Supplemental digital content is available in the text.
Key words/Abbreviations: depression, human, irritable bowel syndrome, melatonin, mental health, BMI = body mass index, EC = enterochromaffin, GI = gastrointestinal, GSRS = Gastrointestinal Symptoms Rating Scale, IBS = irritable bowel syndrome, IQR = interquartile range, MADRS-S = Montgomery Åsberg Depression Rating Scale–Self-Assessment, OC = oral contraceptives, OR = odds ratio, SNRI = serotonin-noradrenaline reuptake inhibitors, SSRI = selective serotonin reuptake inhibitors
ABSTRACT
Objective
The pathophysiology of irritable bowel syndrome (IBS) is not completely understood, although we do know that patients with IBS have a high prevalence of psychiatric comorbidity (mainly depression and anxiety disorders). Melatonin, produced in the gastrointestinal tract, influences gut motility. Psychiatric conditions are associated with circadian disturbances in peripheral melatonin levels. This study aimed to investigate associations between daytime salivary melatonin and gastrointestinal symptoms in young adult psychiatric patients.
Methods
Ninety-six patients (86% women), aged 18–25 years (M (SD) = 21 (2)), seeking psychiatric care with primarily anxiety disorders, affective disorders, or both were included in the study. Total scores from the Gastrointestinal Symptoms Rating Scale - IBS were compared with salivary melatonin measured at three time points (30 minutes after waking up, at 11:00 hours and 30 minutes after lunch) during the waking hours of 1 day.
Results
After adjustment for potential confounders, melatonin levels in saliva 30 minutes after lunch remained significantly correlated to the total Gastrointestinal Symptoms Rating Scale - IBS score after correction for multiple testing (B = 0.016, SE = 0.006, p = .015, q = 0.045). In a post hoc analysis, symptoms of gastrointestinal pain and bloating contributed most to this association.
Conclusions
In young adult psychiatric patients, salivary melatonin levels after lunch are associated with gastrointestinal symptoms, which is consistent with the proposed effect of elevated levels of gastrointestinal melatonin on gut motility. This result suggests a link between IBS symptoms and regulation of melatonin in patients with psychiatric disorders.
INTRODUCTION
Melatonin is a neuroendocrine hormone known to regulate sleep and circadian rhythm. During the dark hours, melatonin in plasma is mainly of pineal origin, whereas during the day, because the production and secretion of melatonin in the pineal gland are effectively inhibited by light, circulating levels of melatonin are believed to be of peripheral origin (1). The enterochromaffin (EC) cells are neuroendocrine cells located throughout the gastrointestinal (GI) tract that produce and secrete serotonin and melatonin—two enzymatic steps away from each other. EC cells are thus a major source of melatonin in the GI tract and the amount may greatly surpass the amount of melatonin in the pineal gland (1,2).
Melatonin affects GI motility via membrane receptors that include melatonin (MT1 and MT2) and serotonin (5-HT) receptors (3). Both MT1 and MT2 are expressed in several cell types throughout the GI tract, with the highest levels in the large intestine (4). Melatonin induces contraction of cultured gastric smooth muscle cells, likely via MT1 receptor signaling given that this effect is blocked with a nonselective MT1/MT2 antagonist but not with a MT2-specific antagonist (5). Binding of melatonin to 5-HT4 receptors can cause smooth muscle relaxation, whereas stimulation of 5-HT3 receptors may result in smooth muscle contraction (3). Melatonin may also influence gut smooth muscle via the inhibition of nicotinic receptor channels regulating smooth muscle contraction (3). Furthermore, melatonin can inhibit the activity of the serotonin transporter (6). The reported effects of melatonin on GI motility seem to be dose dependent, because administration of pharmacological doses of melatonin seems to decrease motility and increase colonic transit time, whereas melatonin in lower concentrations seems to increase motility and decrease colonic transit time (3,7–10). EC cells also produce serotonin and a recent review highlights the complexity of its role in regulating GI motility (11). Increased serotonin signaling has been shown in the gut in patients with irritable bowel syndrome (IBS), including increased levels of serotonin in plasma, (12) and may contribute to the changed motility and sensation in IBS (13). At physiological levels, melatonin most likely acts as an antagonist of serotonin in regulating gut motility (10).
