The introduction of tenofovir alafenamide (TAF), a tenofovir dipivoxil fumarate (TDF) pro-drug, modified the approach to HAART in HIV-experienced and naive patients. Podany et al. [1] showed a 2.41-fold increase of tenofovir intracellular metabolite (TFV-diphosphonate) and a massive decrease in tenofovir plasma concentrations in patients switching from elvitegravir/cobicistat/emtricitabine/TDF (E/C/F/TDF) to E/C/F/TAF, explaining the lower renal and bone toxicity in patients using the TAF-based regimen. TAF has demonstrated a more powerful antiviral activity, higher intracellular levels and lower plasma levels than those of TDF. It is used in combination with E/C/dF instead of tenofovir fumarate. The coadministration of E/C/F/TAF and warfarin has not been studied. Warfarin is metabolized by cytochromes, such as CYP1A2 and CYP3A4 for the R-warfarin enantiomer and CYP2C9 for the S-warfarin enantiomer, which is a different metabolic pathway than that of F/TAF. In contrast, elvitegravir and cobicistat share metabolic pathways with warfarin, as they are a modest inducer of CYP2C9 and a strong inducer of CYP3A4, respectively. In fact, a case report has described a decrease in warfarin exposure, which required an increase in anticoagulant dosage. Consequently, monitoring of the international normalized ratio (INR) is required during coadministration of warfarin, elvitegravir and cobicistat. Here, we describe a case in which the reduced effect of warfarin was not observed during the prolonged coadministration of elvitegravir/cobicistat and TDF, but the reduced effect was observed when TDF was replaced with TAF in combination with elvitegravir/cobicistat.
A 58-year-old man had a history of cardiovascular disease, smoking and intravenous drug use until 2003, when he received a diagnosis of HIV and hepatitis C viruses (HCVs) coinfection. In 2006, he underwent the implantation of a mitral valve mechanical prosthesis, and, since then, he started warfarin to maintain weekly INR levels between 2 and 3. He also began following a strict diet, avoiding all food that may influence warfarin absorption. He was classified as CDC B3 (CD4+ cells count 200 cells/μl and HIV-RNA 97 600 copies/ml). In March 2009, he started a HAART regimen with daily (q.d.) TDF/emtricitabine 245/200 mg and twice-daily raltegravir 400 mg, which was within the study protocol MK 0518-071. In March 2011, at the study conclusion, the HAART regimen was changed to q.d. abacavir/lamivudine 300/300 mg and nevirapine 400 mg, with which he maintained optimal virologic control and had CD4+ cell count recovery (597 CD4+ cells/μl). In August 2013, the patient was diagnosed with Hodgkin's lymphoma and underwent six R-CHOP courses (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone), followed by complete disease remission. During this period, the patient continued the HAART treatment without any adverse effects during chemotherapy. In May 2015, he was treated with oral direct-acting antivirals (DAAs) for hepatitis C and, consequently, the HAART regimen was changed to tenofovir/emtricitabine/raltegravirt. He had a genotype 1a HCV infection, and he underwent a 24-week regimen with ombitasvir/paritaprevir/ritonavir 25/150/100 mg and dasabuvir 250 mg, achieving a sustained virological response at week 12. In January 2016, his last treatment was elvitegravir/cobicistat/emtricitabine/TDF, and no interaction with warfarin was detected. However, in July 2017, after a diagnosis of osteopenia, the patient's treatment was modified to E/C/F/TAF. Before the change, his last INR was 2.7. After 7 days of E/C/F/TAF, the INR increased to 4.5, using the usual warfarin dosage. Despite a reduction in the warfarin dose, according to the cardiologist's suggestion, the INR rose to 6.5 over the next 7 days. Therefore, E/C/F/TAF was stopped and replaced with abacavir/lamivudine/dolutegravir q.d. The change in therapy was followed by an immediate return of INR to the therapeutic range at the usual warfarin dosage. During this drug interaction period, the HIV plasma viral load remained undetectable (Fig. 1).
Fig. 1.
Trend of CD4+ cell number/μl (black line), HIV-RNA in copies/ml (dark grey line) and INR (light grey line) values at the different time points of HAART strategies changes.
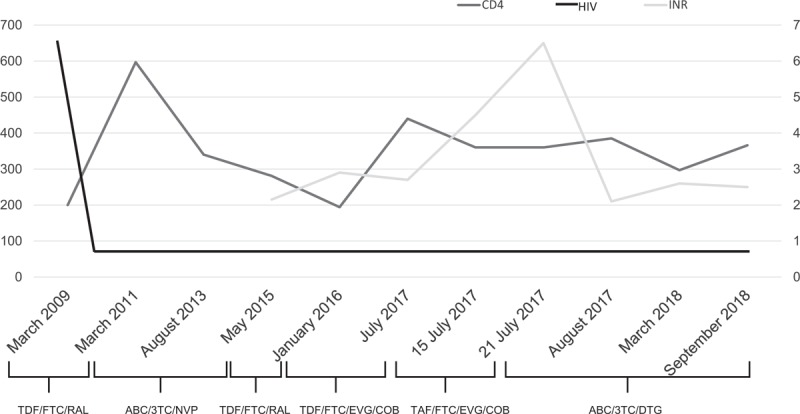
3TC, lamivudine; ABC, abacavir; COB, cobicistat; DTG, dolutegravir; EVG, elvitegravir; FTC, emtricitabine; RAL, raltegravir; TAF, tenofovir alafenamide; TDF, tenofovir dipivoxil fumarate.
The use of new drugs such as TAF has ensured an improvement in the management of antiretroviral treatment. To our knowledge, this is the first case reporting a drug interaction between warfarin and TAF. Good et al. [2] described a similar case, demonstrating an interaction with the elvitegravir-based antiretroviral therapy (ART) regimen. In contrast with this case, our patient did not show an alteration in INR during E/C/F/TDF treatment continuously taken for 18 months. Therefore, it is likely that TAF, rather than the E/C/F association, is the cause of the interference with warfarin. There is very little information about the possible interaction between TAF and cobicistat coadministered with other classes of drugs, and in particular, no data exist regarding coadministration of E/C/F/TAF and warfarin (https://www.hiv-druginteractions.org/checker [Accessed 10 October 2018]). The data in the literature suggest that CYP2C9 induction by elvitegravir has a stronger effect on warfarin metabolism than CYP3A inhibition by cobicistat. However, emtricitabine and TAF do not interact with this metabolic pathway. Further studies are needed to verify interactions with non-HIV drugs to avoid adverse effects in patients taking multiple therapies for comorbidities.
Abstract was presented at the 10th ICAR Congress, 22–24 May 2018, Rome [Abstract ID: 74].
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Podany AT, Bares SH, Havens J, Dyavar SR, O’Neill J, Lee S, et al. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS 2018; 32:761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Good BL, Gomes DC, Fulco PP. An unexpected interaction between warfarin and cobicistat-boosted elvitegravir. AIDS 2015; 29:985–986. [DOI] [PubMed] [Google Scholar]


