Abstract
Previous studies have shown that spinosin was implicated in the modulation of sedation and hypnosis, while its effects on learning and memory deficits were rarely reported. The aim of this study is to investigate the effects of spinosin on the improvement of cognitive impairment in model mice with Alzheimer’s disease (AD) induced by Aβ1–42 and determine the underlying mechanism. Spontaneous locomotion assessment and Morris water maze test were performed to investigate the impact of spinosin on behavioral activities, and the pathological changes were assayed by biochemical analyses and histological assay. After 7 days of intracerebroventricular (ICV) administration of spinosin (100 µg/kg/day), the cognitive impairment of mice induced by Aβ1–42 was significantly attenuated. Moreover, spinosin treatment effectively decreased the level of malondialdehyde (MDA) and Aβ1–42 accumulation in hippocampus. Aβ1–42 induced alterations in the expression of brain derived neurotrophic factor (BDNF) and B-cell lymphoma-2 (Bcl-2), as well as inflammatory response in brain were also reversed by spinosin treatment. These results indicated that the ameliorating effect of spinosin on cognitive impairment might be mediated through the regulation of oxidative stress, inflammatory process, apoptotic program and neurotrophic factor expression, suggesting that spinosin might be beneficial to treat learning and memory deficits in patients with AD via multi-targets.
Keywords: Spinosin, Semen Ziziphi spinosae, Alzheimer’s disease, Neuroprotection
INTRODUCTION
Alzheimer’s disease (AD) is an important age-related neurodegenerative disease typically leading to progressive cognitive decline, behavioral impairments, and finally dementia (Chu et al., 2012). It is assumed that the number of AD patients worldwide will quadruple from the current number of 36 million people by 2050 (Gomez-Ramirez and Wu, 2014). For the development of anti-AD drugs in the decade of 2002 through 2012, 244 compounds were assessed and the success rate for advancing agents for regulatory approval was only 0.4% (99.6% attrition) (Cummings et al., 2014). Thus, searching for effective agents for the treatment or alleviation of AD is urgently needed.
AD is characterized by the over-production of amyloid-beta (Aβ), which is regarded as one of the essential pathologic markers of AD (Yoon and Jo, 2012). Overproduction or lack of clearance of Aβ leads to an increased aggregation of Aβ, which is associated with the pathogenesis of AD (Hardy and Selkoe, 2002). Moreover, oxidative stress is involved in the development of AD (Butterfield and Stadtman, 1997) and is accompanied by the activation of microglial inflammation in the presence of Aβ-mediated inflammatory response (Zhang et al., 2013).
Semen Ziziphi spinosae (SZS), the mature seed of Ziziphus jujube Mill var spinosa (Bunge) Hu ex H F Chou (Rhamnaceae), is widely applied in China, Japan, Korea and other oriental countries to treat insomnia and anxiety symptoms (You et al., 2010). As one of the major active C-glycoside flavonoids in SZS, abundant literatures have demonstrated the sedative and hypnotic effects of spinosin (Shin et al., 1978; Jiang et al., 2007; Wang et al., 2008), which may be related to the regulation of postsynaptic 5-HT1A receptors (Wang et al., 2010). Many studies suggested that sleep is likely linked with learning and memory function (Drummond and Brown, 2001; Graves et al., 2003), and spinosin was also reported to exert therapeutic potential in the treatment of cognitive impairment (Jung et al., 2014; Ko et al., 2015; Lee et al., 2016). Even so, the mechanism of neuroprotection by spinosin is still unclear.
Intracerebroventricular (ICV) injection of Aβ was reported to cause oxidative stress and inflammation, finally resulting in apoptosis of neuronal cells (Nagele et al., 2004); the neurotoxicity of Aβ ensured its effectiveness in producing animal models of AD (Palop and Mucke, 2010). In the current study, we investigated whether spinosin could ameliorate cognitive impairment in a mouse model of AD induced by ICV injection of Aβ1–42, and further determined the underlying mechanisms - including the effect of spinosin on accommodating malondialdehyde (MDA) and brain-derived neurotrophic factor (BDNF) dysregulation, Aβ accumulation, neuronal apoptosis, and neuroinflammation.
MATERIALS AND METHODS
Animals and materials
Male specific pathogen-free Kun-Ming mice (35–40 g) were provided by the Experimental Animal Center of Shenyang Pharmaceutical University (Shenyang, China). All the studies were performed in compliance with the Guideline for Animal Experimentation and the protocol was subject to approval by the Animal Ethics Committee of Shenyang Pharmaceutical University. All animals were housed under conventional conditions with appropriate temperature (22 ± 0.5°C), humidity (50–60%) control and a 12/12 h light/dark cycle with free access to food and water.
