activation of the MAPK pathway (Fig. 3). Moreover, ER stress can trigger apoptosis via ER stress-specific cell-death signals, including CHOP and caspase-12 (Nakagawa et al., 2000). In addition, the ER is a major storage site for Ca2+, and excessive leakage of ER Ca2+ stores into the cytoplasm is involved in ER stress-mediated apoptosis (Ryoo, 2015). In the present study, shikonin treatment significantly increased the accumulation of Ca2+ in the mitochondrial matrix (Fig. 4). In addition, we investigated specific markers of ER stress. When unfolded or misfolded proteins accumulate, ER chaperone-binding immunoglobulin protein binds to these abnormal proteins, releasing its inhibitory hold on PERK, ATF6, and IRE1, which form a signaling pathway to resolve ER stress (Yu et al., 2013). Moreover, ER stress-induced homo-dimerization and transauto-phosphorylation activates IRE1 endoribonuclease activity, allowing it to excise a 26-nucleotide intron from the XBP1 mRNA to generate the spliced form, XBP1s (Li et al., 2010). Activated PERK phosphorylates eIF2α, which attenuates protein synthesis and alleviates ER protein overload (Yu et al., 2013). Here, we showed that shikonin induced PERK/eIF2α phosphorylation, IRE1α phosphorylation, XBP1 splicing, caspase-12 cleavage, and CHOP overexpression. Further, we observed that ER stress was effectively activated by shikonin treatment (Fig. 4).
Recent studies have reported that this apoptotic pathway is related to alterations in the Δψm, which can be triggered by various stimuli (Yang et al., 2015; Burke, 2017). The loss of Δψm in SNU-407 human colon cancer cells upon shikonin treatment was verified by an increase in the fluorescence intensity of the dye JC-1 (Fig. 5). The pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-2 play a role in the control of mitochondrial membrane permeability, which is a fundamental event in apoptosis signaling (El-Najjar et al., 2008). We found that an increase in Bax and a decrease in Bcl-2 were related with the induction of apoptosis in the SNU-407 colon cancer cell line. In human bladder cancer cells and Hela cells, shikonin induces apoptosis via the activation of caspase-3 (Wu et al., 2004; Yeh et al., 2007). Similarly, the expression levels of active caspase-9 and active caspase-3 were increased upon shikonin treatment in the present study.
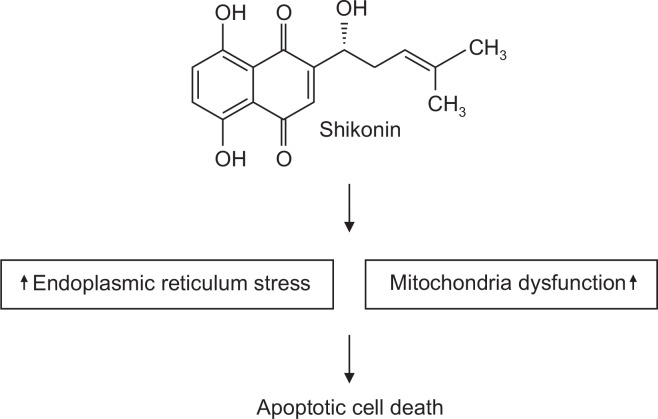
In summary, shikonin exerts anticancer effects on SNU-407 cells by activating apoptosis via MAPK signaling and ER- and mitochondria-mediated pathways as evidenced by the accumulation of mitochondrial Ca2+, induction of ER stress markers, induction of Δψm loss, activation of caspases, and alteration of Bcl-2 family protein expression (Fig. 6).
Fig. 6.
Shikonin induces apoptosis through ER stress and the mitochondria-mediated pathway. Shikonin induces ER stress and mitochondrial dysfunction, leading to apoptotic cell death.
These distinct mechanisms render the natural compound, shikonin, a potentially useful chemotherapeutic candidate that might effectively suppress colon cancer growth.
Acknowledgments
This research was supported by the 2018 scientific promotion program funded by Jeju National University.