IBS is a functional bowel disorder in which the main symptoms consist of abdominal pain and altered stool consistency and frequency. IBS patients often exhibit greater postprandial abdominal pain, discomfort, urge, and greater colonic motility (gastrocolic reflex), as well as increased stress response and visceral hypersensitivity compared with healthy controls (14). Functional imaging of IBS patients has shown abnormalities in areas of the brain involved in pain and arousal states (anterior cingulate cortex and the amygdalae) (15). Four possible IBS subtypes include IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), mixed IBS (IBS-M), and unsubtyped IBS (IBS-U), depending on the predominant stool pattern (16,17). The pathogenesis and pathophysiology of IBS are poorly understood. Low-grade inflammation and disturbances in the brain-gut axis that affect afferent signaling and central processing of nociceptive signals have been proposed to play a role (18–20). In a prospective, community-based study, GI infection as well as predisposing factors (e.g., female sex, vulnerability to diarrhea under stress, illness anxiety, and somatic symptom burden) were found to predict the development of IBS (21). Sex hormones have been suggested to have a potential mechanism in sex differences (3,22,23).
Evidence for a role of abnormalities in serotonin metabolism in IBS has been reported (24). Some suggest that IBS patients with diarrhea might have reduced serotonin reuptake and those with IBS with constipation might have impaired release of serotonin (24,25). There is also evidence of decreased melatonin metabolites in IBS-D versus IBS-C and healthy controls (26).
A high prevalence of psychiatric comorbidity—predominantly major depression and anxiety—has been reported in patients with IBS (27). One study found that depression and generalized anxiety disorder comorbidity is linked to increased symptom severity in patients with IBS (28), whereas another study showed that patients in remission from depression have no more IBS symptoms than controls (29). Sleep disorders are also prevalent in IBS patients (26,30–33), possibly mediated by alterations in tryptophan metabolism (26,30–32).
Although causality has been difficult to establish (whether psychopathology is predisposing for IBS or vice versa), there is evidence that in some patients, functional GI symptoms arise first and that mood disorders develop later, suggesting that primary gut disturbances might be the underlying driver of the mood disorder in at least a subgroup of patients (24). Subgrouping IBS patients based on a combination of GI symptoms and psychological and extraintestinal somatic symptoms common in IBS has been suggested for both clinical management and research (34). One study proposed that the IBS-C might be associated with higher levels of anxiety and depression (35); however, other studies have found no differences between IBS symptom subtypes in this regard (36).
Studies of melatonin administration in patients with IBS have reported ameliorated abdominal pain and reduced rectal pain threshold, as well as improvements in overall IBS scores and quality of life when melatonin was given orally in the evening (37–39). However, no improvement in sleep disturbances was seen in IBS patients with melatonin treatment (38,39).
We recently described the expression of melatonin in EC cells in both normal human GI tract (4) and in tumors derived from these cells (40). Patients with high tumor expression of melatonin reported less diarrhea and high daytime plasma levels of melatonin were associated with nausea (40). Considering the known actions on GI motility, high local accumulation of melatonin in the GI tract could be expected to dampen gut motor activity, whereas a more moderate rise in local melatonin likely would increase motor activity. Although there is large variability in melatonin levels between individuals, the normal melatonin rhythm is very stable over time in a given individual, rather like a hormonal fingerprint (41). Melatonin in saliva is correlated to melatonin in serum (42). Our group has previously demonstrated that levels of melatonin in saliva are highly variable, with low levels at bedtime associated with higher severity of depressive symptoms (43). The present study is conducted on data from the same cohort of young adult psychiatric patients.
Here, we examine the relationship between saliva-melatonin levels measured at three time points per individual for 1 day and self-reported GI symptoms in young adult patients primarily experiencing anxiety and affective disorders. Given the knowledge of melatonin's actions in the GI tract, we hypothesize that IBS symptoms are correlated to daytime melatonin levels in saliva.
MATERIAL AND METHODS
The study was reviewed and approved by the Regional Ethics Committee in Uppsala (Dnr 2012/81 Dnr 2012/81/1 and 2013/219) and all patients signed an informed consent.