Spinosin (purity>99.0%) was provided by the National Institutes for Food and Drug Control of Pharmaceutical and Biological Products (Shenyang, China) and was suspended in physiological saline solution at concentrations of 0.2 and 2 µg/µl. Recombinant human Aβ1–42 (Sigma-Aldrich, St Louis, MO, USA) was dissolved and diluted in physiological saline to a stock concentration of 1.0 mg/mL, which was then sealed and incubated for 120 h at 37°C to allow the peptide to aggregate (Mao et al., 2015). Commercial kits for detection of MDA, B-cell lymphoma-2 (Bcl-2), BNDF, interleukin-6 (IL-6) and Aβ1–42 were purchased from Nanjing Jiancheng Bio engineering Institute (Nanjing, China).
Treatment and experimental design
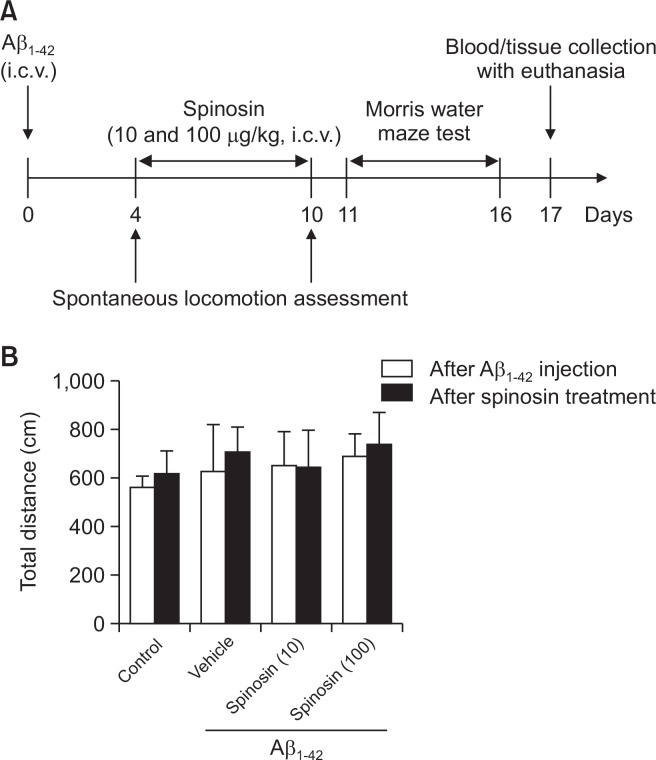
Mice were randomly divided into the following 4 groups (n=10 in each group): (I) control; (II) Aβ1–42; (III) Aβ1–42+10 µg/kg spinosin; (IV) Aβ1–42+100 µg/kg spinosin. Each mouse in groups II-IV was anesthetized with intraperitoneal injection of chloral hydrate (4 g/kg), and 3 µL Aβ1–42 was injected into left hippocampus under the stereotaxic apparatus (AP: −0.5 mm; ML: −1.1 mm; DV: −3.0 mm). Aβ1–42 injection was performed over 3 min, and the needle was still in place for an additional 2 min before retracting. Then, animals were implanted with cannula (8.0 mm) located 5 mm above the right ventricle (AP: −0.2 mm; ML: −1.0 mm; DV: −3.0 mm). The cannula was fixed to the skull with dental cement. Mice were given ICV injection of spinosin (10 and 100 µg/kg) daily for 7 days after surgery. The timeline for animal experiments was provided in Fig. 1A.
Fig. 1.
Schematic representation of the experimental design (A), and locomotor activity of mice in each group (B). Values are means ± SD (n=10).
Behavioral tests
Spontaneous locomotor activity: The spontaneous locomotor activity of mice was measured with a Multi-autonomous Activity Instrument (Huaibei Zhenghua Bioscience Technology Limited Company, Anhui, China) consisting nine chambers (25 cm diameter×13 cm height) using a video-recorded analytical system (Shanghai Jiliang Software Technology Co. Ltd., Shanghai, China). The locomotor activity of each animal was determined by measuring the total distance of movements over a 5-min period.
