Abstract
Background
Successful pancreas transplantation requires surgical expertise and multidisciplinary medical management. The impact of transplant center volume on pancreas allograft survival remains unclear.
Methods
We examined Organ Procurement and Transplantation Network data on 11 568 simultaneous pancreas-kidney (SPK) and 4308 solitary pancreas (pancreas transplant alone and pancreas after kidney) transplants between 2000 and 2013.
Results
Average annual transplant center volume was categorized by tertiles into low, medium, and high volume, respectively, as follows: 1 to 6 (n = 3861), 7 to 13 (n = 3891), and 14 to 34 (n = 3888) for SPK, and 1 to 3 (n = 1417), 4 to 10 (n = 1518), and 11 to 33 (n = 1377) for solitary pancreas transplants. Favorable donor characteristics were seen in low-volume centers. For SPK transplantation, low (adjusted hazard ration [aHR], 1.55, 95% confidence interval [CI], 1.34-1.8) and medium (aHR, 1.24; 95% CI, 1.07-1.44) center volumes were associated with a higher risk of early pancreas graft failure at 3 months. The increased risk associated with low center volume extended to 1, 5, and 10 years. For solitary pancreas transplants, low, but not medium, center volume was associated with a higher risk of early pancreas graft failure at 3 months (aHR, 1.56; 95% CI, 1.232-1.976), and this risk persisted over 10 years. Patients transplanted at high-volume centers had better pancreas survival rates across all categories of the Pancreas Donor Risk Index.
Conclusion
On average, low center volume were associated with higher risk for pancreas failure. Future studies should seek to identify care processes that support optimal outcomes after pancreas transplantation irrespective of center volume.
Impact of transplant center volume on pancreas allograft survival is analyzed on 11 568 simultaneous pancreas-kidney and 4308 solitary pancreas transplants and patients transplanted at high volume centers had better pancreas survival rates across all categories of the pancreas donor risk index. Supplemental digital content is available in the text.
Pancreas transplantation is the only available treatment that reinstates normal glucose homeostasis long term without the risk of significant hypoglycemia or hyperglycemia.1,2 Receiving a pancreas allograft may prevent further diabetic complications and even reverse some of them.2-5 It has been well documented that simultaneous pancreas-kidney (SPK) transplantation provides better recipient and allograft survival for patients with type 1 diabetes mellitus and end-stage renal disease compared with deceased donor kidney transplant alone.6-8 In 1 report, patients with type 1 diabetes who received an SPK transplant had a significant survival advantage at 8-year follow-up compared with deceased donor kidney alone transplant recipients (72% vs 55%).9
Despite data to support improved survival with SPK transplantation in patients with type 1 diabetes and end-stage renal disease, there has been a decline in the number of SPK transplant procedures performed in recent years. The annual number of SPK transplants in the United States peaked in 1999 and has steadily decreased since this time. The total annual number of pancreas transplants (SPK and solitary pancreas, pancreas after kidney [PAK], pancreas transplant alone [PTA]) in the United States peaked at 1484 transplant in 2004 and has decreased by 36% to 944 in 2014, which is the lowest annual total since 1994.10 The cause for this decline is likely multifactorial, including lack of referral sources (especially for PTA), underrecognition of appropriate candidates for SPK transplantation, insufficient knowledge of the survival benefits of pancreas transplantation, and frequent decreased donor pancreas discards.11,12 Some centers are very selective when assessing pancreata for transplantation secondary to lower local demand and the regulatory requirements to maintain excellent outcomes. In addition to the importance of center-specific donor and recipient selection, other center factors, such as volume, management protocols, and follow-up, may influence clinical outcomes. Numerous studies in other organ transplants have demonstrated that centers performing relatively fewer solid organ transplants have inferior allograft outcomes, whereas conversely higher-volume centers are associated with improved survival outcomes (the so-called center effect). The center effect has been shown for lung, liver, heart and kidney transplantation.13-19 A remote study in the United States more than a decade ago suggested that low center volume (defined by < 10 pancreas transplants per year) was associated with reduced pancreas allograft survival compared to higher-volume centers.20 Since then, there has been no large scale analysis of the effect of center volume on pancreas transplant outcomes in the United States.21 The purpose of this study was to determine the association of center volume with pancreas allograft outcomes in the modern era including consideration of the Pancreas Donor Risk Index (PDRI).
