Abstract
Purpose/Background
Loss of gray matter after stroke has been associated with cognitive impairment. This pilot study aimed to investigate the therapeutic potential of lithium, a putative neurotrophic agent, in the stroke recovery process within a year of stroke occurrence.
Methods
Twelve stroke patients (mean ± SD age, 71.1 ± 11.9 years) were recruited to the study, and eligible participants were prescribed open-label lithium for 60 days. Magnetic resonance imaging was used to assess global gray matter at baseline and end of treatment; global cognition was assessed using the standardized Mini-Mental State Examination and Montreal Cognitive Assessment, and verbal memory was evaluated using the Hopkins Verbal Learning Test—Revised.
Findings/Results
There was no difference in global gray matter volume between baseline and follow-up (t = 1.977, P = 0.074). There was a significant interaction between higher lithium dose and increased global gray matter volume (F = 14.25, P = 0.004) and a correlation between higher lithium dose and improved verbal memory (r = 0.576, P = 0.05).
Implications/Conclusions
Lithium pharmacotherapy may be associated with gray matter volume change and verbal memory improvement in stroke patients, providing a rationale for future trials assessing therapeutic potential of lithium in a poststroke population.
Key Words: stroke, lithium, neuroimaging, gray matter volume, cognition
Approximately 795,000 strokes occur in the United States each year, and stroke remains a leading cause of death globally.1 Of the different types of stroke, ischemic stroke is by far the most common, accounting for 87% of stroke cases.1 Immediately after the ischemic event, excitotoxicity, oxidative stress, and inflammation all contribute to neuronal damage and atrophy.2 These apoptotic processes culminate in observable changes in gray matter volume after stroke.3 Diminishing gray matter volume is associated with cognitive impairment4 and notable in a large number of stroke survivors,5 and the presence of cerebral infarcts increases the risk of dementia.6 Although there is some spontaneous recovery after stroke,7 poststroke cognitive impairment does not improve in a large proportion of patients.8
Currently, pharmacotherapy after stroke is limited to acute thrombolysis9 and prophylaxis addressing underlying risk factors of stroke (eg, hypertension, dyslipidemia, and atrial fibrillation).1,10 Addressing cognitive and functional impairment has largely been left to rehabilitation programs. Although rehabilitation is associated with better functional outcomes, there is limited evidence on its benefits for cognition.11 Previously published guidelines have also highlighted the limited benefits of pharmacotherapies such as cholinesterase inhibitors for poststroke cognitive impairment.12 Therefore, it is prudent to develop new strategies to complement and strengthen current poststroke treatments.
Given that clinical outcomes after stroke are attributable to neuroanatomical changes,13 pharmacological agents that target neuroanatomical remodeling are a promising avenue of study. Lithium is recommended as a first-line option when it comes to prophylaxis of mood disorders.14 Lithium has been shown to protect against neuronal apoptosis, promote factors associated with neurogenesis, and increase global gray matter volumes, making it an attractive candidate to study in poststroke neuronal atrophy.15,16 Lithium therapy has also been reported to be associated with cognitive changes, including improvement in verbal memory.17–19
Our pilot study aimed to assess changes in gray matter volume and cognitive function in a lithium-treated poststroke population.
MATERIALS AND METHOD
Study Design
Men and women older than 40 years who met the World Health Organization MONICA Project and National Institute of Neurological Disorders and Stroke criteria for a recent (<1 year) ischemic cortical stroke (evidenced by magnetic resonance imaging [MRI] report from stroke neurologist) were recruited. All participants were required to speak and understand English. We excluded individuals who had evidence of hemorrhagic stroke, aphasia, significant acute medical illness, acute neurological illness, or psychiatric illnesses other than depression; individuals who met inclusion criteria but had contraindications to lithium treatment were enrolled in the study but were not initiated on lithium treatment. This study was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre, and we received written informed consent from all patients enrolled in the study; this study is registered on clinicaltrials.gov (NCT01112813).
Treatments and Monitoring
After obtaining informed consent from eligible patients, lithium carbonate (Carbolith; Valeant Canada Ltd, Montreal, Canada) was administered open label for 60 days, with target serum lithium concentrations of 0.4 to 0.8 mmol/L. However, participants who did not reach target serum lithium concentrations were not withdrawn from the study. Participants were monitored over the course of treatment of adverse events using the Udvalg for Kliniske Undersogelser Side Effects Rating Scale20; in the event of intolerable adverse events, lithium pharmacotherapy was discontinued, but participants were followed up.