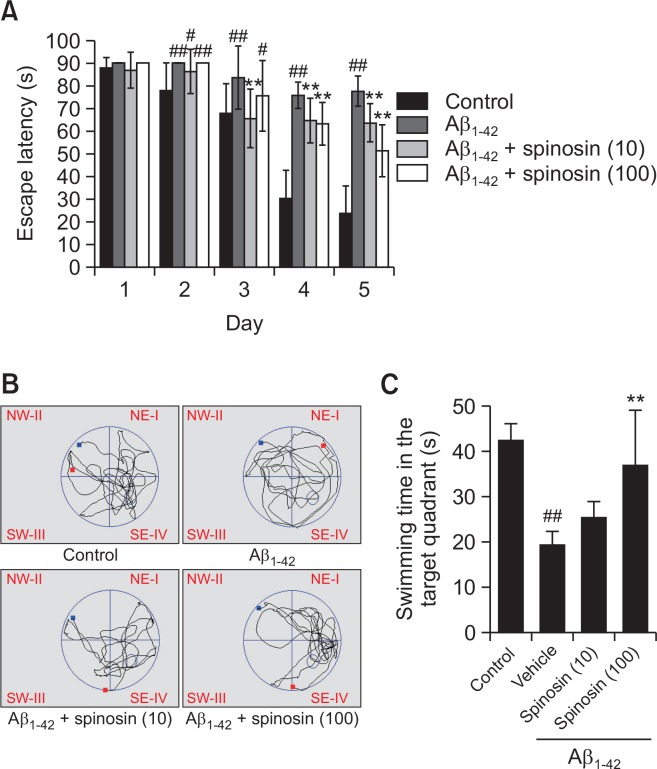
Morris water maze test: Morris maze test was conducted according to the procedure established by Morris with slight modifications (Morris, 1984). The Morris water maze is a dark circular pool, 90 cm in diameter and 45 cm in height. It was filled with dark water (25–27°C) to a depth of 40 cm and the black platform (8 cm in diameter and 10 cm in height) was then placed in the same quadrant (Q4) on every trial with 1 cm below the water so that it was a featureless inner surface. On the first day of Morris maze test, mice were trained for two trials with a trial interval of 10 min to find the hidden escape platform. From the second day, mice received four trials per day for 4 consecutive days. They were randomly placed in the different quadrant and were allowed to swim for 90 s to seek the hidden platform. If the mice found the platform within 90 s and remained on it for at least 3 s, then the track was terminated. If the mice failed to find the platform within 90 s, the mice were guided to the platform and were allowed to stay on it for 15 s. On the 6th day, the platform was removed and the spatial probe test was given by allowing the mice to swim freely for 90 s. The time spent by the animal in the target quadrant was evaluated (Mao et al., 2015).
Biochemical analyses
Mice were deeply anesthetized after behavioral tests, the cerebral cortex and hippocampus of 6 mice in each group were dissected out and immediately stored at −80˚C. For biochemical analysis, the cerebral cortex and the hippocampus tissue were homogenized in ice-cold saline and centrifuged at 2000 rpm for 10 min to collect the supernatant. Levels of MDA were measured by the thiobarbituric acid reaction method at a wavelength of 532 nm according to manufacturer’s instruction. Bcl-2, BNDF, IL-6 and Aβ1–42 in brain tissues were assessed by commercial sandwich enzyme-linked immunosorbent assay (ELISA) kits. In brief, protein samples from brain tissues and biotinylated antibodies targeting Bcl-2, BNDF, IL-6 or Aβ1–42 were added to microwells pre-coated with corresponding nonlabled antibodies. After incubation, streptavidin-HRP was added to bind to biotinylated antibodies. Following incubation with 3,3′,5,5′-tetramethyl-benzidine substrate, stop solution was added and absorbance at 450 nm (OD450) of each sample was recorded by a microplate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA).
Histopathological examination
Four mice in each group were perfused transcardially with saline followed by 4% paraformaldehyde (PFA) in phosphate buffer saline (0.1 mol/L PBS, pH 7.2) after anesthesia. The entire brains were postfixed in 4% paraformaldehyde (PFA) solution for 48 h, and then transferred to 30% sucrose in 0.1 mol/l PBS (pH 7.4) for at least 16 h until sectioning. Serial (neighboring) sections of 10 µm thickness were sectioned and stained with hematoxylin and eosin (H&E) staining (Ahmad et al., 2005).
Statistical analyses
Results are expressed as mean ± SD. The significances among different groups were determined using SPSS 19.0 (IBM Corp., New York, NY) where statistical significance were assessed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test, p<0.05 was considered statistically significant.
RESULTS
The spontaneous locomotor activity of mice
The influence of surgery and ICV administration of Aβ1–42 or spinosin on spontaneous locomotor activity of the mice was investigated. As shown in Fig. 1B, there was no significant difference in the spontaneous locomotor activity indicated by exploratory behavior among different groups after Aβ1–42 or spinosin treatment (p>0.05), which suggested that surgeries or ICV injection of Aβ1–42 or spinosin did not have impact on locomotive activity of mice.