MATERIALS AND METHODS
Study Population
The study cohort was composed of all adult patients who received SPK, PTA, and PAK transplants between January 1, 2000, and December 31, 2013, based on the Organ Procurement and Transplantation Network (OPTN) database, as previously described.22 Average annual transplant center volume of SPK was categorized using tertiles into low-volume, medium-volume, and high-volume centers as follows: 1 to 6 (n = 3861), 7 to 13 (n = 3891), and 14 to 34 (n = 3888). Similarly, the average annual transplant center volume of PAK or PTA transplants was categorized using tertiles into low-volume, medium-volume, and high-volume centers as follows: 1 to 3 (n = 1417), 4 to 10 (n = 1518), and 11 to 33 (n = 1377).
Outcome and Covariate Definitions
Pancreas allograft failure was defined as allograft loss (as reported to the OPTN) or patient death. Patient death was included as allograft loss regardless of the functional status of the pancreas allograft at the time of death. Time to allograft failure was considered from the time of transplantation to the time of reported graft loss or patient death.
Peak panel reactive antibody (PRA) level was calculated based on the higher level of class 1 or class 2 antibodies before transplantation. Preservation time was defined as the sum of cold ischemia time and recipient warm ischemia time. Pancreas Donor Risk Index was calculated using 10 donor factors and 1 transplant factor including donor age, sex, race, body mass index (BMI), height, cause of death, preservation time, donation after cardiac death, terminal creatinine, and cold ischemia time as previously described.22,23 The PDRI was categorized in the SPK setting into 5 groups: 0.85 or less, 0.86 to 1.16, 1.16 to 1.56, 1.57 to 2.11, and ≥ 2.12, in a similar manner to Axelrod et al.23 PDRI was categorized in the solitary pancreas (PAK and PTA) transplant setting into 4 categories: 0.85 or less, 0.86 to 1.16, 1.16 to 1.56, and ≥ 1.57 in a similar manner to Axelrod et al.23
Statistical Methods
Patient characteristics were described using proportions for categorical variables and means and standard deviations for continuous variables. Group differences were compared with the χ2 test for categorical variables and analysis of variance test or Kruskal Wallis tests for continuous variables, depending on the distribution of the variable. Multivariate Cox regression analyses were used to assess the independent association of center volume categories with pancreas allograft failure after adjustment for all recipient and donor characteristics including donor and recipient age, race and sex, recipient BMI and sex, pretransplant dialysis, HLA mismatching, level of sensitization, donor hypertension and cause of death. Separated modules were done at each time point (3 months, 1, 5, and 10 years) in SPK and solitary pancreas transplant. Variation in pancreas allograft survival at 1 year posttransplantation according to center volume, within each PDRI category, was assessed by the Kaplan-Meier method. Pancreas allograft survival at 1 year was also reported in each individual center by the Kaplan-Meier method. A P value less than 0.05 was considered significant for all tests. All analyses were performed using SAS 9.4 software (Cary, NC).
RESULTS
A total of 11 568 SPK and 4308 PAK and PTA transplants were performed between 2000 and 2013. The majority of transplant centers (n = 112, 71%) were categorized in the low-volume group based on performance of an average of 1 to 6 SPK procedures per year. There were 31 transplant centers (20%) in the medium-volume center group (performing 7 to 13 SPK transplants per year), and 15 transplant centers (9%) in the high-volume center group (performing 14 to 34 SPK cases per year). For PTA and PAK, 117 transplant centers (80%) were categorized in the low-volume group based on performance of 1 to 3 cases per year; 25 transplant centers (17%) in the medium-volume center (4 to 10 cases per year), and 5 transplant high-volume centers (3%) (11 to 33 cases per year).
There were no significant differences in recipient sex or BMI (P = 0.12 for SPK; P = 0.24 for PAK and PTA) between groups categorized by center volume (Table 1). Compared with transplants at high-volume centers, transplants at low-volume centers had younger donors; fewer white, female and hypertensive donors; and shorter pancreas preservation times. Transplants at low-volume centers had more HLA mismatches for PAK and PTA but not for SPK transplant procedures. Low-volume centers had longer wait times and transplanted more African-American and Hispanic recipients among all types of pancreas transplants. Low-volume centers had fewer nonsensitized recipients and transplanted patients with longer dialysis vintage in the SPK transplant category only. Finally, low-volume centers transplanted a greater number of low PDRI pancreata compared to high-volume centers.
TABLE 1.