Patient Samples
Patients in the study were part of “Uppsala Psychiatry Patient Samples,” a project designed to collect biological material from patients seeking psychiatric care at the Department of General Psychiatry, Uppsala University Hospital, Sweden. Patients from 18–25 years of age, who met the criteria for any psychiatric diagnosis (mainly affective and anxiety disorders), according to the DSM-IV, were asked to participate in the study. The cohort is described in detail elsewhere (44). The patients in this study were recruited from January 2013 to May 2014. In general, the patients in the cohort were somatically healthy; however, one patient reported celiac disease, one reported rheumatoid arthritis, but none reported inflammatory bowel disease or any other inflammatory disorder.
The self-rating version of the Montgomery-Åsberg Depression Rating Scale Self-Assessment (MADRS-S) (45–47) was used to estimate depressive symptoms. The MADRS-S contains nine questions rated on a six-point (0–6) Likert-like scale, with an overall score ranging from 0 to 54.
The Swedish translation of the Gastrointestinal Symptom Rating Scale for IBS (GSRS-IBS) was used to evaluate GI symptoms. The GSRS was originally developed to evaluate treatment for IBS and peptic ulcer disease. The GSRS-IBS differs from the GSRS as to how the questions and the answer scale are constructed. The GSRS-IBS comprises 13 items addressing only IBS symptoms that occurred in the past week (48). Symptoms were rated on a seven-point (1–7) Likert-like scale, scored from 1 (no symptoms) to 7 (very severe symptoms). The total score could range from 13 to 91 points. The GSRS questions are grouped into the following five categories: pain syndrome (question 1 and 2), bloating syndrome (question 3, 4, and 13), constipation syndrome (question 5 and 8), diarrhea syndrome (question 6, 7, 9, and 10) and satiety (question 11 and 12).
In addition to the GSRS-IBS, patients filled out questionnaires for sociodemographics, medical history, and heredity; all questions are identical to those used in LifeGene (http://www.lifegene.se) and EpiHealth (http://www.epihealth.se). One item from the following question was used in this study. “How likely are you to slumber or fall asleep in the following situation: just after eating lunch (without alcohol), - as opposed to just feeling tired? It relates to your usual way of living lately. Use the following scale to select the most suitable number for each situation: 0 = would never sleep, 1 = slight risk of sleep, 2 = moderate risk of sleep, and 3 = high risk of sleep.”
This study is a subgroup of a previously described cohort (43). Because the GSRS-IBS questionnaire was added at a later date to the Uppsala Psychiatry Patient Samples protocol, the period of the current study is different. For this study, 621 consecutive patients were eligible to participate and 264 (42.5%) consented. Saliva sampling was completed by 102 (38.6%) of these patients. Six patients were omitted because they did not fulfill criteria for any DSM-IV Axis 1 diagnosis. The present study included patients for which both GSRS-IBS scores and saliva were available (n = 96).
Saliva Collection and Analysis
Data were available from saliva samples at six time points during the waking hours of one day: when waking up, 30 minutes after waking up but before breakfast, at 11.00 hours, 30 minutes after lunch, at 22.00 hours and just before going to bed. The three time points representing daytime measurements (30 minutes after waking up but before breakfast, at 11.00 hours and 30 minutes after lunch) were selected for analysis in this study. Participants were carefully instructed and received written guidance on the method of sample collection. Saliva was collected using inert polymer cylindrical swabs (10 × 30 mm), which were then placed in a storage tube (swabs and tubes from Salimetrics Europe Ltd, Suffolk, United Kingdom) and kept in the refrigerator until delivery to the laboratory within 48 hours. Participants were instructed not to eat or drink 30 minutes before sampling. The participants documented collection times. To ensure compliance, the research assistant verified collection times and sampling method with the patient upon receipt (samples not collected as instructed were excluded). Of maximally 288 (3 × 96) hormone measurements, 9 (3%) were missing because of mistakes in saliva sampling or insufficient saliva volume.
Biochemical Analysis
Upon receipt, tubes were centrifuged and stored at −20°C until analysis. Salivary melatonin was measured with competitive ELISA (Direct Salivary Melatonin Elisa EK-DSM; Bühlmann Laboratories AG, Schönenbuch, Switzerland). Analyses were performed at the routine laboratory of the Department of Clinical Chemistry, Uppsala University Hospital, Uppsala, Sweden. The laboratory is accredited by a Swedish government authority (Swedac). Total assay variability was less than 11%. Where melatonin levels were more than 50 ng/l, limited saliva volume did not allow further dilutions. Calculations for these participants were conducted using the value 50 ng/l.