Spinosin improved Aβ1–42-induced cognitive impairment in the Morris water maze test
For the long-term memory evaluated by Morris maze test, mice with Aβ1–42 injection spent longer time searching for the hidden platform (latency time) than the control group during the five days training sessions. On the other hand, mice treated with spinosin (10, 100 µg/kg) after Aβ1–42 injection showed an improved learning and memory performance as compared to mice in model group only received Aβ1–42 injection (Fig. 2A). Accordantly, the time spending in the target quadrant in Aβ1–42 group was lower than those in control group in the probe trial on the 6th day of the test (p<0.01), while the treatment of high dose of spinosin (100 µg/kg) significantly restored the time of mice spending in the target quadrant (p<0.01) (Fig. 2B, 2C).
Fig. 2.
Effects of spinosin on escape latency in the training sessions (A), and search strategy (B) and swimming time in target quadrant in probe trail sessions (C) of the Morris water maze test. Values are means ± SD (n=10). #p<0.05 and ##p<0.01 compared with control group; **p<0.01 compared with Aβ1–42 group.
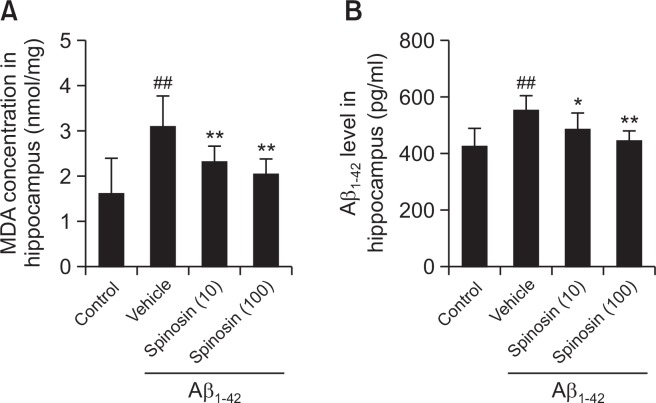
Spinosin decreased the level of MDA in hippocampus of mice with Aβ1–42 injection
Oxidative stress was evaluated by analyzing the level of MDA in hippocampus of mice. Compared with the control group, Aβ1–42 injected mice in model group showed a significant elevation of hippocampus level of MDA (p<0.01). After 5 days treatment with spinosin (10, 100 µg/kg), the MDA content was significantly reduced in the mice treated with both low and high doses of spinosin compared to model group (Fig. 3A).
Fig. 3.
Effect of spinosin on MDA and Aβ1–42 levels in the hippocampus of Aβ1–42 injected mice. Values are mean ± SD (n=6). ##p< 0.01 compared with control group; *p<0.05 and **p<0.01 compared with Aβ1–42 group.
Spinosin inhibited Aβ1–42 accumulation in the hippocampus of mice with Aβ1–42 injection
The Aβ1–42 levels in hippocampus homogenate were evaluated, and the results showed that ICV injection of Aβ1–42 significantly increased the Aβ1–42 level in the hippocampus of mice in model group (p<0.01). Compared with model group, the levels of Aβ1–42 were significantly decreased in the spinosin (10, 100 µg/kg) treatment groups (p<0.01) (Fig. 3B).
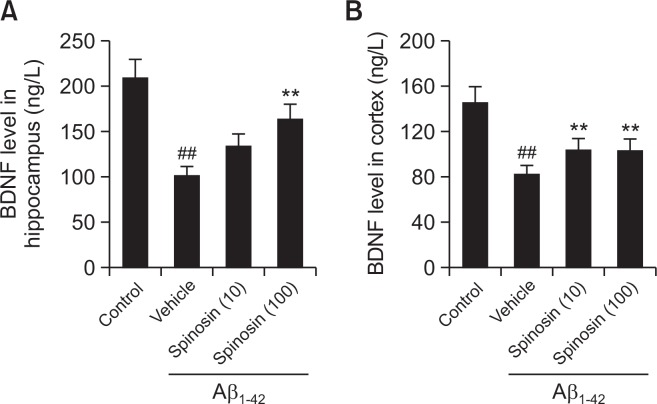
Spinosin restored the level of BDNF in the hippocampus and cerebral cortex of mice with Aβ1–42 injection
The level of BDNF was determined in the hippocampus and cerebral cortex of mice by ELISA. As shown in Fig. 4, the level of BDNF in both hippocampus and cortex of Aβ1–42 treated mice was lower than that of control mice (p<0.01), however, 100 µg/kg spinosin treatment significantly reversed BDNF level in both hippocampus and cortex (p<0.01), while ICV injection of low dose of spinosin (10 µg/kg) only increased BDNF expression in the cortex (p<0.01).