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- Burke PJ. Mitochondria, bioenergetics and apoptosis in cancer. Trends Cancer. 2017;3:857–870. doi: 10.1016/j.trecan.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SW. Structural insights into the transcription-independent apoptotic pathway of p53. BMB Rep. 2014;47:167–172. doi: 10.5483/BMBRep.2014.47.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Najjar N, Dakdouki S, Darwiche N, El-Sabban M, Saliba NA, Gali-Muhtasib H. Anti-colon cancer effects of Salograviolide A isolated from Centaurea ainetensis. Oncol. Rep. 2008;19:897–904. [PubMed] [Google Scholar]
- Fogarty CE, Bergmann A. Killers creating new life: caspases drive apoptosis-induced proliferation in tissue repair and disease. Cell Death Differ. 2017;24:1390–1400. doi: 10.1038/cdd.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Hiromura M, Yasui H, Sakurai H. Direct reaction between shikonin and thiols induces apoptosis in HL60 cells. Biol. Pharm. Bull. 2002;25:827–832. doi: 10.1248/bpb.25.827. [DOI] [PubMed] [Google Scholar]
- Gross A. BCL-2 family proteins as regulators of mitochondria metabolism. Biochim. Biophys. Acta. 2016;1857:1243–1246. doi: 10.1016/j.bbabio.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Xu M, Masuda Y, Aiuchi T, Nakajo S, Cao J, Miyakoshi M, Ida Y, Nakaya K. β-Hydroxyisovaleryl shikonin inhibits the cell growth of various cancer cell lines and induces apoptosis in leukemia HL-60 cells through a mechanism different from those of Fas and etoposide. J. Biochem. 1999;125:17–23. doi: 10.1093/oxfordjournals.jbchem.a022255. [DOI] [PubMed] [Google Scholar]
- Je HD, Kim HD, La HO. The inhibitory effect of shikonin on the agonist-induced regulation of vascular contractility. Biomol. Ther. (Seoul) 2015;23:233–237. doi: 10.4062/biomolther.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon HL, Yi JS, Kim TS, Oh Y, Lee HJ, Lee M, Bang JS, Ko K, Ahn IY, Ko K, Kim J, Park HK, Lee JK, Sohn SJ. Development of a test method for the evaluation of DNA damage in mouse spermatogonial stem cells. Toxicol. Res. 2017;33:107–118. doi: 10.5487/TR.2017.33.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB Rep. 2008;41:11–22. doi: 10.5483/BMBRep.2008.41.1.011. [DOI] [PubMed] [Google Scholar]
- Kim AY, Kwak JH, Je NK, Lee YH, Jung YS. Epithelial-mesenchymal transition is associated with acquired resistance to 5-fluorocuracil in HT-29 colon cancer cells. Toxicol. Res. 2015;31:151–156. doi: 10.5487/TR.2015.31.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. Cytotoxicity evaluation of essential oil and its component from Zingiber officinale Roscoe. Toxicol. Res. 2016;32:225–230. doi: 10.5487/TR.2016.32.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Korennykh AV, Behrman SL, Walter P. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc. Natl. Acad. Sci. U.S.A. 2010;107:16113–16118. doi: 10.1073/pnas.1010580107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Cui J, Zhang K, Xi H, Cai A, Li J, Gao Y, Hu C, Liu Y, Lu Y, Wang N, Wu X, Wei B, Chen L. Shikonin induces ROS-based mitochondria-mediated apoptosis in colon cancer. Oncotarget. 2017;8:109094–109106. doi: 10.18632/oncotarget.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CC, Kolb EA, Sampson VB. Development of chemotherapy with cell-cycle inhibitors for adult and pediatric cancer therapy. Cancer Res. 2018;78:320–325. doi: 10.1158/0008-5472.CAN-17-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Osada S, Gotoh A, Yokoi R, Tsuchiya H, Sakuratani T, Sasaki Y, Okumura N, Hayashi H, Mukai T. Effective timing of surgical resection of colorectal cancer liver metastases during chemotherapy. Anticancer Res. 2018;38:737–743. doi: 10.21873/anticanres.12279. [DOI] [PubMed] [Google Scholar]