Outcome Measures
Demographics
Demographic data, including age, sex, marital status, level of education, living situation, employment, and history of depression, were collected at screening. Date of stroke, current medications, National Institutes of Health stroke score (NIHSS), and medical history were extracted from patient charts once written informed consent was received from patients.
Global Gray Matter Volume
Enrolled patients underwent MRI at baseline and termination visits. Images were acquired using a 3.0-T General Electric MR750 scanner (GE Healthcare) with an 8-channel head coil. After a 3-plane localizer scan, T1-weighted images were obtained using a 3-dimensional fast spoiled gradient echo protocol (repetition time, 8.1 milliseconds; echo time, MinFull; flip angle, 8 degrees; field of view, 22 cm; slice thickness, 1.0 mm). T2/Proton density images were also obtained using a dual-echo 2-dimensional fast spin echo protocol (repetition time, 2500 milliseconds; echo time, 11.1 and 90 milliseconds; field of view, 22 cm; slice thickness, 3.0 mm). Using Semi-Automatic Brain Region Extraction,21 MRI images were assessed by research staff and focal stroke tracing was verified by an experienced neuroradiologist. Briefly, baseline masks for supratentorial intracranial vault and regional parcellation were coregistered to follow-up space to keep total global and regional brain volumes invariant, for better identification of subtle regional changes in relative gray matter, white matter, and stroke volume tissue segmentations.
Cognitive Function
Cognitive function was assessed at baseline and termination visits. We evaluated global cognition using the standardized Mini-Mental State Examination (sMMSE)22 and the Montreal Cognitive Assessment (MoCA).23 Cognitive domains were assessed using the National Institute of Neurological Disorders and Stroke–Canadian Stroke Network 30-minute neuropsychological protocol,24 specifically using the revised Hopkins Verbal Learning Test (HVLT-R) to assess verbal memory.25
Statistical Analyses
All data analyses were conducted using IBM SPSS Statistics 24. Using descriptive statistics, demographics such as age and sex were characterized; days since stroke, NIHSS score, stroke volume, sMMSE, and MoCA were also calculated for the entire group. Demographic data are presented as means or medians, as appropriate.
Lithium treatment was analyzed in 2 ways. Cumulative lithium dose (ie, sum of lithium doses across 60 days) was calculated for all participants. Because we followed up all patients, regardless of lithium treatment, we also stratified participants by average daily lithium dose. Because 300 to 600 mg of lithium per day is considered a therapeutic dose in patients with bipolar disorder,26 we selected 300 mg/d as the cutoff value between our 2 dose groups: less than 300 mg/d (low-dose group) and at least 300 mg/d (high-dose group). Demographic data between dose groups were compared using a 2-tailed independent t test for continuous variables and a 1-tailed Fisher exact test for discrete variables.
Change in global gray matter volume was determined using a 2-tailed paired t test comparing global gray matter volumes at baseline and termination. Because potential changes in global gray matter volume may be associated with lithium dose, correlational analysis was conducted on cumulative lithium dose and percent change in global gray matter volume using Pearson correlation. Furthermore, interaction between dose groups and change in global gray matter volume was assessed using a repeated-measures analysis of variance, with dose group as the between-subject factor; direction of the interaction was evaluated by comparing mean percent global gray matter change between dose groups using a 2-tailed independent t test. A repeated-measures analysis of covariance was conducted to adjust for effects of age and baseline cognition.
Changes in verbal memory were evaluated using z scores obtained from the delayed recall component of the HVLT-R. z Scores were compared between baseline and termination using a 2-tailed paired t test. The interaction between cumulative lithium dose changes in this score was analyzed using a repeated-measures analysis of covariance, with cumulative lithium dose as a covariate. Direction of interaction was evaluated using Pearson correlation. Furthermore, the interaction between dose group and change in this score was assessed using a repeated-measures analysis of variance comparing baseline and termination, with dose group as the between-subject factor.
