A phase 3 study of a levonorgestrel 52-mg intrauterine system in U.S. females shows high 5-year contraceptive efficacy and low hormonal adverse effect rates.
Abstract
OBJECTIVE:
To assess the 5-year contraceptive efficacy and safety of a levonorgestrel (LNG) 52-mg intrauterine system (IUS) from an ongoing 10-year phase 3 contraceptive trial.
METHODS:
Study investigators enrolled 1,751 nulliparous and parous females aged 16–45 years and desiring contraception to receive a novel LNG 52-mg IUS at 29 centers in the United States, including reproductive health clinics, private offices, and university centers. Participants had scheduled follow-up visits four times during the first year. After year 1, study visits occurred every 6 months, with phone contact at the 3-month point between visits. We assessed the primary outcome of pregnancy rate (Pearl Index) in females aged 16–35 years at enrollment through 60 months. The safety evaluation included all females for their entire duration of participation.
RESULTS:
The 1,751 enrollees included 1,600 females aged 16–35 years and 151 aged 36–45 years. Successful IUS placement occurred in 1,714 (97.9%) participants. At the time of the data evaluation, 495 participants finished 5 years and 176 had entered the seventh year of IUS use. Nine pregnancies occurred, six of which were ectopic. The Pearl Indices for years 1 and 5 were 0.15 (95% CI 0.02–0.55) and 0.20 (95% CI 0.01–1.13) pregnancies per 100 women-years, respectively. The cumulative life-table pregnancy rate was 0.92% (0.46–1.82%) through 5 years. Participants aged 16–35 years at enrollment were significantly more likely to report new or worsening acne, dyspareunia, pelvic pain, and dysmenorrhea; participants aged 36–45 years at enrollment were more likely to report new or worsening weight increase. Discontinuation for adverse events occurred in 322 (18.8%) participants, most commonly related to expulsion (n=65 [3.8%]). Only 39 (2.2%) IUS users discontinued as a result of bleeding symptoms. Pelvic infection was diagnosed in 14 (0.8%) participants.
CONCLUSION:
This LNG 52-mg IUS is highly effective and safe over 5 years of use in U.S. females.
CLINICAL TRIAL REGISTRATION:
FUNDING SOURCE:
Medicines360.
In the United States, 45% of pregnancies each year are unintended.1 Females who use long-acting reversible contraception (LARC) methods, specifically the currently available copper intrauterine device, levonorgestrel intrauterine system (LNG-IUS), or single-rod contraceptive implant, have substantially lower rates of unintended pregnancy than those using other methods of reversible contraception.2,3 These methods are not new in the United States with U.S. Food and Drug Administration approval of the currently available copper intrauterine device in 1986, the first LNG-IUS in 2000, and the single-rod implant in 2002. However, for many years, multiple barriers to access limited their use. The Contraceptive CHOICE study in St. Louis3 and two population-based clinical intervention projects4,5 aimed at decreasing barriers to access for LARC methods showed substantial increases in LARC uptake and decreases in unintended pregnancy and abortion rates.
Long-acting reversible contraception use has increased in the United States over the past decade, with data demonstrating an increase from 6% (2008 data) to 14% (2014 data) of contracepting females.6 Approximately two thirds of females obtaining a LARC method choose a hormonal IUS.7 Even with the introduction of mandated contraceptive coverage without cost sharing for insured individuals through the 2010 Affordable Care Act, females without insurance, with insurance through entities claiming religious exemptions, or who do not qualify for medical assistance still encounter significant financial barriers.8 Individual cost for contraception is a significant public health concern because socioeconomic level has an important relationship to unintended pregnancy. In 2011, females with household incomes below 200% of the federal poverty level had a 5.6-fold greater risk of unintended pregnancy than those with higher incomes.1 This disparity has increased from a 2.5-fold difference in 1994.9
To address these barriers, Medicines360, a nonprofit pharmaceutical company, developed a new LNG 52-mg IUS, which was approved by the U.S. Food and Drug Administration in 2015 for 3 years of contraception based on an ongoing phase 3 study. The IUS was brought to market at a $50 price for public sector clinics participating in the Federal 340B drug discount program. Four-year U.S. Food and Drug Administration approval was granted in October 2017. In this evaluation, we aim to report the data supporting a 5-year contraceptive indication.