Recipient, donor, and transplant characteristics among SPK transplant and pancreas alone recipients stratified according to center volume
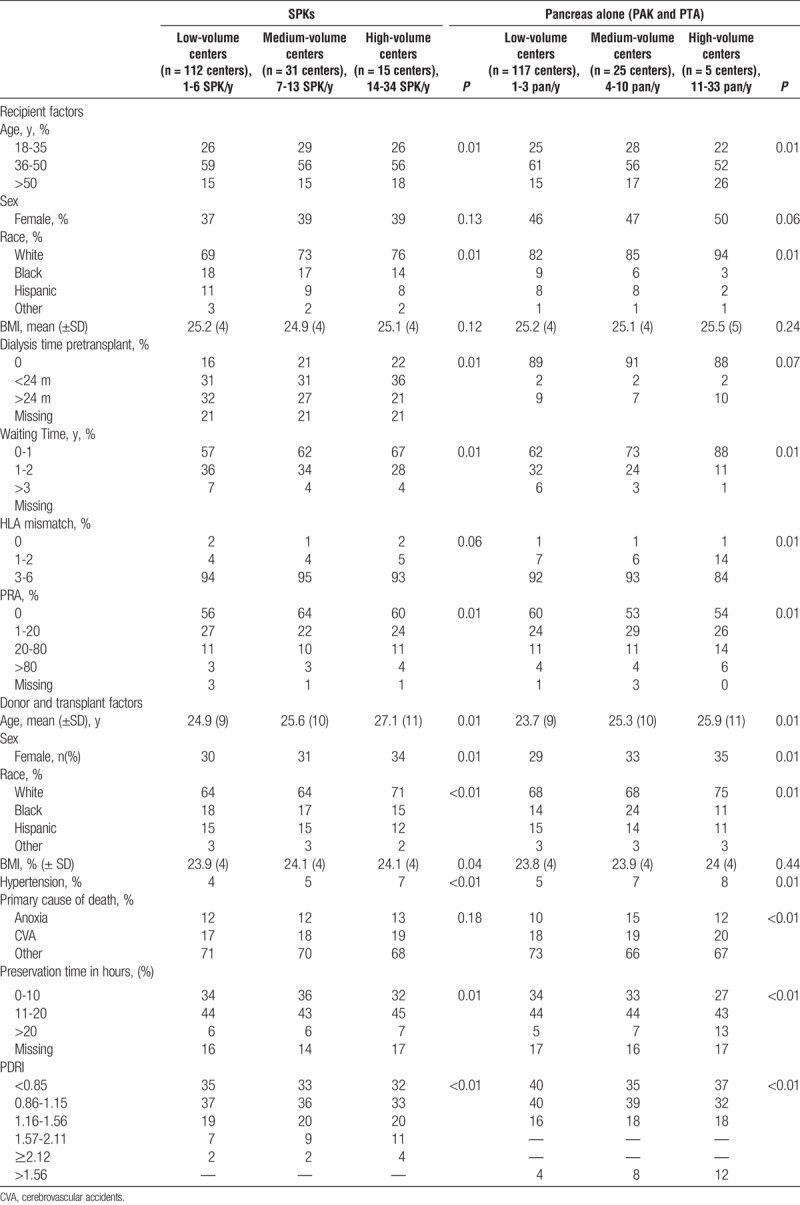
Pancreas Allograft Failure
For SPK transplantation, compared with high center volume, low (hazards ratio [HR], 1.42; 95% confidence interval [CI], 1.23-1.63) and medium (HR, 1.42; 95% CI, 1.23-1.63) center volume was associated with a significantly increased unadjusted risk of pancreas graft failure at 3 months. After multivariate adjustment for recipient, donor and transplant factors, low (adjusted HR [aHR], 1.55; 95% CI, 1.34-1.80) and medium (aHR, 1.24; 95% CI, 1.07-1.44) center volume was associated with worse pancreas allograft survival at 3 months compared with high center volume. A similar pattern was noted for low center volume at 1, 5, and 10 years (Figure 1).
FIGURE 1.
Association of center volume and pancreas allograft failure at 3 months, 1, 5, and 10 years in the SPK setting.