RESULTS
Demographic Features
Twelve patients with ischemic stroke were recruited to the study. Participants had a mean age of 71.1 ± 11.9 years and were evenly split between men and women. Six participants had left hemispheric stroke, 2 had right hemispheric stroke, and 4 had bilateral stroke. On average, each participant was on 5.8 ± 2.3 medications and were 90.2 ± 65.3 days removed from their stroke at baseline. Median NIHSS score at enrollment was 1 (interquartile range [IQR], 0–1), and the average stroke volume at baseline was 6.2 ± 8.8 mL. Median sMMSE and MoCA scores were 28 (IQR, 25–29) and 22.5 (IQR, 16–25.5), respectively (Table 1). Baseline values were comparable between dose groups (<300 or ≥300 mg/d) for all demographic features except age (t = 2.45, P = 0.035) and baseline MoCA score (t = −4.33, P = 0.002), with patients in the higher-dose group scoring higher.
TABLE 1.
Gray Matter and Cognitive Outcomes, Baseline and Follow-Up
Of the 12 participants, 8 completed 60 days of lithium treatment, 3 participants discontinued pharmacotherapy early because of adverse events (2 cases of nausea/vomiting, 1 case of constipation), and 1 participant was excluded because of contraindication to lithium therapy (impaired renal function). On average, participants received 231.0 ± 150.5 mg of lithium per day and maintained an average serum lithium level of 0.275 ± 0.190 mM.
Global Gray Matter Volume and Cognitive Function
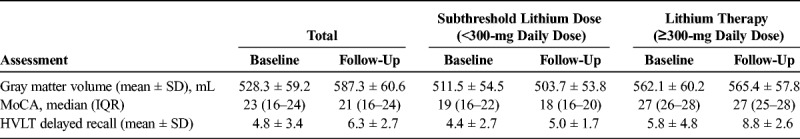
There was no significant difference in gray matter volumes between baseline (528.3 ± 59.2 mL) and termination (524.3 ± 60.6 mL; t = 1.977, P = 0.074). There was no correlation between cumulative lithium dose and percent change in gray matter volume (r = 0.447, P = 0.145). However, comparing change in global gray matter volume over time between dose groups, there was a significant interaction between time and dose group (F = 14.25, P = 0.004). This interaction remained significant after adjusting for age (F = 10.72, P = 0.01), baseline sMMSE (F = 21.66, P = 0.001), or MoCA scores (F = 16.13, P = 0.003). Mean percent change in global gray matter volume was significantly different between the 2 dose groups: at least 300 mg/d (0.63 ± 0.61) and less than 300 mg/d (−1.52 ± 1.03; t = −3.80, P = 0.003; Fig. 1).
FIGURE 1.
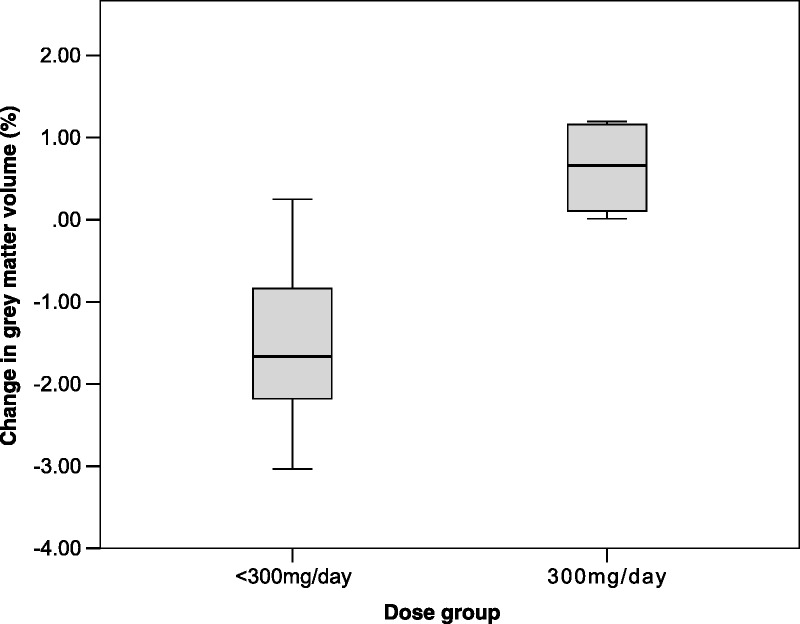
Percent change in global gray matter volume was significantly different between the 2 dose groups: at least 300 mg/d and less than 300 mg/d group (t = −3.80, P = 0.003).