The Sponsor, Medicines360, designed the study and oversees its conduct, including funding the trial, and provided all study product free of charge to participants.
ROLE OF THE FUNDING SOURCE
The Sponsor, Medicines360, designed the study and oversees its conduct, including funding the trial, and provided all study product free of charge to participants.
METHODS
ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) was designed to assess efficacy and safety of a branded LNG 52-mg IUS (Liletta) in a diverse population of females. This phase 3 clinical trial is ongoing at 29 U.S. sites, including reproductive health clinics, private offices, and university centers. The study was approved by a central or local institutional review board for each center, as applicable, and performed in accordance with International Conference on Harmonisation-Good Clinical Practice guidelines. All participants signed written informed consent before study participation and received compensation for time and travel. An independent data safety monitoring committee reviews data every 6 months.
The study methodology, sample size rationale, and study population characteristics have been previously published.10 Briefly, enrollment began in December 2009 and participants receiving the LNG 52-mg IUS included healthy, sexually active (at least four times monthly), nulliparous and parous females aged 16–45 years (inclusive) with regular menstrual cycles (21–35 days when not using hormones). Exclusion criteria ensured good general health, potential fertility, and low risk for adverse events with IUS use and did not include any restrictions on weight or body mass index. The 16- to 45-year-old population included 1,011 (57.7%) nulliparous and 438 (25.1%) obese females.10
At the screening visit and after signing informed consent, participants provided a medical history and underwent evaluations to ensure all study criteria were met. Enrollment and IUS placement could occur on the same day as the screening procedures. Intrauterine system placement was attempted only in participants if the uterus was successfully sounded to 5.5 cm or more. Information on how to check for the IUS strings was provided, but the participants were not required to routinely check the strings.
Participants had scheduled follow-up visits four times during the first year (1, 3, 6, and 12 months after IUS placement); after year 1, study visits occurred every 6 months. Study staff questioned the participant about adverse events and concomitant medications and whether she was relying on the IUS as her primary method of contraception. Intrauterine system presence was confirmed by palpation or direct visualization of the strings; participants with missing strings underwent transvaginal ultrasonography at that visit and at subsequent annual visits. Starting at month 9, telephone contacts occurred at the 3-month point between visits to ask the same questions as at study visits. A follow-up visit for urine pregnancy testing and evaluation of ongoing adverse events was scheduled 30–43 days after IUS discontinuation. The study IUS could be removed whenever requested.
Participants completed a diary for the first 24 months for daily recording of other contraceptive use and the greatest amount of bleeding that day as none, spotting, light flow, normal flow, or heavy flow based on their own subjective impression. After 24 months, the diary included only additional contraceptive use. Participants were asked at each visit or telephone contact beginning with month 27 to describe their bleeding pattern over the preceding 3 months.
The efficacy and safety data for this report include all outcomes documented through September 8, 2017. We evaluated pregnancy rates in females aged 16–35 years old at study entry with successful IUS insertion who had at least one follow-up contact during IUS use. The maximum duration of follow-up for the efficacy evaluation was 60 months (65 cycles). Duration of use was based on the last in-person visit, which documented IUS presence and a negative pregnancy test. The primary outcome was on-treatment pregnancy, defined as any pregnancy with a conception date beginning on the day of insertion and through 7 days after IUS discontinuation. We calculated pregnancy rates primarily as the Pearl Index (number of pregnancies per 100 women-years) for individual years, including only months during which participants did not report any other contraceptive use. If a pregnancy occurred during a month with additional contraceptive use, that month was included in the denominator. Pregnancies among participants who experienced an expulsion that was not identified before a pregnancy diagnosis were included as product failures. Secondary efficacy outcomes included cumulative Pearl Indices over 5 years and life-table pregnancy rates calculated using the Kaplan-Meier method.