Statistics
All statistical analyses were conducted with the Statistical Package for the Social Sciences, Version 22.
Before all analyses, the continuous variables were screened for normality using the Shapiro-Wilk test of normality, p > .05, and for a visually estimated normal distribution (histogram, Q-Q plot, box plot). The total GSRS-IBS scores and subscales were not normally distributed. Salivary melatonin was not normally distributed, and hence, analyses were performed as nonparametric tests. Because of the skewed data distribution, the generalized linear model analysis was performed using the gamma distribution.
Generalized linear model analyses of melatonin in relation to GSRS-IBS scores were conducted for the three selected time points. The following possible confounding factors were included in all models: sex, body mass index (BMI), antidepressive medication, and oral contraception. A two-sided p value of less than .05 was considered significant. The Bonferroni method was applied to correct for multiple comparisons and was reported as q values.
RESULTS
Participant Characteristics
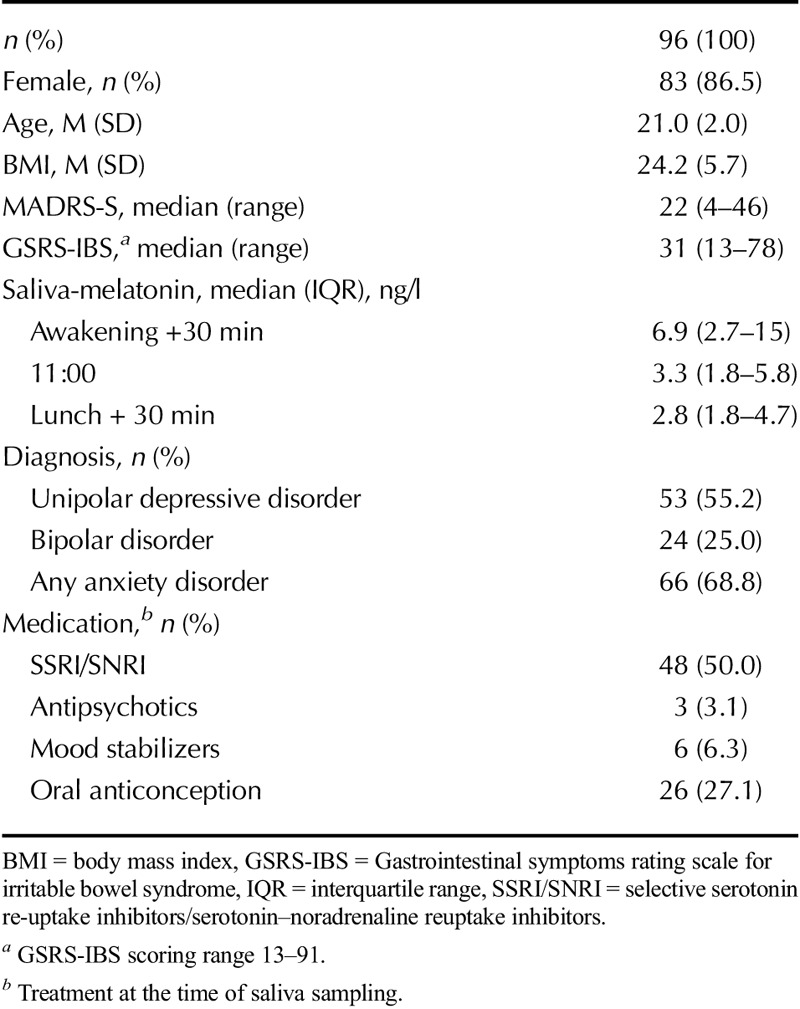
A total of 96 patients were included in the study (83 women, 13 men), with a mean age of 21 years (range = 18–25 years). The patients were diagnosed with current depression (n = 53), bipolar disorder (n = 24), and any anxiety disorder (n = 66) (some of the patients had more than one disorder). MADRS-S total scores ranged between 4 and 46, with a median value of 22 (Table 1 presents participant characteristics).
TABLE 1.
