Supplemental digital content is available in the text.
Abstract
Background and Objectives
Pain scores are routinely reported in clinical practice, and we wanted to examine whether this routinely measured, patient-reported variable provides prognostic information, especially with regard to chronic opioid use, after taking preoperative and perioperative variables into account in a preoperative opioid user population.
Methods
In 32,874 preoperative opioid users undergoing primary total knee arthroplasty at Veterans Affairs hospitals between 2010 and 2015, we compared preoperative and perioperative characteristics in patients reporting lower versus higher acute pain (scores ≤4/10 vs >4/10 averaged over days 1–3). We calculated the propensity for lower acute pain based on all available data. After 1:1 propensity score matching, to identify similar patients differing only in acute pain, we contrasted rates of chronic significant opioid use (mean >30 mg/d in morphine equivalents) beyond postoperative month 3, discharge prescriptions, and changes in postoperative versus preoperative dose categories. Sensitivity analysis examined associations with dose escalation.
Results
Rates of chronic significant opioid use (21% overall) differed in patients with lower versus higher acute pain (36% vs 64% of the overall cohort). After propensity matching (total n = 20,926 patients) and adjusting for all significant factors, lower acute pain was associated with less chronic significant opioid use (rates 12% vs 16%), smaller discharge prescriptions (ie, supply <30 days and daily oral morphine equivalent <30 mg/d), and more reduction in dose, all P < 0.001. In sensitivity analysis, dose escalation was 15% less likely with lower acute pain (odds ratio, 0.85; 95% confidence interval, 0.80–0.91).
Conclusions
Acute pain predicts chronic opioid use. Prospective studies of efforts to reduce acute pain, in terms of long-term effects, are needed.
Pain scores measured on the 0- to 10-point numeric rating scale, despite limitations, are routinely documented as a vital sign in electronic medical records within and outside the Veterans Health Administration.1 Acute postoperative pain scores guide analgesia in the hospital and are also used as outcome measures in randomized trials around operations such as total knee arthroplasty (TKA).2,3 Because of prior studies looking at the association between high pain score reports or inadequately treated pain in the acute setting and its long-term negative consequences, there is a need for studies that directly examine this phenomenon using granular data from electronic medical records in a large at-risk population, particularly one already taking opioids prior to surgery.4–6 We hypothesize that acute pain scores, measured routinely on the 11-point numeric rating scale, have prognostic value independent of other preoperative and perioperative characteristics, leading us to the question of whether acute postoperative pain is a relevant target in actions to “turn the tide” against growing chronic postoperative opioid use.7,8
Millions of Americans undergo surgery annually, but perioperative and postoperative opioid prescribing is not addressed in the 2016 Centers for Disease Control and Prevention guidelines.9,10 Rates of chronic postoperative use are several folds higher in preoperative opioid users, especially veterans, when compared with the preoperatively opioid-naive and nonveteran populations.11–14 Public health implications are serious after TKA because preoperative use is common in this population, and the use of this procedure is growing rapidly.9,12–14 Therefore, we undertook this study to examine the association between acute postoperative pain and chronic opioid use in a large cohort of preoperatively opioid-using veterans undergoing TKA, contrasting significant opioid prescriptions beyond 3 months after discharge in patients with lower versus higher acute pain scores. Because it is ethically irresponsible to randomize patients to lower versus higher acute pain in a clinical trial, this observational study, controlling for significant sources of bias, provides valuable evidence to inform ongoing efforts to counter chronic postoperative opioid overuse.
METHODS
Approval was granted by the institutional review board at the Durham VA Healthcare System, which waived informed consent requirements because of the minimal risk of revealing personal health information in this retrospective study with results presented in aggregate.