Safety analyses included any adverse events reported amongst all enrolled participants regardless of duration of IUS use; based on the study start date, these analyses include participants with more than 7 years of IUS use. We organized adverse events into standardized terms using the Medical Dictionary for Regulatory Activities in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Pelvic infection included all participants diagnosed by a clinician with endometritis or pelvic inflammatory disease.
All efficacy and safety data were censored based on last contact at which data collection occurred. Intrauterine system exposure was calculated in 28-day cycle equivalents. Amenorrhea was defined as no bleeding or spotting over a 90-day interval. We evaluated amenorrhea rates for the last 90-day interval for years 1–5 five and other bleeding data only in participants with available diary or bleeding questionnaire data. Fisher exact tests were used when appropriate. Data were analyzed using SAS 9.3.
Authors' Data Sharing Statement
Will individual participant data be available (includ-ing data dictionaries)? No.
What data in particular will be shared: Not available.
What other documents will be available? Not available.
When will data be available (start and end dates)? Not applicable.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Not applicable.
RESULTS
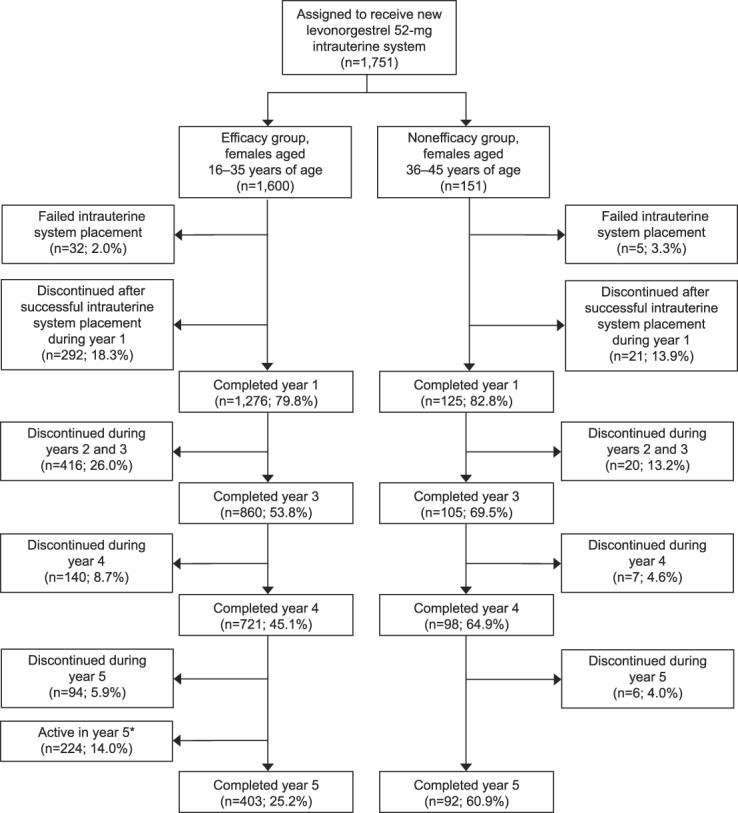
We enrolled 1,751 participants, with successful IUS placement in 1,714 (97.9%), including 1,568 females aged 16–35 years and 146 aged 36–45 years at enrollment. Among the 16- to 35-year-old participants, 30 did not have a follow-up contact after IUS placement, leaving 1,538 participants in the efficacy evaluation. Participant follow-up over the first 5 years of this trial is presented in Figure 1.
Fig. 1. Disposition of females through 5 years in a phase 3 U.S. study investigating a levonorgestrel 52-mg intrauterine system. *The study was initiated in December 2009 and is still ongoing. Not all active females have completed 5 years of participation.
Teal. Levonorgestrel 52-mg IUS at 5 Years. Obstet Gynecol 2019.