Participant Characteristics
Melatonin Levels and Correlations to Gastrointestinal Symptoms
Salivary melatonin levels ranged from 0.5 to greater than 50 ng/l for all time points (median values are shown in Table 1). The median GSRS-IBS score was 31 (range = 13–78), with an interquartile range (IQR) of 19. For the subscales, median and IQR were the following: pain = 3 (1.5), satiety = 2 (2.5), bloating = 3 (1.6), constipation = 1.5 (2), and diarrhea = 2 (1.5).
Melatonin values at three time points (awakening +30 minutes, 11:00 hours and lunch +30 minutes) were evaluated using generalized linear models with potential confounding factors (sex, BMI, use of antidepressants, and oral contraceptives) included in the analyses. A significant relationship between melatonin values after lunch (lunch +30 min) and the total GSRS-IBS score (p = .015, q = 0.045) was found (Table 2). Post hoc exploration of the subscales showed that the association was related to the symptoms of GI pain (p = .047) and bloating (p = .033). Thereafter, these subscale symptoms were tested for associations with melatonin levels at the other two time points, where a significant relationship was found between GI pain (p = .037) and melatonin values 30 minutes after waking up (awakening +30 minutes).
TABLE 2.
Generalized Linear Models for Total GSRS Scores and Melatonin Measured 30 Minutes After Waking up, at 11:00 Hours and 30 Minutes After Lunch
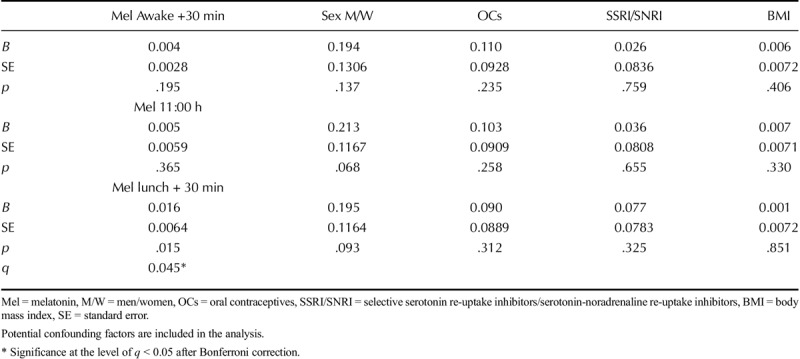
Adjusting for use of antidepressant drugs did not alter the relationship between melatonin and GI symptoms. Oral contraception may also influence melatonin levels and a separate analysis of women alone is listed in Table S1, Supplemental Digital Content 1, to interpret the contribution of oral contraception to the model, http://links.lww.com/PSYMED/A520.
In another separate analysis, a correlation between reported frequency of sleeping after lunch and higher melatonin levels after lunch was revealed. This association remained after adjustment for sex, use of oral contraceptives, and use of antidepressants (p = .028).
DISCUSSION
This study found that higher levels of melatonin after lunch were related to increased IBS symptoms in young adults seeking psychiatric care, even after adjusting for relevant confounders. The subscales GI pain and bloating were those with the highest association to postprandial melatonin levels. In another analysis, melatonin levels in saliva after lunch correlated positively with self-reported sleepiness after meals.
The results confirm the hypothesis that daytime melatonin levels are correlated to GI symptoms as measured by the GSRS-IBS. These results are in line with some research on melatonin expression and proposed function on GI motility in animals (2,10,49–52) and with our own study on patients with neuroendocrine tumors (40). In the present study, the mechanism through which higher levels of melatonin are linked to more GI symptoms may be via decreased motility. Although this study cannot determine the source of the measured postprandial saliva melatonin, there is good cause to believe that saliva melatonin levels are reflective of those in the GI tract during the daytime. EC cells are one potential source of daytime melatonin. An increase in EC cells in patients with IBS has been described (53), and speculatively, this could increase melatonin levels, at least in a subpopulation of patients with IBS. Compellingly, a role for EC cells in psychiatric disease has also been suggested but not yet proven (54).