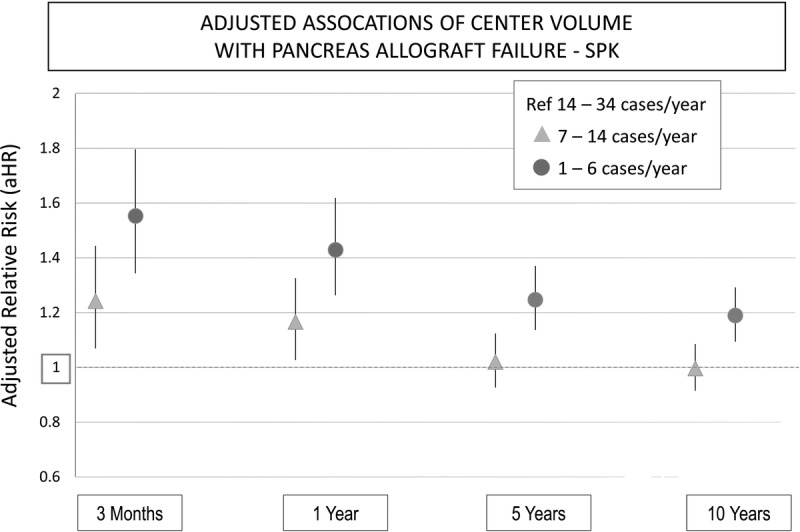
For PAK and PTA, low center volume (HR, 1.41; 95% CI, 1.14-1.76) was associated with a significantly increased unadjusted risk of pancreas graft failure at 3 months compared with high center volume. Medium center volume (aHR, 1.15; 95% CI, 0.92-1.35) was not associated with an increased risk of pancreas graft failure for PAK or PTA. After multivariate adjustment for recipient, donor, and transplant factors, low center volume (aHR, 1.56; 95% CI, 1.23-1.98) was associated with significantly worse allograft survival compared to high center volume, whereas medium center volume (aHR, 1.21; 95% CI, 0.96-1.53) was not associated with an increased risk of graft loss. A similar pattern for low-volume and medium-volume centers was noted at 1, 5, and 10 years (Figure 2). The full multivariate modules including other factors associated with pancreas allograft failure at 3 months are shown in Table S1, SDC (http://links.lww.com/TP/B386). Other modules for 1, 5, and 10 years are not shown.
FIGURE 2.
Association of center volume and pancreas allograft failure at 3 months, 1, 5, and 10 years in the pancreas alone setting (PAK and PTA).
Pancreas Donor Risk Index
To examine relationships between center volume and organ quality, we compared pancreas allograft survival according to center volume across each PDRI category. One-year pancreas allograft survival was higher in high-volume centers compared to low-volume centers within each category of PDRI for both SPK and PTA/PAK groups (Figure 3 and Figure 4).
FIGURE 3.
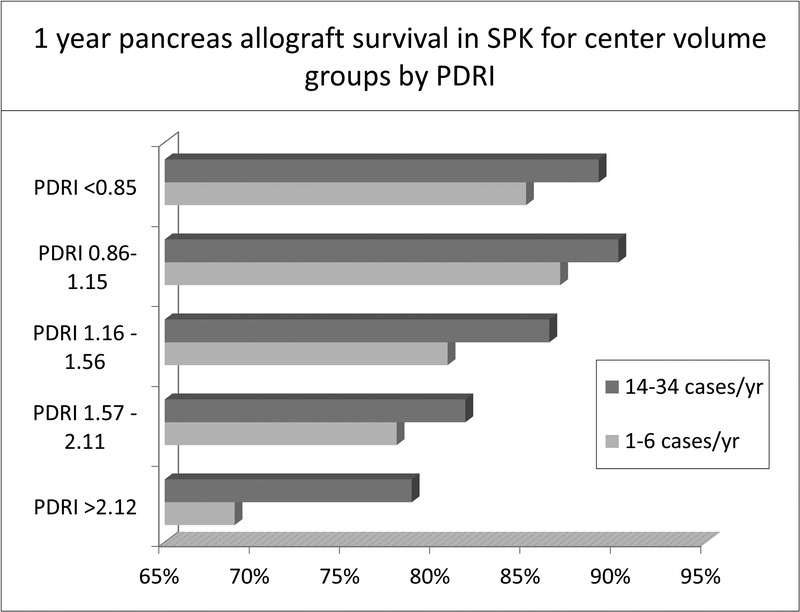
1 year pancreas allograft survival in low-volume and high-volume centers in the SPK setting stratified according to the PDRI groups.
FIGURE 4.