Fig. 4.
Effect of spinosin on the protein expression levels of BNDF in hippocampus (A) and cortex (B) of Aβ1–42 injected mice. Values are mean ± SD (n=6). ##p<0.01 compared with control group; **p<0.01 compared with Aβ1–42 group.
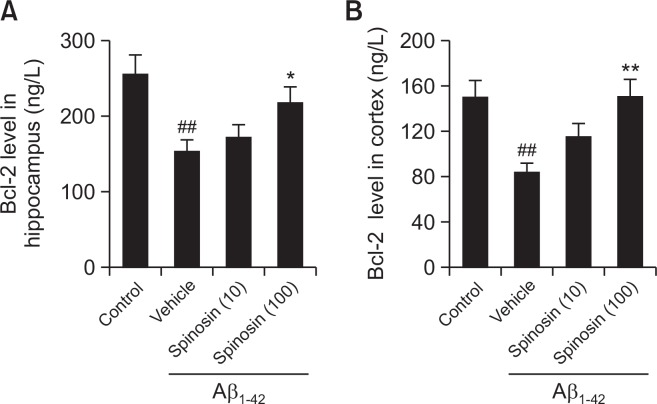
Spinosin reversed the level of Bcl-2 in the hippocampus and cerebral cortex of mice with Aβ1–42 injection
The anti-apoptotic effect of spinosin level of Bcl-2 was examined by ELISA. Compared with control group, a significant decrease in protein expression level of Bcl-2 was found in the hippocampus and cerebral cortex of the mice in model group with Aβ1–42 treatment (p<0.01). However, Bcl-2 was up-regulated significantly in both hippocampus and cerebral cortex following high dose rather than low dose of spinosin treatment compared with model group (Fig. 5), the results indicated that 100 µg/kg spinosin treatment could increase anti-apoptotic molecules level in the hippocampus and cerebral cortex area of Aβ1–42 injected mice.
Fig. 5.
Effect of spinosin on the protein expression levels of Bcl-2 in hippocampus (A) and cortex (B) of Aβ1–42 injected mice. Values are mean ± SEM (n=6). ##p<0.01 compared with control group; *p<0.05 compared with Aβ1–42 group; **p<0.01 compared with Aβ1–42 group.
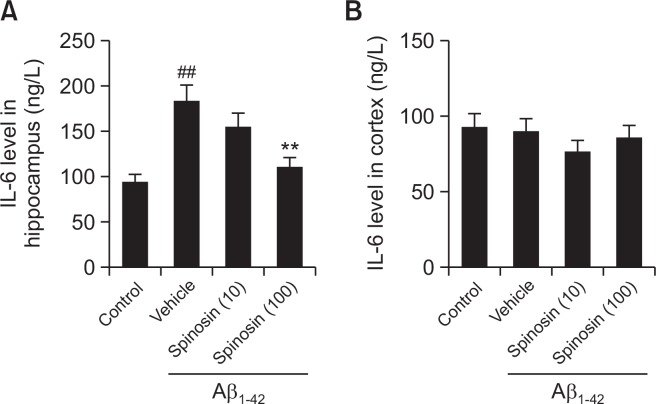
Spinosin decreased the level of IL-6 in the hippocampus of mice with Aβ1–42 injection
To confirm the effect of spinosin on inflammatory response, the level of IL-6 in different groups was examined. IL-6 level in hippocampus was significantly higher in Aβ1–42 injected mice than control mice (p<0.01), whereas IL-6 level was significantly decreased with spinosin (100 µg/kg) treatment (p<0.01). However, no difference was found among different groups for the level of IL-6 in the cerebral cortex (Fig. 6).
Fig. 6.
Effect of spinosin on the protein level of IL-6 in hippocampus (A) and cortex (B) of Aβ1–42 injected mice. Values are mean ± SD (n=6). ##p<0.01 compared with control group; **p<0.01 compared with Aβ1–42 group.