Our finding that melatonin is positively associated with increased bloating and GI pain contrasts with studies showing that melatonin treatment reduces pain (38,39) and administration of melatonin in postmenopausal women with IBS-C reduces bloating (55). In these studies, however, melatonin was administered at night, during fasting, or both, which does not interfere with GI processing of meals and therefore may explain the discrepant results. Indeed, the authors stated that their results suggest that melatonin probably improved IBS symptoms by modifying visceral pain perception rather than influencing gut motility, sleep pattern, or psychological well-being in patients with IBS (38). In a rat model of visceral hyperalgesia, melatonin's antinociceptive effects were demonstrated to have no effect on primary sensory afferents but did exert its effect in supraspinal areas via the central opioid system (56). Accordingly, another possibility is that the experience of symptoms in the gut may be influenced by melatonin centrally. Moreover, centrally acting melatonin may influence gut motility (e.g., through cholecystokinin-induced changes in the ileum) (57). The finding that elevated postprandial melatonin in saliva was associated with sleepiness after meals may indicate central nervous system effects.
Given the connection between IBS symptoms and psychiatric morbidity, our study population would a priori likely exhibit more GI symptoms than healthy controls though fewer symptoms compared with a population diagnosed with IBS (29,48). All patients in this study experience psychiatric conditions, but the association between melatonin and IBS symptoms seems to be independent of a psychiatric diagnosis. A univariate association was found between the variable “any anxiety disorder (Y/N)” and postprandial melatonin but not with GSRS-IBS. Adding depressive symptoms (MADRS-S score) or any anxiety disorder (Y/N) to the model did not change the outcome (data not shown). Generally, patients with depression have been reported to have lower levels of melatonin at night compared with healthy controls (58–60). However, depressed patients are reported to have higher melatonin in saliva than healthy controls during the daytime (61). Melatonin levels in this population are generally high; however, the current cohort consists of young individuals from the ages 18–25 years. We know that melatonin levels decrease with age, which may explain the lower levels found in samples with older adults (62).
An alternative interpretation of the results is that elevations in melatonin may be secondary to increased immune activation and low-grade inflammation. Melatonin is produced as a scavenger of free radicals and does not only neutralize reactive oxygen species but may also modulate immune response (63–68). This is a potentially important point, because both depression and IBS are linked to increased innate immune activity and oxidative stress (66,67).
This study has several limitations. First, saliva testing was conducted at home and may have differed between the participants. Extensive measures, however, were taken to reduce this risk (described elsewhere (43)). Second, inclusion of patients from the eligible patient sample was low. We have recently conducted a study examining factors that contribute to declining participation in the biobank. Major reasons for unwillingness to participate were lack of time or feeling too sick or fatigued to take part in research (unpublished manuscript). We have, additionally, examined factors that differed between patients who completed the saliva sampling as instructed versus those who did not. Patients with executive dysfunction and high impulsiveness were less likely to deliver saliva samples in accordance with the instructions (data not shown). A third limitation is that IBS symptoms are self-reported and not a verified clinical IBS diagnosis. These findings need to be verified in IBS patients. Fourth, information about the menstruation cycle distribution was not known for the time point when the GSRS-IBS was completed and could not be controlled for. Finally, because this is a cross-sectional study, information about the directionality of the correlation between melatonin in saliva and GI symptoms cannot be extracted.
CONCLUSIONS
To our knowledge, this is the first study to examine the relationship between endogenous melatonin and GI symptoms. In a cohort of young adult psychiatric patients, higher levels of melatonin in saliva after lunch are associated with increased GI symptoms, notably bloating and pain, as measured by the GSRS-IBS. Further studies are needed to determine the relationship between melatonin and GI symptoms, which may have relevance for both psychiatric patients and patients with IBS. The source of daytime melatonin in saliva, as well as the central and peripheral mechanisms linking melatonin to GI symptoms merit further investigation.
Supplementary Material
Acknowledgments
The authors thank Ulla Nordén for her excellent technical assistance. The authors also thank Professor Lisa Ekselius and Associate Professor Mats Stridsberg for fruitful scientific discussions. The authors thank Hans Arinell for excellent assistance in the statistical analysis. Sample management has been in collaboration with Uppsala Biobank.
Source of Funding and Conflicts of Interest: This work was funded by grants to JLC from Märta och Nicke Nasvells fund, Anna-Britta Gustafssons stiftelse, Stiftelsen Apotekare Hedbergs Fund, Erik, Karin och Gösta Selanders Stiftelse, and Stiftelsen Söderström-Königska sjukhemmet, The Swedish Society of Medicine and Medical Training and Research Agreement (ALF), funds of Uppsala University Hospital. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The authors declare no competing interests.