Study Population
We identified patients who had undergone primary TKA within the Veterans Health Administration Healthcare System across 80 Veterans Affairs (VA) hospitals using Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) procedure codes and data extracted from the Veterans Health Administration's repository of electronic health records, the Corporate Data Warehouse (CDW).15 To focus on patients at greatest risk of chronic postoperative use, we excluded preoperatively opioid-naive patients and those who died before discharge.12–14 Thus, our study cohort consisted only of preoperative opioid users, which was the majority of patients (~73% of total), undergoing primary TKA at VA hospitals between January 1, 2010, and December 31, 2015. Baseline demographics, patient and facility characteristics, diagnoses, other treatments or procedures, medication use (including details of opioid prescriptions), pain scores before and after surgery, and several other factors were identified for each patient and used as follows.16
Definitions of Exposure, Outcome, and Covariates
All variables are organized temporally with relation to the time of surgery and are described in association with preoperative, in-hospital, at time of discharge, and out-of-hospital postoperative time frames. Acute pain scores were extracted from the “Vital Signs” domain in the CDW, where they are recorded on the 11-point numeric rating scale (as a value between 0 and 10). We examined the proportions of patients with mild (0–4/10), moderate (5–7/10), or severe (8–10/10) postoperative pain on each hospital day between days 0 and 7 (Fig. 1). because acute pain scores stabilized by day 3, we computed the average value of all available scores over hospital days 1 to 3. Patients with mean values of 4/10 or less versus greater than 4/10 were defined, respectively, as “exposed” to lower versus higher acute pain. In addition, we also extracted data on preoperative pain. A mean preoperative score was computed for each patient as the average of all scores recorded in outpatient settings over 90 days prior to admission. Because the location and nature of pain are not discernable from these scores, and back pain is the most common location associated with increased risk,11 we classified each patient as having back pain (vs not) based on ICD-9 diagnosis codes and treatments (epidural steroid injections in the neck or back) or imaging codes (magnetic resonance imaging scans of the spine) over the 2-year period before admission (Appendix 1, Supplemental Digital Content 1, http://links.lww.com/AAP/A258).
FIGURE 1.
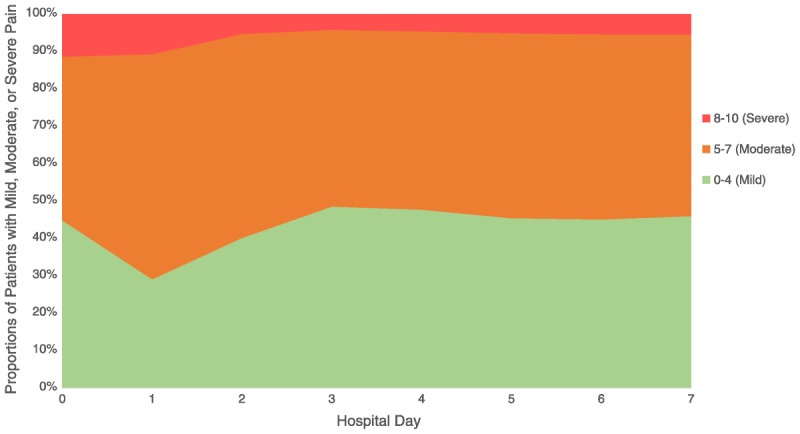
Proportions of patients with mild and moderate or severe postoperative pain on hospital days 0 to 7. Note increased pain from postoperative day (POD) 0 to POD1, possibly consistent with termination of analgesic effects of single-shot nerve blocks.
Outcomes
Chronic significant opioid use was defined based on data extracted from the VA Pharmacy Benefits Management program that oversees the national drug plan for eligible veterans, including outpatient pharmacies.17 In line with prior studies, we first excluded prescriptions in the first 3 months after discharge,7,11,12 defining chronic use based only on prescriptions filled beyond postoperative month 3. Rather than “present” or “absent,” chronic significant use was then defined by the mean daily dose in oral morphine equivalents (OMEs in milligrams per day measured using published dose conversion formulae; Appendix 2, Supplemental Digital Content 2, http://links.lww.com/AAP/A259), calculated by smoothing the total amount dispensed over the 1-year period between postoperative months 4 and 15 (total opioid amount = combination of strength and number of pills in all outpatient prescriptions). Informed by a recent study showing that doses of greater than 30 mg/d were associated with increased risk of overdose death among veterans,18 we categorized patients into groups of less than 5, 6 to 15, 16 to 30, and greater than 30 mg/d. Thus, the primary outcome was the proportion of patients with a mean chronic postoperative dose of greater than 30 mg/d (compared in groups with lower vs higher acute pain scores). Because risk of chronic use may be mediated by discharge prescriptions,19–21 which may in turn be related to acute postoperative pain scores, we identified the average daily amount (<30 or >30 mg/d) and days' supply (<30 or >30 days' supply) in the prescription filled around discharge. As noted previously, these were excluded from calculations of chronic dose. Change in dose category, computed based on differences between mean postoperative versus mean preoperative categories, were also identified as additional outcomes. Thus, we examined all outpatient prescriptions for each patient from 12 months before admission to 12 months beyond postoperative month 3, a 27-month period.