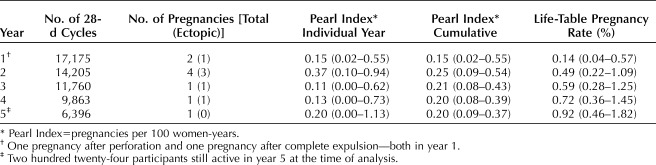
The 5-year life-table pregnancy rate was 0.92% (95% CI 0.46–1.82%) over 59,399 cycles. Efficacy per year and cumulatively over 5 years is presented in Table 1. Nine pregnancies occurred in four nulliparous and five parous participants. The 5-year life-table pregnancy rates in nulliparous and parous females were 0.83% (95% CI 0.28–2.50%) and 1.26% (95% CI 0.51–3.10%), respectively. Six (67%) pregnancies were ectopic, resulting in an ectopic pregnancy rate through 5 years of 0.13 per 100 women-years. No pregnancies occurred in LNG 52-mg IUS users aged 36–45 years of age at enrollment.
Table 1.
Pregnancy Rate Through 5 Years of Levonorgestrel 52-mg Intrauterine System Use in U.S. Females Aged 16–35 Years Old at Insertion
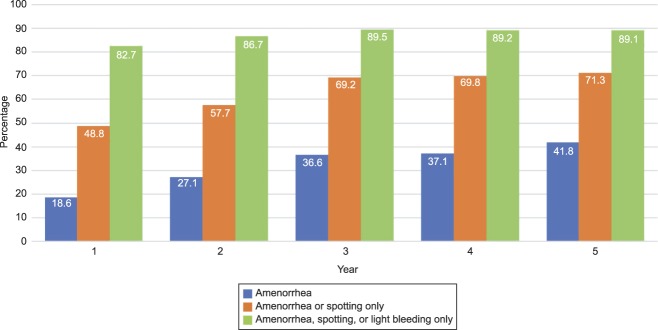
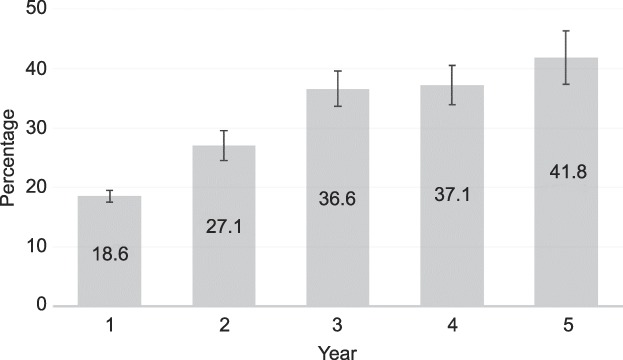
Amenorrhea rates increased over the 5 years of follow-up (Fig. 2). Approximately 50% of participants experienced amenorrhea or spotting only during the last 90 days of the first year of use and 80% experienced amenorrhea, spotting, or light bleeding only (Fig. 3). By the third year, these rates reached 70% and 90%, respectively, and stayed approximately the same through the fifth year. For participants who subjectively reported heavy menstrual flow at baseline, 53 of 145 (36.6%), 53 of 122 (43.4%), and 52 of 102 (51.0%) reported amenorrhea or spotting only at 1, 2, and 3 years, respectively. Similarly, for participants who reported severe menstrual cramping at baseline, 26 of 62 (41.9%), 25 of 52 (48.1%), and 21 of 44 (47.7%) reported none or mild cramping at 1, 2, and 3 years, respectively.
Fig. 2. Amenorrhea rates over 5 years in females using a levonorgestrel 52-mg intrauterine system. Amenorrhea defined as no bleeding or spotting in the preceding 90 days. Data evaluated for 90-day period before end of year.
Teal. Levonorgestrel 52-mg IUS at 5 Years. Obstet Gynecol 2019.
Fig. 3. Amenorrhea, spotting, and light bleeding rates over 5 years in females using a levonorgestrel 52-mg intrauterine system. Amenorrhea defined as no bleeding or spotting in the preceding 90 days. Data evaluated for 90-day period before end of year.
Teal. Levonorgestrel 52-mg IUS at 5 Years. Obstet Gynecol 2019.