There was no significant difference in sMMSE scores between baseline and termination (t = 0.252, P = 0.806), even after adjusting for cumulative lithium dose (F = 0.098, P = 0.760) or comparing between dose groups (F = 0.029, P = 0.868). Likewise, there was no significant difference in MoCA scores between baseline and termination (t = 0.688, P = 0.506), even after adjusting for cumulative lithium dose (F = 0.091, P = 0.769) or comparing between dose groups (F = 0.283, P = 0.606).
There was no significant difference in delayed recall scores between baseline and termination. However, higher cumulative lithium dose was associated with improvement in delayed recall (β = 0.58, P = 0.05), where cumulative lithium dose accounted for 27% of the variation in delayed recall change (adjusted R2 = 0.27, F = 4.97, P = 0.05; Fig. 2). There was no significant interaction between change in delayed recall score and dose group (F = 0.917, P = 0.361).
FIGURE 2.
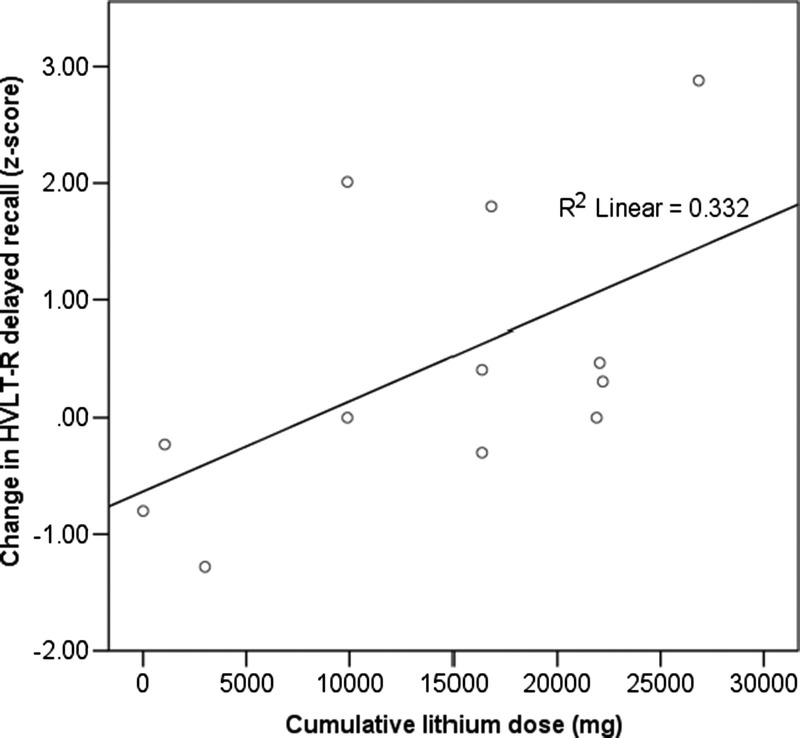
Greater cumulative lithium dose was positively correlated with a more positive change in delayed recall score (r = 0.576, P = 0.05).
DISCUSSION
This study provided preliminary data on the interactions between lithium and physiological and clinical outcomes in a poststroke population. There was an interaction between change in global gray matter and lithium dose group; patients who received the therapeutically significant daily dose of lithium (ie, 300 mg) showed positive change in global gray matter volume, and those who received less than the target daily dose showed negative change in global gray matter volume. Through clinical assessments, we identified a positive association between cumulative lithium dose and verbal memory. Findings from this study provided novel insight into the possible relationship between lithium and neuroanatomical and clinical changes in a poststroke population, which could be used to inform future studies on new pharmacotherapies for stroke recovery.
Our study also highlighted tolerability of lithium as a potential limitation for its therapeutic use in elderly stroke patients. Patients in the higher-dose group were significantly younger than those in the lower-dose group, suggesting lower tolerance in the elderly. Although most were mild, 3 patients were discontinued because of intolerable adverse events: 1 because of constipation and 2 because of nausea and vomiting. None of these were considered related to the lithium.