Similar to postoperative calculations, we also computed a mean preoperative dose for each patient by smoothing the total opioid amount from all prescriptions over the 1-year period before admission. We grouped patients into the same dose categories as postoperatively and examined changes as noted previously, classifying each patient as having a changed dose (escalated or decreased) or not. We classified patients as early versus late initiators based on when the first prescription was filled in the preoperative period. Late initiators were defined as those filling prescriptions in only the last 3 months prior to admission (opioid naive before then), whereas early initiators filled prescriptions before this. We also grouped patients based on whether tramadol was dispensed, because risks of this synthetic μ-receptor agonist may be distinct from other opioids.22 Unlike outpatient use, opioid amounts in the inpatient setting were not readily extractable. Bar-Code Medication Administration records were available, representing administration by a nurse in most inpatient locations, but documentation was variable (making data extraction impractical). Intraoperative and immediate postoperative amounts were not readily extractable either. We did identify each patient as having received a patient-controlled analgesia (PCA) pump in the VA hospital versus not.
Covariates
For each patient, we extracted data on characteristics shown to be associated chronic opioid use from various CDW domains,7,11,14 including age, sex, race, body mass index, and preoperative diagnoses (categories using a modified Charlson Comorbidity Index based on ICD-9 codes; Appendix 1, Supplemental Digital Content 1, http://links.lww.com/AAP/A258). Psychiatric illnesses including posttraumatic stress disorder were identified separately.12 Substance use disorder, smoking, and alcohol use were also identified separately.11–14 In addition to back pain and mean preoperative pain, we extracted data on outpatient visits to a pain clinic in the year before admission. For perioperative treatments, we identified the type of anesthesia (as general vs not) and noted whether nerve blocks were used.15 Perhaps because of the lack of financial incentive to code for nerve blocks within the VA system, these CPT codes were not well captured. To circumvent this lack of accurate CPT coding for nerve blocks, we also extracted data from inpatient pharmacy and order set domains (showing that local anesthetic infusions had been ordered and dispensed during hospitalization). This captures continuous infusion but does not identify single injections (local anesthesia for single-shot nerve blocks is not ordered by clinicians and “dispensed” by pharmacy). Finally, to account for potential effects of other facility-level and surgeon-level characteristics, we identified the geographic location and the annual TKA volume tercile.23
Descriptive, Multivariable, and Sensitivity Analyses
We examined preoperative and perioperative attributes in the overall cohort (Fig. 2). “Raw” postoperative outcomes, prior to adjusting for differences in preoperative and perioperative attributes, were also examined (Appendix 3, Supplemental Digital Content 3, http://links.lww.com/AAP/A260). Then we compared these in the “exposure” groups: patients with lower versus higher acute pain, using standard statistical tests (analysis of variance, t tests, and χ2 tests with 2-tailed P values, threshold for significance set at <0.05; Table 1). We used multivariable logistic regression analysis to examine the propensity for (odds of) lower acute pain based on available preoperative and perioperative characteristics (Appendix 4, Supplemental Digital Content 4, http://links.lww.com/AAP/A261). Model parameters were examined with goodness of fit and area under receiver operating characteristic curve statistics (Appendix 5, Supplemental Digital Content 5, http://links.lww.com/AAP/A262) and then applied to the entire cohort to calculate propensity scores for each patient. Thus, for each patient, we computed the probability of lower acute pain given their specific preoperative and perioperative characteristics. Then we used these propensity scores to adjust for baseline differences.24 Using a 1:1 pairwise matching algorithm (greedy match without replacement approach), we selected patients with similar propensity scores but differing acute pain. This identified patients with comparable probabilities of acute pain (ie, similar preoperative and perioperative characteristics, but with lower vs higher acute pain scores). We reexamined preoperative and perioperative attributes in the propensity score–matched cohort to confirm that all significant baseline differences had been eliminated by the matching algorithm, allowing the comparison of outcomes to be reflective of differences in acute pain scores (Fig. 2).24 We compared chronic significant postoperative opioid use, the opioid prescription at discharge, change in dose category, and length of stay during index hospitalization. In sensitivity analysis (Appendix 6, Supplemental Digital Content 6, http://links.lww.com/AAP/A263), we examined—using a separate multivariable logistic regression analysis—factors associated with an increase in postoperative dose category. In other words, after grouping each patient based on whether the postoperative dose category was higher than the preoperative dose category (escalation of dose) versus not (no change or decrease in dose), we asked what factors are associated with escalation. All analyses were conducted using SQL Server Management Studio (version 2014; Microsoft Corp, Redmond, Washington) and StataMP (version 11; StataCorp, College Station, Texas) in the secure VA informatics and computing infrastructure.