The safety analysis included participants who had used the LNG 52-mg IUS for up to or beyond 7 years. At the time of the data analysis, 236, 176, and 31 participants had completed 6, 7, and 7.5 years, respectively. Overall, 1,041 (66.4%) 16–35-year-olds and 69 (47.3%) 36–45-year-olds discontinued study participation, most frequently for an adverse event (n=293 [18.3%] and n=29 [19.2%], respectively), desiring pregnancy (n=228 [14.5%] and n=4 [2.6%], respectively), lost-to follow-up or withdrawal of consent (n=226 [14.1%] and n=16 [10.6%], respectively), or relocation far from a study site (n=102 [6.5%] and n=4 [2.6%], respectively). Only 555 (31.7%) participants discontinued for events that would be seen in clinical practice such as product-related reasons (eg, adverse event, new medical contraindication) or personal reasons, including pregnancy desire. For participants requesting removal of an IUS in the uterine cavity, only two procedures could not be completed in the office.
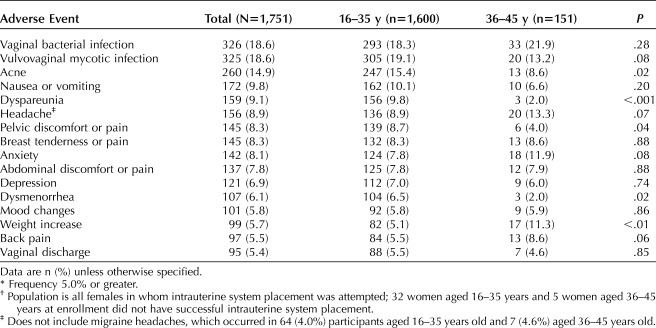
Table 2 includes adverse reactions reported with a frequency of 5% or greater. At least one adverse event was noted in 1,438 (89.9%) 16–35-year-olds and 136 (90.1%) 36–45-year-olds. A serious adverse event was reported in 73 (4.2%) participants, of which eight were probably related or related; these eight included seven ectopic pregnancies (six ectopic pregnancies through 60 months and one ectopic pregnancy after 60 months) and one ovarian cyst treated surgically. Pelvic infection was uncommon, reported in only 14 (0.8%) participants. Four participants received this diagnosis in the first 2 months, four between 6 and 12 months, two after 12 months through 24 months, and four after 24 months. The 1-year pelvic infection rate was 0.46% (95% CI 0.14–0.77%). Only two (0.1%) participants had the IUS removed for a pelvic infection.
Table 2.
Adverse Events* Occurring in U.S. Females and Potentially Related to Using a Levonorgestrel 52-mg Intrauterine System for More than 7 Years†
Overall, 322 (18.8%) participants discontinued use as a result of an adverse event. The most frequent event leading to discontinuation among the 1,714 participants with successful insertion was partial or complete expulsion (n=65 [3.8%]). Expulsion rates did not differ between participants aged 16–35 years old (58/1,568 [3.7%]) and those aged 36–45 years old (7/146 [4.8%]) at enrollment (P=.49). Most (n=50 [76.9%]) expulsions were diagnosed in the first year of product use and occurred overall less frequently in nulliparous (21/986 [2.1%]) participants than parous (44/728 [6.0%]) participants (P<.001). Complete expulsions comprised 28 (43.1%) of the 65 expulsions. One pregnancy occurred in a woman who experienced complete IUS expulsion during the first year of use; no other pregnancies occurred related to expulsion.
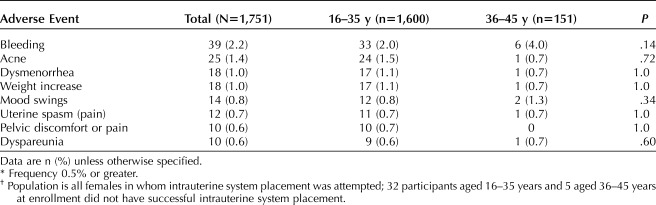
Table 3 describes nonexpulsion adverse events that resulted in at least 0.5% of participants requesting discontinuation during more than 7 years of IUS use. The most frequent of these events, bleeding symptoms, were cited by only 39 (2.2%) participants. The annual rates of discontinuation for bleeding were low in years 1–5: 17 of 1,714 (1.0%) 13 of 1,401 (0.9%), 5 of 1,149 (0.4%), 2 of 965 (0.2%), and 2 of 819 (0.2%) participants, respectively. Only one woman cited amenorrhea as a reason for discontinuation (during year 2).
Table 3.