Footnotes
Supplemental Content
REFERENCES
- 1.Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol 2011;17:3888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bubenik GA. Thirty four years since the discovery of gastrointestinal melatonin. J Physiol Pharmacol 2008;59(Suppl 2):33–51. [PubMed] [Google Scholar]
- 3.Esteban-Zubero E, Lopez-Pingarron L, Alatorre-Jimenez MA, Ochoa-Moneo P, Buisac-Ramon C, Rivas-Jimenez M, Castan-Ruiz S, Antonanzas-Lombarte A, Tan DX, Garcia JJ, Reiter RJ. Melatonin's role as a co-adjuvant treatment in colonic diseases: a review. Life Sci 2017;170:72–81. [DOI] [PubMed] [Google Scholar]
- 4.Soderquist F, Hellstrom PM, Cunningham JL. Human gastroenteropancreatic expression of melatonin and its receptors MT1 and MT2. PLoS One. 2015;10:e0120195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed R, Mahavadi S, Al-Shboul O, Bhattacharya S, Grider JR, Murthy KS. Characterization of signaling pathways coupled to melatonin receptors in gastrointestinal smooth muscle. Regul Pept 2013;184:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matheus N, Mendoza C, Iceta R, Mesonero JE, Alcalde AI. Melatonin inhibits serotonin transporter activity in intestinal epithelial cells. J Pineal Res 2010;48:332–9. [DOI] [PubMed] [Google Scholar]
- 7.Lu WZ, Song GH, Gwee KA, Ho KY. The effects of melatonin on colonic transit time in normal controls and IBS patients. Dig Dis Sci 2009;54:1087–93. [DOI] [PubMed] [Google Scholar]
- 8.Drago F, Macauda S, Salehi S. Small doses of melatonin increase intestinal motility in rats. Dig Dis Sci 2002;47:1969–74. [DOI] [PubMed] [Google Scholar]
- 9.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci 2002;47:2336–48. [DOI] [PubMed] [Google Scholar]
- 10.Thor PJ, Krolczyk G, Gil K, Zurowski D, Nowak L. Melatonin and serotonin effects on gastrointestinal motility. J Physiol Pharmacol 2007;58(Suppl 6):97–103. [PubMed] [Google Scholar]
- 11.Keating DJ, Spencer NJ. What is the role of endogenous gut serotonin in the control of gastrointestinal motility? Pharmacol Res 2018. [DOI] [PubMed] [Google Scholar]
- 12.Stasi C, Bellini M, Bassotti G, Blandizzi C, Milani S. Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Tech Coloproctol 2014;18:613–21. [DOI] [PubMed] [Google Scholar]
- 13.Lordal M, Wallen H, Hjemdahl P, Beck O, Hellstrom PM. Concentration-dependent stimulation of intestinal phase III of migrating motor complex by circulating serotonin in humans. Clin Sci (Lond) 1998;94:663–70. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Y, Kanazawa M, Palsson OS, Tilburg MAV, Gangarosa LM, Fukudo S, Drossman DA, Whitehead WE. Increased postprandial colonic motility and autonomic nervous system activity in patients with irritable bowel syndrome: a prospective study. J Neurogastroenterol Motil 2018;24:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 2011;140:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology 2016;150:1262–79. [DOI] [PubMed] [Google Scholar]
- 17.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480–91. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med 2012;367:1626–35. [DOI] [PubMed] [Google Scholar]
- 19.Chen JY, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res 2011;1392:121–31. [DOI] [PubMed] [Google Scholar]
- 20.Lawal A, Kern M, Sidhu H, Hofmann C, Shaker R. Novel evidence for hypersensitivity of visceral sensory neural circuitry in irritable bowel syndrome patients. Gastroenterology 2006;130:26–33. [DOI] [PubMed] [Google Scholar]
- 21.Lowe B, Lohse A, Andresen V, Vettorazzi E, Rose M, Broicher W. The development of irritable bowel syndrome: a prospective community-based cohort study. Am J Gastroenterol 2016;111:1320–9. [DOI] [PubMed] [Google Scholar]
- 22.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol 2012;107:991–1000. [DOI] [PubMed] [Google Scholar]