FIGURE 2.
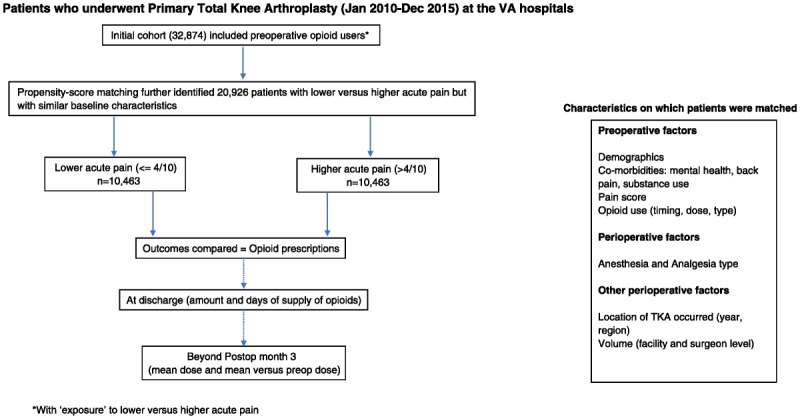
Study design with initial comparison between the 2 exposure groups followed by outcomes examined in propensity score–matched patients.
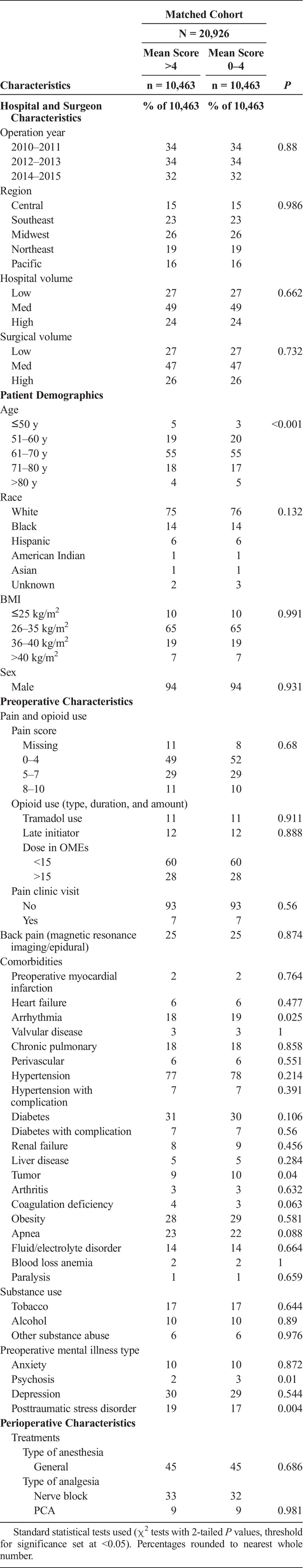
TABLE 1.
Preoperative Characteristics and Perioperative Treatments in Patients With Lower Versus Higher Acute Pain Scores After Propensity Score Matching
RESULTS
Our study cohort of 32,874 veterans (Fig. 2) had 11,747 patients (36%) with lower acute pain scores (≤4/10) and 21,127 patients (64%) with higher acute pain scores (>4/10). Chronic significant postoperative opioid use (mean dose, >30 mg/d) was present in 21% of the overall cohort (n = 6856). Opioid prescriptions at discharge exceeded 30 days in more than half of all patients and exceeded 30 mg/d in approximately 3 of 5 patients. Overall, comparing postoperative versus preoperative categories, a majority of patients (54%) had no significant change, a modest percentage (28% [n = 9170]) decreased dose, whereas a smaller percentage (18% [n = 6034]) escalated dose. Comparing baseline characteristics in the 2 exposure groups (Appendix 7, Supplemental Digital Content 7, http://links.lww.com/AAP/A264), the following differences were notable. Patients with lower acute pain were older, had surgery performed in earlier years (2010–2011 vs 2014–2015), had significantly lower preoperative pain, had less preoperative opioids (both dose and duration of use) and more tramadol use, were less likely to visit pain clinics, and had significantly lower rates of back pain, substance abuse, tobacco or alcohol abuse, and lower rates of psychiatric illness. Percentage of patients with chronic significant postoperative opioid use was 11% versus 26%, respectively, with lower versus higher acute pain. Opioid prescriptions at discharge exceeded 30 days and 30 mg/d in well under half of the patients with lower pain scores (Appendix 3, Supplemental Digital Content 3, http://links.lww.com/AAP/A260).