Given tolerability of lithium in our participants, the average serum lithium level (0.27 mM) was low and well below our target range (0.4–0.8 mM). This may limit observed effects over time in both neuroanatomical and clinical measures. However, previous studies have shown that prolonged exposure to even minute amounts of lithium may be neuroprotective,27–29 suggesting that greater length of exposure may be needed to detect an effect at lower dose. Furthermore, the sample size of this pilot study was small, limiting power: based on existing literature,16 26 patients would be needed to detect a change in global gray matter volume over time with 80% power. In addition, there may be mediating factors that affect the correlation of cumulative lithium dose and change in HVLT-delayed recall. A larger sample would also allow multiple covariates to be added to the analyses. Given that this was an exploratory study, we did not include a placebo control arm and lithium was prescribed open label, which can lead to bias and practice effect confounds. However, our data can be used to inform design of a larger randomized, double-blind placebo-control trial that would be required to validate the findings from this preliminary study. The lack of placebo arm also means that we cannot account for natural healing through the passage of time. However, based on the literature, this is unlikely to have been the case, for neither the gray matter volume finding nor the improvement in delayed recall. Yu et al30 found that gray matter volume decreased from the time of stroke to 6 months after stroke in most brain structures, and Cai et al31 found the same in patients 1 year after stroke. Similarly, studies found that natural improvement of cognition after stroke was rare.8,32
Considering the increase in stroke prevalence over time and impact of stroke on daily function, it is imperative to develop new strategies to improve poststroke outcomes. Our study suggested an interaction between change in global gray matter and lithium dose. Furthermore, we identified a positive association between lithium dose and verbal memory. Doses of at least 300 mg/d seemed to be required for these effects. Findings from this study provide a preliminary signal suggesting future studies assessing the therapeutic potential of lithium in a poststroke population and invite further research in this area.
AUTHOR DISCLOSURE INFORMATION
Yue Ran Sun, Christopher Scott, Richard Swartz, and Julia Hopyan report no disclosures.
Nathan Herrmann is supported by peer-reviewed grants from the Alzheimer Society of Canada, Canadian Institute of Health Research, Heart and Stroke Foundation, Brain Canada, Ontario Ministry of Health and Long Term Care AFP Provincial Innovation Fund, and Ontario Brain Institute. He has received consultation fees from Pfizer, Lilly, Merck, and Astellas.
Sandra E. Black is supported by peer-reviewed grants from the Canadian Institutes of Health Research, Heart and Stroke Foundation; Alzheimer's Drug Discovery Fund; Brain Canada Foundation; Ontario Brain Institute; and US National Institutes of Health. She is involved in contract research funded by Lilly-Avid, Biogen, and Genentech-Roche. She has been a continuing medical education speaker for Novartis and Lilly-Avid. She had personal support from the Brill Chair in Neurology and currently from the Department of Medicine, Sunnybrook Health Sciences Centre, Sunnybrook Research Institute, and Toronto Dementia Research Alliance.
Krista L. Lanctôt is supported by peer-reviewed grants from the Alzheimer Society of Canada, Alzheimer's Drug Discovery Foundation, Canadian Institute of Health Research, National Institute on Aging of the National Institutes of Health, Alzheimer's Association, and the Heart and Stroke Foundation in addition to research contracts funded by AbbVie Pharmaceuticals and consultation fees from AbbVie and Lundbeck-Otsuka.
Footnotes
Funding support was provided by Heart and Stroke Foundation (NA 7220).