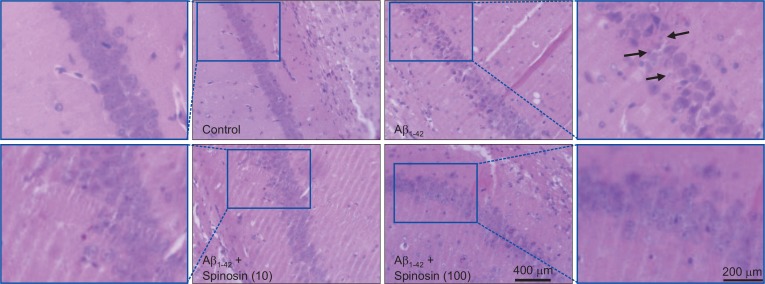
Histopathology findings
In the control group, the neurons in CA1 region of hippo-campus exhibited clear nuclei and intact neuronal bodies, while swollen and dispersed neuronal bodies were found in hippocampus of Aβ1–42 injected mice. After low or high dose spinosin treatment, the morphological aspects of neurons were recovered significantly compared with the model group (Fig. 7).
Fig. 7.
Histopathological changes of neurons in hippocampus of mice after Aβ1–42 injection. Representative images of neurons in hippocampal CA1 region of mice from control, Aβ1–42, Aβ1–42+10 μg/kg spinosin, and Aβ1–42+100 μg/kg spinosin groups. The arrows indicated neuronal degeneration of hippocampal CA1 region.
DISCUSSION
AD is characterized by cognitive impairment and neurodegeneration (De Strooper and Karran, 2016), and Aβ peptide deposition in the brain has been recognized as a major event during the neuropathogenesis of AD (Ianiski et al., 2012). Oral administration of spinosin was shown to improve memory in Aβ- or scopolamine-treated and normal naïve mice with cognitive dysfunction by inhibiting the 5-hydroxytriptamine 1A (5-HT1A) receptor, increasing choline acetyltransferase expression, reducing the number of glial cell, up-regulating adult hippocampal neurogenesis, and activating the ERK-CREB-BDNF signaling pathway (Jung et al., 2014; Ko et al., 2015; Lee et al., 2016). In our study, spinosin was directly administrated into the brains of Aβ1–42 injected mice to avoid the possible metabolic effect after oral administration, and the other potential targets of spinosin for the treatment of AD were identified.
Morris water maze test was conducted to confirm the effectiveness of spinosin in control of cognitive impairment of mice with AD induced by Aβ1–42. The ICV administration of spinosin was shown to improve cognitive deficits in Aβ1–42 injected mice as evidenced by shorter escape latency and increased time spent in the target quadrant (Fig. 2). The results in the behavioral task indicated a neuroprotective effect of spinosin in the brain. In addition, the spontaneous locomotor activity reflected by exploratory behavior was not affected by Aβ1–42 or spinosin injection (Fig. 1).
Pathological conditions of AD are evidenced to link with oxidative stress, and the level of lipid peroxidation is considered as an important factor in AD (Gao et al., 2012). The level of MDA, a marker of lipid peroxidation index, was evaluated in hippocampus rather than cortex since a high level of MDA is induced in hippocampus after ICV injection of Aβ1–42 (Ziech et al., 2010; Wu et al., 2017). Our results showed that spinosin significantly attenuated the up-regulation of MDA in hippocampus induced by ICV injection of Aβ1–42. The evidence indicated that cognitive impairments improved by spinosin might be associated with its anti-oxidative effects (Fig. 3A).
As the increase of Aβ1–42 was considered as an index of neurotoxicity (Pauwels et al., 2012), and the accumulation of deposits of Aβ is one of the classical neuropathological hall-marks (Kowalska, 2004). The level of Aβ1–42 in hippocampus, the region received Aβ1–42 injection, was evaluated in the current study. Our study showed that ICV injection of Aβ1–42 significantly increased Aβ1–42 level in the hippocampus of mice in model group, while spinosin significantly decreased the Aβ1–42 level compared with model mice. These results indicated a potential improving effect of spinosin in AD via inhibition of Aβ1–42 accumulation (Fig. 3B).
The reports of association of neuroinflammation with AD pathology have led to increased interest in pursuing anti-inflammatory therapy for AD (Tuppo and Arias, 2005). Singh and Guthikonda (1997) compared plasma concentrations of IL-6, IL-12, IFN-7, and IFN-γ in AD patients with control subjects, and IL-6 was found to be selectively elevated in AD patients. Our results showed an increased level of IL-6 in hippocampus of mice after Aβ1–42 injection, while the administration of spinosin decreased IL-6 level in the hippocampus (Fig. 6). Accordantly, Ko et al. (2015) reported an anti-inflammatory activity of spinosin by reducing the number of activated microglia and astrocytes in mice with Aβ1–42 injection. Thus, the anti-amnesic activity of spinosin was, in part, mediated by inhibiting inflammation.