In the propensity score model (Appendix 4, Supplemental Digital Content 4, http://links.lww.com/AAP/A261), multivariable logistic regression showed that lower acute pain was more likely with later preoperative initiation of opioids, use of tramadol, Southeast or Central US regions (vs the Pacific), Hispanic race, age older than 60 years, obesity, TKA in facilities in the lower (vs intermediate) volume tercile or by surgeons in the lower or higher (vs intermediate) volume tercile, receipt of PCA, and nerve blocks. Conversely, lower acute pain was less likely with higher preoperative opioid doses (>15 mg/d over the year before admission), greater preoperative pain, TKA in later years (2012–2013 or 2014–2015 vs 2010–2011) or in the Midwest (vs the Pacific), black race, chronic pulmonary disease, tobacco or substance abuse, psychiatric illnesses, back pain, or receipt of general anesthesia. Model parameters were good (area under receiver operating characteristic curve ~71% and satisfactory goodness of fit, Appendix 5, Supplemental Digital Content 5, http://links.lww.com/AAP/A262), so we used the model to compute propensity scores for each patient. Matching patients with a 1:1 greedy match algorithm, we identified 10,463 pairs of patients for inclusion in the propensity-matched group (Fig. 2). Reexamining baseline differences in this propensity-matched cohort (Table 1), in nearly two-thirds of the study cohort (20,926 of 32,874 patients), we saw that all clinically significant differences were removed. In other words, propensity matching effectively allowed the comparison of postoperative outcomes to be reflective of differences in acute pain scores (Fig. 2).
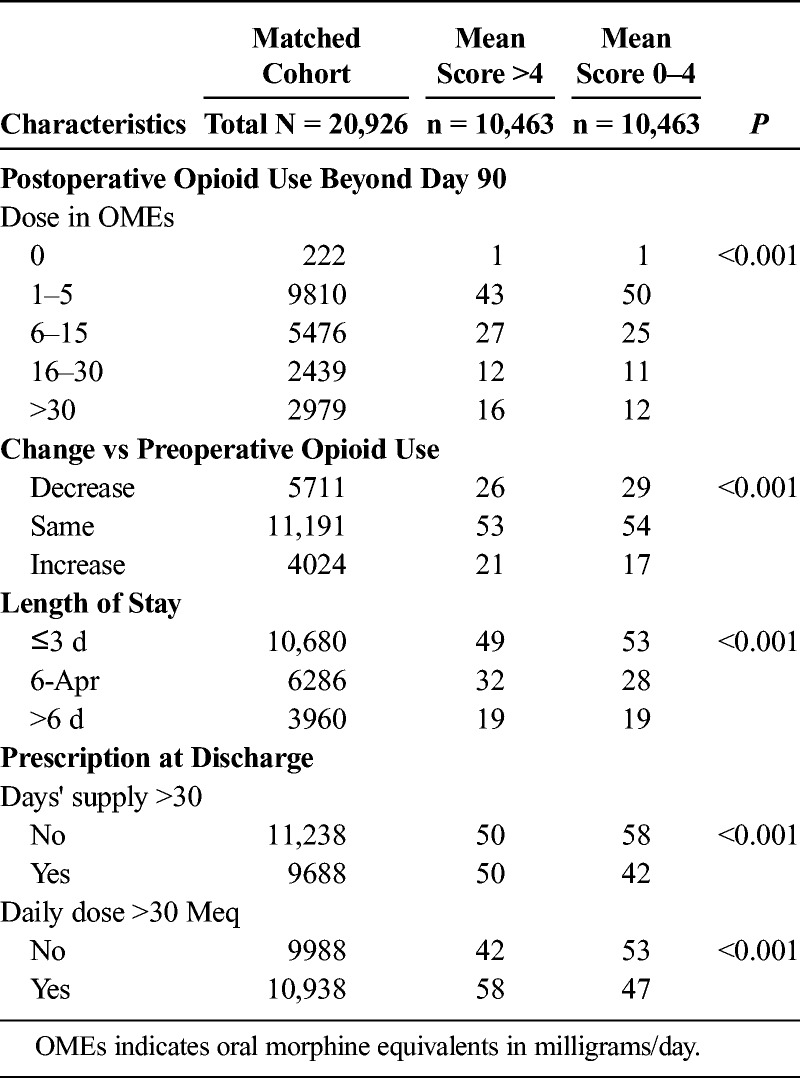
In the large propensity-matched cohort (Fig. 2 and Table 2), we found that the percentage of patients with chronic significant postoperative opioid use (mean dose, >30 mg/d),18 was 12% in those with lower acute pain scores versus 16% in those with higher scores, P < 0.001. Discharge prescriptions at more than 30 days and greater than 30 mg/d were filled by 50% and 58% of patients with higher pain scores (vs 42% and 47%, respectively, by patients with lower pain scores, P < 0.001). In terms of change in dose categories, mean postoperative opioid use increased in 17% of patients with lower acute pain versus in 21% of patients with higher acute pain, P < 0.001. Conversely, a larger percentage of patients with lower acute pain scores, 29%, decreased postoperative (vs preoperative) dose when compared with patients with higher acute pain (26%), P < 0.001. Taken together, the overall difference in change in chronic opioid use in absolute terms was 7% (4% difference in dose escalation plus a 3% difference in dose decrease). In terms of the numbers needed to treat, this meant that for every 15 patients reporting lower versus higher acute pain there was 1 more patient with a desirable change in chronic postoperative opioid use. Lengths of stay were longer than 3 days in 51% of patients with higher scores and 47% of patients with lower scores, P < 0.001. Sensitivity analysis (Appendix 6, Supplemental Digital Content 6, http://links.lww.com/AAP/A263) showed that acute pain scores reduced the odds of an increase in postoperative dose category by 15% (odds ratio, 0.85; 95% confidence interval [CI] 0.80–0.91), independent of all other factors including discharge prescriptions. A larger daily dose (>30 mg/d) in the discharge prescription was more important (odds ratio, 1.68; 95% CI, 1.55–1.81) than supply for more days (>30 days; odds ratio, 1.25; 95% CI, 1.16–1.35).