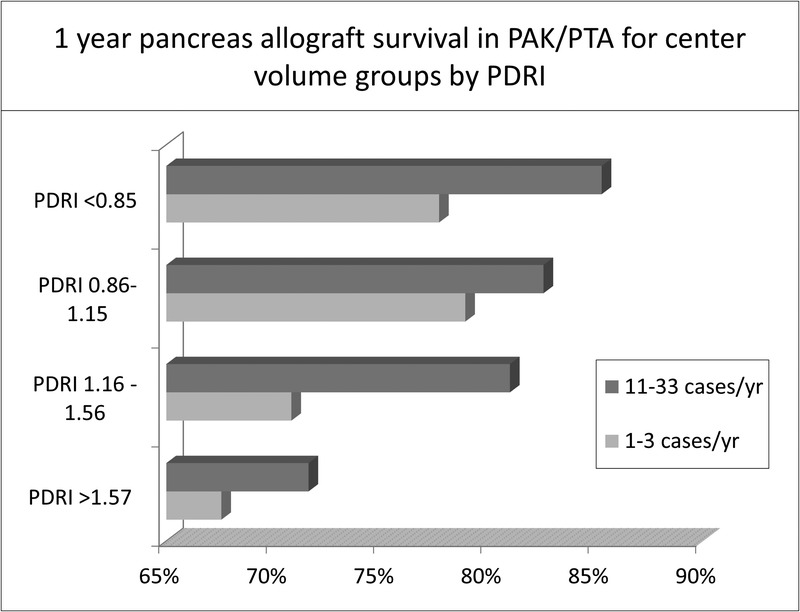
1 year pancreas allograft survival in low-volume and high-volume centers in the pancreas alone setting (PAK and PTA) stratified according to the PDRI groups.
Individual Center Survival
Several centers with low volume had better 1 year pancreas allograft survival than medium and high center volume in both SPK and solitary pancreas transplant. In the same time, 25% to 35% of these centers had lower survival than medium and high center volume (Figure 5 and Figure 6).
FIGURE 5.
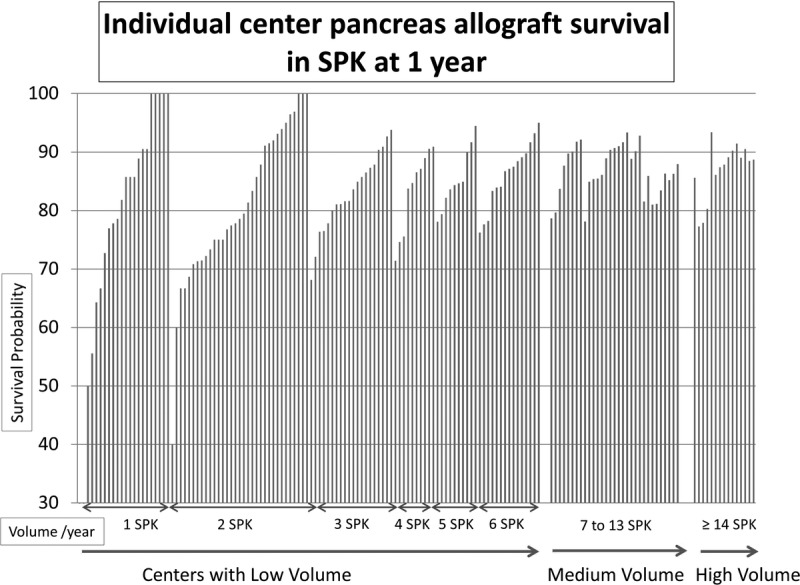
Individual center pancreas allograft survival in SPK at 1 year. Each bar represents an individual center. Y axis shows survival probability and x-axis sorted by increasing volume (SPK/year) and then by survival (if centers have the same volume).
FIGURE 6.
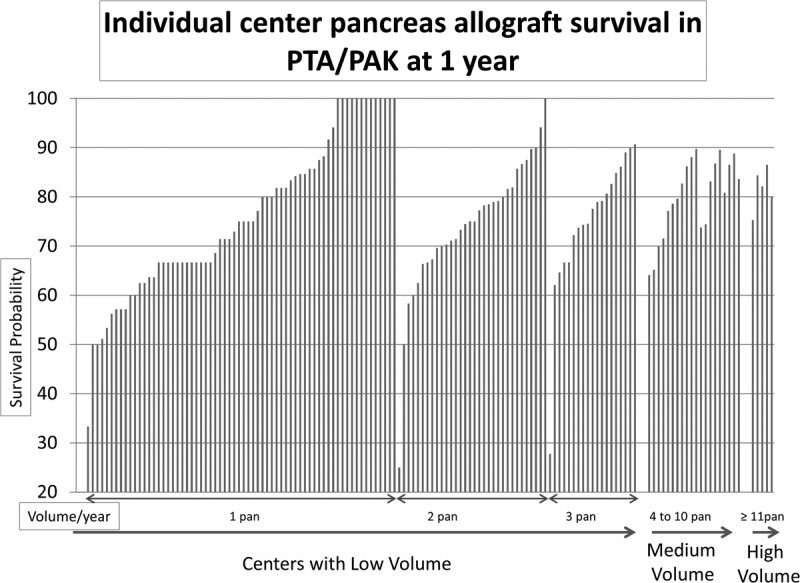
Individual center pancreas allograft survival in PTA/PAK at 1 year. Each bar represents an individual center. Y axis shows survival probability and x axis sorted by increasing volume (solitary pancreas/year) and then by survival (if centers have the same volume).