BDNF, a key molecule in the maintenance of synaptic plasticity and memory storage in the hippocampus, has been shown to protect neurons from a variety of brain diseases (Numakawa et al., 2010). Additionally, the neuroprotection by BDNF is partly mediated by the anti-apoptotic effect through the up-regulation of Bcl-2 (Almeida et al., 2005). In our study, spinosin was found to exert anti-apoptotic effect through the regulation of Bcl-2 (Fig. 5), and the results also demonstrated a neuroprotective effect of spinosin associated with the upregulation of BDNF in both hippocampus and cortex (Fig. 4), which is consistent with a previous study showing the activation of ERK-CREB-BDNF signaling pathway in the hippocampus after spinosin stimulation (Lee et al., 2016). Therefore, spinosin might protect the neurons by regulating the expression of BDNF as its potential target.
In conclusion, in addition to previously reported targets of spinosin, neuroprotective effects of spinosin were found through the regulation of oxidative stress, Aβ1–42 accumulation, as well as apoptotic program in brain. These findings suggest that spinosin may represent a potential therapeutic agent to improve cognitive function in patients with AD through the regulation of multi-targets.
Acknowledgments
This research was supported by National Natural Science Foundation of China (81573580; 81503229), China Postdoctoral Science Foundation (2017M621161), Foundation of Liaoning Education Committee (201610163L26), Doctoral Starting up Foundation of Liaoning Science and Technology Department (201601139), Key Laboratory of Polysaccharide Bioactivity Evaluation of TCM of Liaoning Province and Key Laboratory of Quality Control of TCM of Liaoning Province (17-137-1-00).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Ahmad M, Saleem S, Ahmad AS, Yousuf S, Ansari MA, Khan MB, Ishrat T, Chaturvedi RK, Agrawal AK, Islam F. Ginkgo biloba affords dose-dependent protection against 6-hydroxydopamine-induced Parkinsonism in rats: neurobehavioural, neurochemical and immunohistochemical evidences. J. Neurochem. 2005;93:94–104. doi: 10.1111/j.1471-4159.2005.03000.x. [DOI] [PubMed] [Google Scholar]
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Grãos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Stadtman ER. Protein oxidation processes in aging brain. Adv. Cell Aging Gerontol. 1997;2:161–191. doi: 10.1016/S1566-3124(08)60057-7. [DOI] [Google Scholar]
- Chu YF, Chang WH, Black RM, Liu JR, Sompol P, Chen Y. Crude caffeine reduces memory impairment and amyloid β1–42 levels in an Alzheimer’s mouse model. Food Chem. 2012;135:2095–2102. doi: 10.1016/j.foodchem.2012.04.148. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res. Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Karran E. The cellular phase of Alzheimer’s disease. Cell. 2016;164:603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- Drummond SPA, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology. 2001;25:S68–S73. doi: 10.1016/S0893-133X(01)00325-6. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn. Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Zhou H, Hong JS. NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol. Sci. 2012;33:295–303. doi: 10.1016/j.tips.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ramirez J, Wu J. Network-based biomarkers in Alzheimer’s disease: review and future directions. Front. Aging Neurosci. 2014;6:12. doi: 10.3389/fnagi.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Ianiski FR, Alves CB, Souza ACG, Pinton S, Roman SS, Rhoden CRB, Alves MP, Luchese C. Protective effect of meloxicam-loaded nanocapsules against amyloid-β peptide-induced damage in mice. Behav. Brain Res. 2012;230:100–107. doi: 10.1016/j.bbr.2012.01.055. [DOI] [PubMed] [Google Scholar]
- Jiang JG, Huang XJ, Chen J, Lin QS. Comparison of the sedative and hypnotic effects of flavonoids, saponins, and polysaccharides extracted from Semen Ziziphus jujube. Nat. Prod. Res. 2007;21:310–320. doi: 10.1080/14786410701192827. [DOI] [PubMed] [Google Scholar]