TABLE 2.
Outcomes in the Propensity Score–Matched Cohort
DISCUSSION
We examined the association between acute postoperative pain and chronic opioid use in a large population at significant risk, preoperatively opioid-using veterans undergoing TKA. Prior studies have looked at opioid-naive and nonnaive patient populations undergoing surgery and its direct correlation with chronic postoperative opioid use and found much higher incidences of persistent opioid use in those patients taking opioids prior to surgery.11,14 Given these prior findings, we focused on preoperative opioid users because the study outcome of chronic postoperative opioid use is more common in this population. Hence, the effects of variations in acute pain can be better examined. Comparing rates of chronic use beyond 3 months after discharge, at mean doses exceeding 30 mg/d (risk of overdose death in veterans),18 in patients with lower versus higher acute postoperative pain scores, we found that patients with lower acute pain had significantly less chronic opioid use when compared with similar patients who had higher acute pain scores. Furthermore, postoperative dose categories were more likely to have decreased and less likely to have increased (vs preoperative categories) in patients with lower acute pain scores. The discharge prescription was also significantly smaller with lower acute pain scores, but the association between acute pain and chronic opioid use was independent of the duration and dose of the discharge prescription.
Particularly important is our finding that patients with lower acute pain scores (≤4/10) had a rate of 23% versus 28% in patients with higher acute pain scores (>4/10), for the receipt of a chronic postoperative opioid dose of greater than 15 mg/d in OMEs beyond 3 months after discharge. Amounts less than this are associated with a low risk of adverse events.18 This 5% difference in absolute terms translates to a number needed to treat of 20 patients. In other words, for every 20 patients who has an acute pain score of greater than 4 (rather than ≤4) on the 11-point scale, 1 more patient will go on chronic opioid use at a dose of greater than 15 mg/d. Given the volume of TKAs, the population-level implications of this difference are significant.
These findings are consistent with previous reports11–14,25 and to the best of our knowledge are the first to specifically identify intensity of acute pain as a potentially modifiable risk factor in ongoing efforts to reduce chronic opioid use.7 This is notable for several reasons. We studied patients undergoing TKA: the leading elective inpatient operative procedure.9,13,14 We examined a hypothesis that cannot be directly tested in randomized trials, using rigorous propensity score–based methods to minimize baseline differences before comparing chronic opioid use in large groups of patients with differing acute pain scores. Thus, observed associations are less likely to be an epiphenomenon. Lastly, the favorable exposure—lower acute postoperative pain scores (on average ≤4/10)—is not common. Lower scores were present in only 36% of all patients and declined recently (ie, a smaller proportion reported lower acute pain scores in 2014–2015 vs 2010–2011).