Nonexpulsion-Related Adverse Events* Resulting in Discontinuation in U.S. Females Using a Levonorgestrel 52-mg Intrauterine System for More Than 7 Years†
Of note, seven (0.4%) participants attributed vaginal infection to the IUS and requested removal (six bacterial vaginosis and one candidiasis). Two IUS perforations were discovered during the first year; no other perforations were noted thereafter. Although ovarian cysts were diagnosed in 78 (4.5%) participants during follow-up, only six (0.3%) requested IUS removal. One death occurred during the first year of use as a result of a preexisting illness and was unrelated to the IUS.
DISCUSSION
The LNG 52-mg IUS evaluated in this study is highly effective for contraception through 5 years and has low discontinuation rates for infectious or systemic hormonal adverse events. The results presented in this report represent ongoing follow-up of the single largest phase 3 study of an LNG 52-mg IUS in U.S. females. The study population, which includes approximately 58% nulliparous females,10 provides important information about safety and efficacy of a hormonal IUS in a typical population of U.S. females.
This report is the first to describe amenorrhea rates for the LNG 52-mg IUS through 5 years of continued use. Amenorrhea, defined as no bleeding or spotting for the preceding 90 days, increased throughout the evaluation period, exceeding 40% of participants at 5 years. With the large number of obese and nulliparous participants in this trial,10 we have previously shown that amenorrhea rates over the first 3 years do not differ based on parity or obesity status.11 Most participants who are not amenorrheic are experiencing spotting or light bleeding. The proportion of participants experiencing amenorrhea, spotting, or light bleeding remains relatively constant in years 3–5. Very few participants discontinued use for bleeding (only 2% over 5 years), implying high satisfaction with the bleeding pattern experienced by participants using an LNG 52-mg IUS. Most (77%) discontinuations for bleeding occurred in year 1 or 2.
The trial size and length of follow-up for this study allowed us to evaluate adverse events over extended use in U.S. females. Only one (0.06%) related serious adverse event, an ovarian cyst requiring surgical intervention, occurred exclusive of ectopic pregnancy. Most notably, events that could be affected by hormones, such as acne, mood changes, and weight increase, led to discontinuation in a combined 62 (3.5%) participants over a period of more than 7 years. Ovarian cysts have long been a concern with LNG-IUS products,12,13 primarily because studies looked for and reported asymptomatic small cysts. In our study, we performed ultrasound examinations only when clinically indicated (eg, missing IUS string or pelvic pain); symptomatic ovarian cysts were diagnosed in less than 5% of participants during this extended follow-up. Only six (0.3%) participants requested IUS removal related to an ovarian cyst. The low incidence of clinically apparent ovarian cysts as well as the very low discontinuation for this event provides objective evidence that the LNG 52-mg IUS does not cause significant issues related to ovarian cysts. Intrauterine system counseling should not continue to include information related to an increase in problems with ovarian cysts.
The study size and length of follow-up also allowed us to assess differences in adverse event incidence among females typically evaluated in a contraceptive trial (35 years and younger at enrollment) and women later in the reproductive years who would also use such a product (36–45 years at enrollment). Younger females are significantly more likely to report new or worsening acne, dyspareunia, pelvic pain, and dysmenorrhea, whereas older women are more likely to report new or worsening weight increase. None of the adverse events resulting in discontinuation differed by age. However, this analysis is limited by the significant difference in the number of participants and continuation rates in each age cohort (Fig. 1). Accordingly, nonsignificant differences may not be generalizable to other populations and require additional study. Importantly, these data demonstrate that further assessments of adverse event rates in hormonal IUS users should include evaluation of outcomes by age.
The findings of this pivotal phase 3 study through 5 years confirm the high efficacy, safety, and tolerability of the LNG 52-mg IUS in a large cohort of U.S. females. The clinical data in this report build on the previously published 3-year efficacy and safety results10 and pharmacokinetic data showing hormone release through 5 years.14 The ongoing trial is currently planned to evaluate the LNG 52-mg IUS for up to 10 years of continuous use.15 Use of the same product for a longer period of time would allow for fewer procedures, which will both decrease health care costs and result in less discomfort and risk for females to endure. Continued study of the LNG 52-mg IUS can address some of the barriers that continue to limit full access to intrauterine contraception.