Etiologies of Allograft Failure
There was a trend of higher allograft failure secondary to rejection at medium-volume and high-volume centers compared to the low-volume centers in the SPK setting, and a higher rate of pancreatitis at the high-volume centers in the PTA/PAK setting. In the same period, unspecified etiologies of graft failure were higher in the low-volume centers in SPK and PTA/PAK settings (Figure 7 and Figure 8).
FIGURE 7.

Causes of pancreas allograft failure within 3 months of transplantation in the SPK setting.
FIGURE 8.
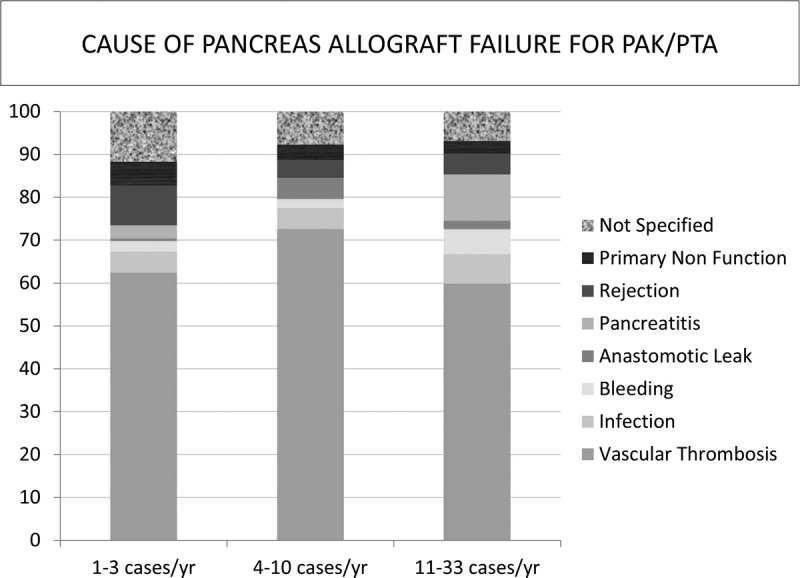
Causes of pancreas allograft failure within 3 months of transplantation in the pancreas alone setting (PAK and PTA).
DISCUSSION
Pancreas transplantation is technically challenging both from a donor and recipient perspective. Advances in immunosuppression, medical care, and refinements in surgical techniques have led to steadily improving outcomes over time. We found that SPK and PAK/PTA recipients transplanted at low-volume centers have a 50% higher risk of pancreas allograft failure at 3 months compared with those transplanted at higher-volume centers, even after adjustment for donor, recipient, and transplant factors. This pattern persists at 1 year and has a continued effect at 5 and 10 years follow-up. This finding is consistent with a recent publication from the Eurotransplant region that showed a 30% higher risk of pancreas allograft failure at 3 years in low-volume centers, defined as less than 5 pancreas transplants per year, compared to high-volume centers, defined by ≥ 13 pancreas transplants per year.24 This observation is also consistent with findings in other organ transplants.13-19 Although there was significant association between low center volume and pancreas graft failure at long-term follow up (5 and 10 years), the major association was seen in the short-term follow-up (3 months and 1 year) (Figure 1 and Figure 2).
In this study, transplants performed at low-volume centers had more favorable donor characteristics compared with organs transplanted at high-volume centers, as assessed by the PDRI, including younger donor age, shorter pancreas preservation times, as well as less frequent use of hypertensive donors. Despite more favorable donor selection at the low-volume centers, their outcomes remained inferior to high-volume centers. The PDRI was developed to identify factors associated with an increased risk of allograft failure at 1 year posttransplant.23 Regardless of the PDRI category, our results show that low-volume centers have inferior graft survival compared to high-volume centers at 1 year follow-up.
The explanation for inferior outcomes in low-volume centers is likely to be complex, but center volume could be a surrogate marker for surgical expertise, adequate recipient selection, multidisciplinary preoperative and postoperative inpatient and outpatient care, and appropriate long-term follow-up.25,26 Good support structures including specialized transplant coordinators, other allied health professionals and ready access to other surgical and medical specialties to help manage transplant-related complications are needed to improve pancreas transplant outcomes. Centers performing fewer pancreas transplants are more likely to be smaller hospitals where such support structures may not be robust.