But how can lower acute pain (over 3 days in the hospital) have an effect on chronic opioid use? First, discharge prescriptions are a key pathway. We found that more patients with higher acute pain scores had longer and larger discharge prescriptions (Table 2). It is intuitive that surgeons might be guided by proximal pain scores and opioid use.26,27 However, the link between postoperative prescriptions and chronic use has been recognized only recently.19–21,26 Thus, there may be an opportunity to reduce variations in the dose and duration of discharge prescriptions after surgery—by lowering acute pain.27 Second, the association between acute pain and chronic pain may explain increased chronic opioid use.28 Complex mechanistic pathways, operating in both the peripheral and central nervous systems, have been described in the transition from acute to chronic pain.28 We also found that association (between acute pain and chronic opioid use) to be independent of discharge prescriptions (Appendix 6, Supplemental Digital Content 6, http://links.lww.com/AAP/A263). Hence, lowering acute pain scores may reduce long-term opioid use independent of discharge prescriptions.28 Third, scores reported in the hospital after surgery may represent pain at operative and nonoperative sites.29 Over a third of the propensity-matched patients received continuous nerve blocks (Table 1), but this may not address pain outside the specific areas “blocked” by local anesthetics. Back pain was prevalent in 25% of our cohort (based on ICD diagnosis codes or preoperative treatment or imaging codes) and, when managed with opioids, may predispose to long-term use with increased rates of addiction and loss of efficacy secondary to the emergence of opioid tolerance and opioid-induced hyperalgesia.29 The presence of chronic pain at another site may reconcile apparent differences between our findings and reports showing that nerve blocks did not lower chronic opioid use.30
Our study has several strengths in addition to those described previously. We also found that late initiation of opioids before surgery (in the last 3 preoperative months rather than earlier), lower preoperative opioid doses (<15 mg/d), and use of tramadol increased the likelihood of lower acute pain scores (Appendix 4, Supplemental Digital Content 4, http://links.lww.com/AAP/A261). Nerve blocks had modest benefit on long-term opioid-sparing effects, but consistent with known effects of local anesthetic infusions (vs single injections),30 we observed an increase in acute pain from day 0 to day 1 (Fig. 1), suggesting that nerve blocks when used were likely to be for short durations in our study. Our study also has several limitations. As with a majority of studies pertaining to the veteran population, this study cohort is dominated by a male majority (>90%), so generalizability to a more equal sex-distributed population is limited. Pain scores, as a single number between 0 and 10, do not reflect the biopsychosocial complexity of pain. Unmeasured confounding cannot be ruled out, and an independent factor, such as less pain catastrophizing, may lower both acute pain and chronic opioid use, creating the appearance of an association where none exists. Another limitation is the use of pain scores that simply represent general intensity of pain by patient report on an 11-point scale in the inpatient and outpatient setting without granular information with respect to activity, site, nature, character, and other attributes of pain. Finally, we cannot make causal inferences, and generalizability to non-VA settings may be limited.
IMPLICATIONS AND CONCLUSIONS
We conclude that in a population exposed to preoperative opioid use acute postoperative pain was associated with chronic opioid use after TKA, independent of discharge prescriptions. Scores reported by patients in the first few days after surgery can thus have prognostic value. Whether interventions to reduce acute pain will be effective in reducing chronic postoperative opioid overuse is unclear because confounding cannot be completely ruled out in any observational study. However, our finding provides reasonable support for aggressive efforts to reduce acute postoperative pain. Future prospective studies should look at comprehensive strategies to address both operative and nonoperative pain in ways that may mitigate the long-term use of opioids.
Supplementary Material
ACKNOWLEDGMENTS
The following individuals contributed to the initial conception, and to certain aspects of data interpretation, in this study: Alan R. Ellis, PhD, assistant professor, Department of Social Work, North Carolina State University, Raleigh, NC; Atilio Barbeito, MD, MPH, and Rebecca Schroeder, MD, MMCi, Department of Anesthesiology, and Juliann Hobbs, MD, MPH, Department of Anesthesiology, Duke University Medical Center/Durham VAMC, Durham, NC; and Edward Mariano, MD, MAS (Clinical Research), Anesthesiology and Perioperative Care Service, VA Palo Alto Health Care System, and professor of Anesthesiology, Perioperative and Pain Medicine Stanford University School of Medicine, Palo Alto, CA.
Footnotes
This work was funded by the VA National Center for Patient Safety, Field Office 10A4E, through the Patient Safety Center of Inquiry at the Durham VA Medical Center.
The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.rapm.org).