Footnotes
Supported by Medicines360.
Financial Disclosure Stephanie B. Teal has served on the Advisory Board for Merck & Co. Her university department receives contraceptive research funding from Bayer HealthCare, Medicines360, Merck & Co, and Sebela. David K. Turok has received speaking honoraria from Allergan and Medicines360. He also served on the Advisory Boards for Allergan, Bayer, Pharmanest, and Teva and has been a consultant for Bioceptive. His university department receives contraceptive research funding from Bayer HealthCare, Cooper Surgical, Medicines360, Merck & Co, Sebela, and Teva. Beatrice A. Chen has served on the Advisory Board for Merck & Co. Her university department receives contraceptive research funding from Medicines360, Merck & Co, and Sebela. Thomas Kimble has received speaking honoraria from Allergan and Merck and is a consultant for Medicines360. His university department receives contraceptive research funding from Allergan, Antiva, Bayer HealthCare, Chemo, Invovio, Medicines360, Mithra, and Sebela. Andrea I. Olariu is a Medicines360 employee. Mitchell D. Creinin has received speaking honoraria from Gedeon Richter and Merck & Co. He has served on the Advisory Boards for Lupin and Merck & Co. He has been a consultant for Estetra, Gedeon Richter, Icebreaker Health, and Medicines360, and his university department receives contraceptive research funding from Daré Bioscience, HRA Pharma, Medicines360, Merck & Co, and Sebela.
Presented in part as a poster at the American College of Obstetricians and Gynecologists' Annual Clinical and Scientific Meeting, April 27–30, 2018, Austin, Texas.
The authors thank the participating investigators and coordinators at the 29 study centers for conduct of the clinical trial and submission of data.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews are available at http://links.lww.com/AOG/B227.
REFERENCES
- 1.Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med 2016;374:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013;62:1–60. [PubMed] [Google Scholar]
- 3.Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012;366:1998–2007. [DOI] [PubMed] [Google Scholar]
- 4.Lewis C, Darney P, Thiel de Bocanegra H. Intrauterine contraception: impact of provider training on participant knowledge and provision. Contraception 2013;88:226–31. [DOI] [PubMed] [Google Scholar]
- 5.Ricketts S, Klingler G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline in births among young, low-income women. Perspect Sex Reprod Health 2014;46:125–32. [DOI] [PubMed] [Google Scholar]
- 6.Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception 2018;97:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among U.S. women, 2009–2012. Obstet Gynecol 2015;126:917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broecker J, Jurich J, Fuchs R. The relationship between long-acting reversible contraception and insurance coverage: a retrospective analysis. Contraception 2016;93:266–72. [DOI] [PubMed] [Google Scholar]
- 9.Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011;84:478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD, et al. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception 2015;92:10–6. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber CA, Teal SB, Blumenthal PD, Keder LM, Olariu AI, Creinin MD. Bleeding patterns for the Liletta® levonorgestrel 52 mg intrauterine system. Eur J Contracept Reprod Health Care 2018;23:116–20. [DOI] [PubMed] [Google Scholar]
- 12.Gemzell-Danielsson K, Schellschmidt I, Apter D. A phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril 2012;97:616–22.e1–3. [DOI] [PubMed] [Google Scholar]
- 13.Nelson A, Apter D, Hauck B, Schmelter T, Rybowski S, Rosen K, et al. Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstet Gynecol 2013;122:1205–13. [DOI] [PubMed] [Google Scholar]
- 14.Creinin MD, Jansen R, Starr RM, Gobburu J, Gopalakrishnan M, Olariu A. Levonorgestrel release rates over 5 years with the Liletta® 52-mg intrauterine system. Contraception 2016;94:353–6. [DOI] [PubMed] [Google Scholar]
- 15.Medicines360. A study of a levonorgestrel-releasing intrauterine system for long-term, reversible contraception. Available at: https://www.clinicaltrials.gov/ct2/show/NCT00995150?term=00995150&rank=1. Retrieved June 20, 2018.