One method that has been used in previous studies to assess the effect of surgical expertise on allograft outcomes is to analyze the early technical failure rate. In pancreas transplantation, technical failure is usually defined as allograft failure secondary to allograft thrombosis, allograft pancreatitis, intra-abdominal infections, enteric or exocrine leaks, and bleeding. Low-volume centers were more likely to report allograft loss as “not specified,” which makes the frequencies of other etiologies of graft failure inaccurate. Regardless, vascular thrombosis remained the major cause of early pancreas allograft failure in the first 3 months posttransplant across all centers.
With the steady decline in the volume of pancreas transplantation, many transplant providers may not maintain or acquire the necessary experience to manage the unique medical and surgical complications of pancreas transplantation. It is not unreasonable to postulate that this decline might have a more substantial impact on low-volume centers.
It is notable that 15 centers performed one-third of the SPK transplants in the United States and only 5 centers performed one-third of the PTA/PAK transplants between 2000 and 2013. Some insurance companies define centers performing 15 or more pancreas transplants per year as “centers of excellence.” This designation might sustain a continuous referral source for high-volume centers by directing patients to receive transplants at these centers exclusively. However, examination of individual center pancreas allograft survival at 1 year (Figure 5 and Figure 6) demonstrates that several low-volume centers had better survival than medium-medium and high-volume centers. Thus, although volume is relevant to quality, performance metrics should also consider outcomes independent of volume. Identification of care processes that support optimal outcomes after pancreas transplantation, both in relation to and distinct from center volume, is an important priority.
There are a number of limitations to this study. First, it is a retrospective study based on registry data with missing values for certain variables, for example, PRA and HLA mismatches, although we attempted to reduce the impact of missing values by adjusting for “missing” status, as per the methodology used by the Scientific Registry of Transplant Recipients in developing risk adjustment models for transplant center performance. We examined center-reported pancreas graft failure as the major outcome measure and reporting practices for graft failure may vary across centers. Recently, the United Network for Organ Sharing has defined uniform criteria for center reporting of pancreas graft failure to include: recipient's transplanted pancreas is removed, recipient re-registers for a pancreas transplant, recipient registers for an islet transplant after receiving a pancreas transplant, recipient’s total insulin use is 0.5 or greater units/kg per day for a consecutive 90 days, or the recipient dies (OPTN policy 1.2 Definitions; effective upon implementation and notice to members).27 Conversely, our study has several important strengths. First, this is the one of the first studies in the United States to highlight the outcome implications of pancreas center volume in the current era. Second, we used a national database that allowed us to include a large number of pancreas transplants with long-term follow-up. Moreover, we were able to incorporate the PDRI as a measure of donor quality for further stratification.
In conclusion, patients transplanted at centers performing low volumes of pancreas transplants have inferior short-term and long-term allograft survival rates than those transplanted at higher-volume centers. This is true for both SPK and solitary pancreas transplants regardless of the PDRI. However, graft survival at some low-volume centers is excellent. Future studies should seek to identify care processes that support optimal outcomes after pancreas transplantation irrespective of center volume.
Supplementary Material
Footnotes
The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
The authors declare no conflicts of interest.