- Jung IH, Lee HE, Park SJ, Ahn YJ, Kwon G, Woo H, Lee SY, Kim JS, Jo YW, Jang DS, Kang SS, Ryu JH. Ameliorating effect of spinosin, a C-glycoside flavonoid, on scopolamine-induced memory impairment in mice. Pharmacol. Biochem. Behav. 2014;120:88–94. doi: 10.1016/j.pbb.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Ko SY, Lee HE, Park SJ, Jeon SJ, Kim B, Gao Q, Jang DS, Ryu JH. Spinosin, a C-glucosylflavone, from Zizyphus jujuba var. spinosa ameliorates Abeta1–42 oligomer-induced memory impairment in mice. Biomol. Ther. (Seoul) 2015;23:156–164. doi: 10.4062/biomolther.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska A. The beta-amyloid cascade hypothesis: a sequence of events leading to neurodegeneration in Alzheimer’s disease. Neurol. Neurochir. Pol. 2004;38:405–412. [PubMed] [Google Scholar]
- Lee Y, Jeon SJ, Lee HE, Jung IH, Jo YW, Lee S, Cheong JH, Jang DS, Ryu JH. Spinosin, a C-glycoside flavonoid, enhances cognitive performance and adult hippocampal neurogenesis in mice. Pharmacol. Biochem. Behav. 2016;145:9–16. doi: 10.1016/j.pbb.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Mao X, Liao Z, Guo L, Xu X, Wu B, Xu M, Zhao X, Bi K, Jia Y. Schisandrin C ameliorates learning and memory deficits by Abeta1–42-induced oxidative stress and neurotoxicity in mice. Phytother. Res. 2015;29:1373–1380. doi: 10.1002/ptr.5390. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Nagele RG, Wegiel J, Venkataraman V, Wang KC, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol. Aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010;25:237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-beta induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat. Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels K, Williams TL, Morris KL, Jonckheere W, Vandersteen A, Kelly G, Schymkowitz JS, Rousseau F, Pastore A, Serpell LC, Broersen K. Structural basis for increased toxicity of pathological aβ42: aβ40 ratios in Alzheimer disease. J. Biol. Chem. 2012;287:5650–5660. doi: 10.1074/jbc.M111.264473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin KH, Lee CK, Woo WS, Kang SS. Sedative action of spinosin. Arch. Pharm. Res. 1978;1:7–11. doi: 10.1007/BF02856299. [DOI] [Google Scholar]
- Singh VK, Guthikonda P. Circulating cytokines in Alzheimer’s disease. J. Psychiatr. Res. 1997;31:657–660. doi: 10.1016/S0022-3956(97)00023-X. [DOI] [PubMed] [Google Scholar]
- Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Wang LE, Bai YJ, Shi XR, Cui XY, Cui SY, Zhang F, Zhang QY, Zhao YY, Zhang YH. Spinosin, a Cglycoside flavonoid from semen Zizhiphi Spinozae, potentiated pentobarbital-induced sleep via the serotonergic system. Pharmacol. Biochem. Behav. 2008;90:399–403. doi: 10.1016/j.pbb.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Wang LE, Cui XY, Cui SY, Cao JX, Zhang J, Zhang YH, Zhang QY, Bai YJ, Zhao YY. Potentiating effect of spinosin, a C-glycoside flavonoid of Semen Ziziphi spinosae, on pentobarbital-induced sleep may be related to postsynaptic 5-HT1A receptors. Phytomedicine. 2010;17:404–409. doi: 10.1016/j.phymed.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Wu L, Tong T, Wan S, Yan T, Ren F, Bi K, Jia Y. Protective effects of puerarin against Aβ1–42-induced learning and memoryimpairments in mice. Planta Med. 2017;83:224–231. doi: 10.1055/s-0042-111521. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Jo SA. Mechanisms of Amyloid-β peptide clearance: potential therapeutic targets for Alzheimer’s disease. Biomol. Ther. (Seoul) 2012;20:245–255. doi: 10.4062/biomolther.2012.20.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZL, Xia Q, Liang FR, Tang YJ, Xu CL, Huang J, Zhao L, Zhang WZ, He JJ. Effects on the expression of GABAA receptor subunits by jujuboside A treatment in rat hippocampal neurons. J. Ethnopharmacol. 2010;128:419–423. doi: 10.1016/j.jep.2010.01.034. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhen YF, Pu-Bu-Ci-Ren, Song LG, Kong W. N., Shao T. M., Li X., Chai XQ. Salidroside attenuates beta amyloid-induced cognitive deficits via modulating oxidative stress and inflammatory mediators in rat hippocampus. Behav. Brain. Res. 2013;244:70–81. doi: 10.1016/j.bbr.2013.01.037. [DOI] [PubMed] [Google Scholar]
- Ziech D, Franco R, Georgakilas AG, Georgakila S, MalamouMitsi V, Schoneveld O, Pappa A, Panayiotidis MI. The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem. Biol. Interact. 2010;188:334–339. doi: 10.1016/j.cbi.2010.07.010. [DOI] [PubMed] [Google Scholar]