- Radha G, Raghavan SC. BCL2: a promising cancer therapeutic target. Biochim. BiophysActa. 2017;1868:309–314. doi: 10.1016/j.bbcan.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Rajasekar S, Park da J, Park C, Park S, Park YH, Kim ST, Choi YH, Choi YW. In vitro and in vivo anticancer effects of Lithospermum erythrorhizon extract on B16F10 murine melanoma. J. Ethnopharmacol. 2012;144:335–345. doi: 10.1016/j.jep.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Ryoo HD. Drosophila as a model for unfolded protein response research. BMB Rep. 2015;48:445–453. doi: 10.5483/BMBRep.2015.48.8.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi A, Talatappe BS, Pourahmad J. Xylene induces oxidative stress and mitochondria damage in isolated human lymphocytes. Toxicol. Res. 2017;33:233–238. doi: 10.5487/TR.2017.33.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery ML, Pellatt DF, Wolff RK, Lundgreen A. Genes, environment and gene expression in colon tissue: a pathway approach to determining functionality. Int. J. Mol. Epidemiol. Genet. 2016;7:45–57. [PMC free article] [PubMed] [Google Scholar]
- Theodoratou E, Timofeeva M, Li X, Meng X, Ioannidis JPA. Nature, nurture, and cancer risks: genetic and nutritional contributions to cancer. Annu. Rev. Nutr. 2017;37:293–320. doi: 10.1146/annurev-nutr-071715-051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TC, Lai KH, Su JH, Wu YJ, Sheu JH. 7-Acetylsinumaximol B induces apoptosis and autophagy in human gastric carcinoma cells through mitochondria dysfunction and activation of the PERK/eIF2α/ATF4/CHOP signaling pathway. Mar. Drugs. 2018;16:E104. doi: 10.3390/md16040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakita A, Motoyama S, Sato Y, Koyota S, Usami S, Yoshino K, Sasaki T, Imai K, Saito H, Minamiya Y. REG Iα activates c-Jun through MAPK pathways to enhance the radiosensitivity of squamous esophageal cancer cells. Tumour Biol. 2015;36:5249–5254. doi: 10.1007/s13277-015-3183-y. [DOI] [PubMed] [Google Scholar]
- Wu Z, Wu LJ, Li LH, Tashiro S, Onodera S, Ikejima T. Shikonin regulates HeLa cell death via caspase-3 activation and block age of DNA synthesis. J. Asian Nat. Prod. Res. 2004;6:155–166. doi: 10.1080/1028602032000169622. [DOI] [PubMed] [Google Scholar]
- Xiong S, Mu T, Wang G, Jiang X. Mitochondria-mediated apoptosis in mammals. Protein Cell. 2014;5:737–749. doi: 10.1007/s13238-014-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Guo S, Wang S, Qian Y, Tai H, Chen Z. Advanced glycation end products-induced chondrocyte apoptosis through mitochondrial dysfunction in cultured rabbit chondrocyte. Fundam. Clin. Pharmacol. 2015;29:54–61. doi: 10.1111/fcp.12094. [DOI] [PubMed] [Google Scholar]
- Yeh CC, Kuo HM, Li TM, Lin JP, Yu FS, Lu HF, Chung JG, Yang JS. Shikonin-induced apoptosis involves caspase-3 activity in a human bladder cancer cell line (T24) In Vivo. 2007;21:1011–1019. [PubMed] [Google Scholar]
- Yingkun N, Lvsong Z, Huimin Y. Shikonin inhibits the proliferation and induces the apoptosis of human HepG2 cells. Can. J. Physiol. Pharmacol. 2010;88:1138–1146. doi: 10.1139/Y10-085. [DOI] [PubMed] [Google Scholar]
- Yu M, Melissa DT, Richard AC, Xiao YT. Endoplasmic reticulum stress and related pathological processes. J. Pharmacol. Biomed. Anal. 2013;1:1000107. [PMC free article] [PubMed] [Google Scholar]
- Zarour LR, Anand S, Billingsley KG, Bisson WH, Cercek A, Clarke MF, Coussens LM, Gast CE, Geltzeiler CB, Hansen L, Kelley KA, Lopez CD, Rana SR, Ruhl R, Tsikitis VL, Vaccaro GM, Wong MH, Mayo SC. Colorectal cancer liver metastasis: evolving paradigms and future directions. Cell Mol. Gastroenterol. Hepatol. 2017;3:163–173. doi: 10.1016/j.jcmgh.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhu Y, Duan W, Feng C, He X. Allicin induces apoptosis of the MGC-803 human gastric carcinoma cell line through the p38 mitogen-activated protein kinase/caspase-3 signaling pathway. Mol. Med. Rep. 2015;11:2755–2760. doi: 10.3892/mmr.2014.3109. [DOI] [PubMed] [Google Scholar]