REFERENCES
- 1.Lorenz KA, Sherbourne CD, Shugarman LR, et al. How reliable is pain as the fifth vital sign? J Am Board Fam Med. 2009;22:291–298. [DOI] [PubMed] [Google Scholar]
- 2.Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126:923–937. [DOI] [PubMed] [Google Scholar]
- 3.Karlsen AP, Wetterslev M, Hansen SE, Hansen MS, Mathiesen O, Dahl JB. Postoperative pain treatment after total knee arthroplasty: a systematic review. PLoS One. 2017;12:e0173107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinatra R. Causes and consequences of inadequate management of acute pain. Pain Med. 2010;11:1859–1871. [DOI] [PubMed] [Google Scholar]
- 5.Lindberg MF, Miaskowski C, Rustøen T, et al. The impact of demographic, clinical, symptom and psychological characteristics on the trajectories of acute postoperative pain after total knee arthroplasty. Pain Med. 2017;18:124–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunwoody CJ, Krenzischek DA, Pasero C, Rathmell JP, Polomano RC. Assessment, physiological monitoring, and consequences of inadequately treated acute pain. J Perianesth Nurs. 2008;23:S15–S27. [DOI] [PubMed] [Google Scholar]
- 7.Murthy VH. Ending the opioid epidemic—a call to action. N Engl J Med. 2016;375:2413–2415. [DOI] [PubMed] [Google Scholar]
- 8.Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176:1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDermott KW, Sun R. Trends in hospital inpatient stays in the United States, 2005–2014. HCUP Stat Brief. 2017;225. [Google Scholar]
- 10.Dowell D, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1–49. [DOI] [PubMed] [Google Scholar]
- 11.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in us adults. JAMA Surg. 2017;152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mudumbai SC, Oliva EM, Lewis ET, et al. Time-to-cessation of postoperative opioids: a population-level analysis of the Veterans Affairs Health Care System. Pain Med. 2016;17:1732–1743. [DOI] [PubMed] [Google Scholar]
- 13.Bedard NA, Pugely AJ, Westermann RW, Duchman KR, Glass NA, Callaghan JJ. Opioid use after total knee arthroplasty: trends and risk factors for prolonged use. J Arthroplasty. 2017;32:2390–2394. [DOI] [PubMed] [Google Scholar]
- 14.Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Memtsoudis SG, Poeran J, Cozowicz C, Zubizarreta N, Ozbek U, Mazumdar M. The impact of peripheral nerve blocks on perioperative outcome in hip and knee arthroplasty—a population-based study. Pain. 2016;157:2341–2349. [DOI] [PubMed] [Google Scholar]
- 16.Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47:739–750. [DOI] [PubMed] [Google Scholar]
- 17.Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements. 2004–2014. J Manag Care Spec Pharm. 2016;22:1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohnert AS, Logan JE, Ganoczy D, Dowell D. A detailed exploration into the association of prescribed opioid dosage and overdose deaths among patients with chronic pain. Med Care. 2016;54:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill MV, Stucke RS, Billmeier SE, Kelly JL, Barth RJ., Jr Guideline for discharge opioid prescriptions after inpatient general surgical procedures. J Am Coll Surg. 2017. [DOI] [PubMed] [Google Scholar]
- 20.Scully RE, Schoenfeld AJ, Jiang W, et al. Defining optimal length of opioid pain medication prescription after common surgical procedures. JAMA Surg. 2018;153:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babalonis S, Lofwall MR, Nuzzo PA, Siegel AJ, Walsh SL. Abuse liability and reinforcing efficacy of oral tramadol in humans. Drug Alcohol Depend. 2013;129:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald DC, Carlson K, Izrael D. Geographic variation in opioid prescribing in the U.S. J Pain. 2012;13:988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stürmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med. 2014;275:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll I, Barelka P, Wang CK, et al. A pilot cohort study of the determinants of longitudinal opioid use after surgery. Anesth Analg. 2012;115:694–702. [DOI] [PubMed] [Google Scholar]
- 26.Hill MV, McMahon ML, Stucke RS, Barth RJ., Jr Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265:709–714. [DOI] [PubMed] [Google Scholar]
- 27.Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth. 2010;105:i69–i85. [DOI] [PubMed] [Google Scholar]
- 28.Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun EC, Bateman BT, Memtsoudis SG, Neuman MD, Mariano ER, Baker LC. Lack of association between the use of nerve blockade and the risk of postoperative chronic opioid use among patients undergoing total knee arthroplasty: evidence from the Marketscan database. Anesth Analg. 2017;125:999–1007. [DOI] [PubMed] [Google Scholar]
- 30.Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102:248–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