T.A. participated in the study design, data acquisition, analysis, and writing of the article. A.M., D.C.B., R.J.S., S-H.C. J.W., T.H., and K.L.L. participated in the study design, interpretation, and writing of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Cottrell DA, Henry ML, O'Dorisio TM, et al. Hypoglycemia after successful pancreas transplantation in type I diabetic patients. Diabetes Care. 1991;14:1111–1113. [DOI] [PubMed] [Google Scholar]
- 2.Fioretto P, Steffes MW, Sutherland DE, et al. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:69–75. [DOI] [PubMed] [Google Scholar]
- 3.Navarro X, Sutherland DE, Kennedy WR. Long-term effects of pancreatic transplantation on diabetic neuropathy. Ann Neurol. 1997;42:727–736. [DOI] [PubMed] [Google Scholar]
- 4.Koznarová R, Saudek F, Sosna T, et al. Beneficial effect of pancreas and kidney transplantation on advanced diabetic retinopathy. Cell Transplant. 2000;9:903–908. [DOI] [PubMed] [Google Scholar]
- 5.La Rocca E, Fiorina P, di Carlo V, et al. Cardiovascular outcomes after kidney-pancreas and kidney-alone transplantation. Kidney Int. 2001;60:1964–1971. [DOI] [PubMed] [Google Scholar]
- 6.Smets YF, Westendorp RG, van der Pijl JW, et al. Effect of simultaneous pancreas-kidney transplantation on mortality of patients with type-1 diabetes mellitus and end-stage renal failure. Lancet. 1999;353:1915–1919. [DOI] [PubMed] [Google Scholar]
- 7.Tydén G, Bolinder J, Solders G, et al. Improved survival in patients with insulin-dependent diabetes mellitus and end-stage diabetic nephropathy 10 years after combined pancreas and kidney transplantation. Transplantation. 1999;67:645–648. [DOI] [PubMed] [Google Scholar]
- 8.Mohan P, Safi K, Little DM, et al. Improved patient survival in recipients of simultaneous pancreas-kidney transplant compared with kidney transplant alone in patients with type 1 diabetes mellitus and end-stage renal disease. Br J Surg. 2003;90:1137–1141. [DOI] [PubMed] [Google Scholar]
- 9.Reddy KS, Stablein D, Taranto S, et al. Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis. 2003;41:464–470. [DOI] [PubMed] [Google Scholar]
- 10.Gruessner AC, Gruessner RW. Pancreas Transplantation of US and Non-US Cases from 2005 to 2014 as Reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud. 2016:e2016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandaswamy R, Skeans MA, Gustafson SK, et al. OPTN/SRTR 2013 Annual Data Report: pancreas. Am J Transplant. 2015;15(Suppl 2):1–20. [DOI] [PubMed] [Google Scholar]
- 12.Stratta RJ, Gruessner AC, Odorico JS, et al. Pancreas Transplantation: An Alarming Crisis in Confidence. Am J Transplant. 2016. [DOI] [PubMed] [Google Scholar]
- 13.Asrani SK, Kim WR, Edwards EB, et al. Impact of the center on graft failure after liver transplantation. Liver Transpl. 2013;19:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnaoutakis GJ, George TJ, Allen JG, et al. Institutional volume and the effect of recipient risk on short-term mortality after orthotopic heart transplant. J Thorac Cardiovasc Surg. 2012;143:157–167, 67.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilic A, George TJ, Beaty CA, et al. The effect of center volume on the incidence of postoperative complications and their impact on survival after lung transplantation. J Thorac Cardiovasc Surg. 2012;144:1502–1508; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 16.Shuhaiber JH, Moore J, Dyke DB. The effect of transplant center volume on survival after heart transplantation: a multicenter study. J Thorac Cardiovasc Surg. 2010;139:1064–1069. [DOI] [PubMed] [Google Scholar]
- 17.Thabut G, Christie JD, Kremers WK, et al. Survival differences following lung transplantation among US transplant centers. JAMA. 2010;304:53–60. [DOI] [PubMed] [Google Scholar]
- 18.Weiss ES, Allen JG, Meguid RA, et al. The impact of center volume on survival in lung transplantation: an analysis of more than 10,000 cases. Ann Thorac Surg. 2009;88:1062–1070. [DOI] [PubMed] [Google Scholar]
- 19.Axelrod DA, Guidinger MK, McCullough KP, et al. Association of center volume with outcome after liver and kidney transplantation. Am J Transplant. 2004;4:920–927. [DOI] [PubMed] [Google Scholar]
- 20.Mandal AK, Drew N, Lapidus JA. The effect of center volume on pancreas transplant outcomes. Surgery. 2004;136:225–231. [DOI] [PubMed] [Google Scholar]
- 21.Gruessner AC. 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud. 2011;8:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhamad T, Malone AF, Lentine KL, et al. Selected mildly obese donors can be used safely in simultaneous pancreas and kidney transplantation. Transplantation. 2016. [DOI] [PubMed] [Google Scholar]
- 23.Axelrod DA, Sung RS, Meyer KH, et al. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant. 2010;10:837–845. [DOI] [PubMed] [Google Scholar]
- 24.Kopp W, van Meel M, Putter H, et al. Center volume is associated with outcome after pancreas transplantation within the eurotransplant region. Transplantation. 2016. [DOI] [PubMed] [Google Scholar]
- 25.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. [DOI] [PubMed] [Google Scholar]
- 27.Changes UP. OPTN/UNOS Policy 1. Administrative Rules and Definitions. Definition of Pancreas Graft Failure (approved, pending implementation). http://optn.transplant.hrsa.gov/media/1211/policy_notice_07-2015.pdf. Accessed January 7, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