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gold AB, Herrmann N, Lanctôt KL. Lithium and its neuroprotective and neurotrophic effects: potential treatment for post–ischemic stroke sequelae. Curr Drug Targets. 2011;12:243–255. [DOI] [PubMed] [Google Scholar]
- 3.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 4.Stebbins GT, Nyenhuis DL, Wang C, et al. Gray matter atrophy in patients with ischemic stroke with cognitive impairment. Stroke. 2008;39:785–793. [DOI] [PubMed] [Google Scholar]
- 5.Jin YP, Di Legge S, Ostbye T, et al. The reciprocal risks of stroke and cognitive impairment in an elderly population. Alzheimers Dement. 2006;2:171–178. [DOI] [PubMed] [Google Scholar]
- 6.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer SC, Riley JD. Neuroplasticity and brain repair after stroke. Curr Opin Neurol. 2008;21:76–82. [DOI] [PubMed] [Google Scholar]
- 8.Desmond DW, Moroney JT, Sano M, et al. Recovery of cognitive function after stroke. Stroke. 1996;27:1798–1803. [DOI] [PubMed] [Google Scholar]
- 9.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 10.Romero JR, Morris J, Pikula A. Stroke prevention: modifying risk factors. Ther Adv Cardiovasc Dis. 2008;2:287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.das Nair R, Cogger H, Worthington E, et al. Cognitive rehabilitation for memory deficits after stroke. Cochrane Database Syst Rev. 2016;9:CD002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eskes GA, Lanctot KL, Herrmann N, et al. Canadian Stroke Best Practice Recommendations: Mood, Cognition and Fatigue Following Stroke practice guidelines, update 2015. Int J Stroke. 2015;10:1130–1140. [DOI] [PubMed] [Google Scholar]
- 13.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. [DOI] [PubMed] [Google Scholar]
- 14.Coryell W. Maintenance treatment in bipolar disorder: a reassessment of lithium as the first choice. Bipolar Disord. 2009;11(suppl 2):77–83. [DOI] [PubMed] [Google Scholar]
- 15.Malhi GS, Tanious M, Das P, et al. Potential mechanisms of action of lithium in bipolar disorder. Current understanding. CNS Drugs. 2013;27:135–153. [DOI] [PubMed] [Google Scholar]
- 16.Moore GJ, Cortese BM, Glitz DA, et al. A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J Clin Psychiatry. 2009;70:699–705. [DOI] [PubMed] [Google Scholar]
- 17.Wingo AP, Wingo TS, Harvey PD, et al. Effects of lithium on cognitive performance: a meta-analysis. J Clin Psychiatry. 2009;70:1588–1597. [DOI] [PubMed] [Google Scholar]
- 18.Arts B, Jabben N, Krabbendam L, et al. A 2-year naturalistic study on cognitive functioning in bipolar disorder. Acta Psychiatr Scand. 2011;123:190–205. [DOI] [PubMed] [Google Scholar]
- 19.Kessing LV, Forman JL, Andersen PK. Does lithium protect against dementia? Bipolar Disords. 2010;12:87–94. [DOI] [PubMed] [Google Scholar]
- 20.Lingjaerde O, Ahlfors UG, Bech P, et al. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. [DOI] [PubMed] [Google Scholar]
- 21.Dade LA, Gao FQ, Kovacevic N, et al. Semiautomatic brain region extraction: a method of parcellating brain regions from structural magnetic resonance images. Neuroimage. 2004;22:1492–1502. [DOI] [PubMed] [Google Scholar]
- 22.Molloy DW, Standish TI. A guide to the standardized Mini-Mental State Examination. Int Psychogeriatr. 1997;9(suppl 1):87–94; discussion 143–150. [DOI] [PubMed] [Google Scholar]
- 23.Nasreddine ZS, Bédirian NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 24.Benedict RHB, Schretlen D, Groninger L, et al. Hopkins Verbal Learning Test—Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 25.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. [DOI] [PubMed] [Google Scholar]
- 26.Sproule BA, Hardy BG, Shulman KI. Differential pharmacokinetics of lithium in elderly patients. Drugs Aging. 2000;16:165–177. [DOI] [PubMed] [Google Scholar]
- 27.Nunes MA, Viel TA, Buck HS. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer's disease. Curr Alzheimer Res. 2013;10:104–107. [DOI] [PubMed] [Google Scholar]
- 28.Kessing LV, Gerds TA, Knudsen NN, et al. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiat. 2017;74:1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fajardo VA, Fajardo VA, LeBlanc PJ, et al. Examining the relationship between trace lithium in drinking water and the rising rates of age-adjusted Alzheimer's disease mortality in Texas. J Alzheimers Dis. 2018;61:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu X, Yang L, Song R, et al. Changes in structure and perfusion of grey matter tissues during recovery from ischaemic subcortical stroke: a longitudinal MRI study. Eur J Neurosci. 2017;46:2308–2314. [DOI] [PubMed] [Google Scholar]
- 31.Cai J, Ji Q, Xin R, et al. Contralesional cortical structural reorganization contributes to motor recovery after sub-cortical stroke: a longitudinal voxel-based morphometry study. Front Hum Neurosci. 2016;10:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochstenbach JB, den Otter R, Mulder TW. Cognitive recovery after stroke: a 2-year follow-up. Arch Phys Med Rehabil. 2003;84:1499–1504. [DOI] [PubMed] [Google Scholar]