- 23.Meleine M, Matricon J. Gender-related differences in irritable bowel syndrome: potential mechanisms of sex hormones. World J Gastroenterol 2014;20:6725–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol 2016;1:133–46. [DOI] [PubMed] [Google Scholar]
- 25.Esteban-Zubero E, Alatorre-Jimenez MA, Lopez-Pingarron L, Reyes-Gonzales MC, Almeida-Souza P, Cantin-Golet A, Ruiz-Ruiz FJ, Tan DX, Garcia JJ, Reiter RJ. Melatonin's role in preventing toxin-related and sepsis-mediated hepatic damage: a review. Pharmacol Res 2016;105:108–20. [DOI] [PubMed] [Google Scholar]
- 26.Heitkemper MM, Han CJ, Jarrett ME, Gu H, Djukovic D, Shulman RJ, Raftery D, Henderson WA, Cain KC. Serum tryptophan metabolite levels during sleep in patients with and without irritable bowel syndrome (IBS). Biol Res Nurs 2016;18:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, Roger M, Tamouza R, Leboyer M, Boyer L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2014;264:651–60. [DOI] [PubMed] [Google Scholar]
- 28.Lackner JM, Ma CX, Keefer L, Brenner DM, Gudleski GD, Satchidanand N, Firth R, Sitrin MD, Katz L, Krasner SS, Ballou SK, Naliboff BD, Mayer EA. Type, rather than number, of mental and physical comorbidities increases the severity of symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2013;11:1147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karling P, Danielsson A, Adolfsson R, Norrback KF. No difference in symptoms of irritable bowel syndrome between healthy subjects and patients with recurrent depression in remission. Neurogastroenterol Motil 2007;19:896–904. [DOI] [PubMed] [Google Scholar]
- 30.Fass R, Fullerton S, Tung S, Mayer EA. Sleep disturbances in clinic patients with functional bowel disorders. Am J Gastroenterol 2000;95:1195–2000. [DOI] [PubMed] [Google Scholar]
- 31.Wang B, Duan R, Duan L. Prevalence of sleep disorder in irritable bowel syndrome: a systematic review with meta-analysis. Saudi J Gastroenterol 2018;24:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu Q, Heitkemper MM, Jarrett ME, Buchanan DT. Sleep disturbances in irritable bowel syndrome: a systematic review. Neurogastroenterol Motil 2017;29. [DOI] [PubMed] [Google Scholar]
- 33.Elsenbruch S, Harnish MJ, Orr WC. Subjective and objective sleep quality in irritable bowel syndrome. Am J Gastroenterol 1999;94:2447–52. [DOI] [PubMed] [Google Scholar]
- 34.Polster A, Van Oudenhove L, Jones M, Öhman L, Tornblom H, Simren M. Mixture model analysis identifies irritable bowel syndrome subgroups characterised by specific profiles of gastrointestinal, extraintestinal somatic and psychological symptoms. Aliment Pharmacol Ther 2017;46:529–39. [DOI] [PubMed] [Google Scholar]
- 35.Muscatello MR, Bruno A, Pandolfo G, Mico U, Stilo S, Scaffidi M, Consolo P, Tortora A, Pallio S, Giacobbe G, Familiari L, Zoccali R. Depression, anxiety and anger in subtypes of irritable bowel syndrome patients. J Clin Psychol Med Settings 2010;17:64–70. [DOI] [PubMed] [Google Scholar]
- 36.Farzaneh N, Ghobakhlou M, Moghimi-Dehkordi B, Naderi N, Fadai F. Evaluation of psychological aspects among subtypes of irritable bowel syndrome. Indian J Psychol Med 2012;34:144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saha L, Malhotra S, Rana S, Bhasin D, Pandhi P. A preliminary study of melatonin in irritable bowel syndrome. J Clin Gastroenterol 2007;41:29–32. [DOI] [PubMed] [Google Scholar]
- 38.Lu WZ, Gwee KA, Moochhalla S, Ho KY. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind placebo-controlled study. Aliment Pharmacol Ther 2005;22:927–34. [DOI] [PubMed] [Google Scholar]
- 39.Song GH, Leng PH, Gwee KA, Moochhala SM, Ho KY. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut 2005;54:1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soderquist F, Janson ET, Rasmusson AJ, Ali A, Stridsberg M, Cunningham JL. Melatonin Immunoreactivity in Malignant Small Intestinal Neuroendocrine Tumours. PLoS One 2016;11:e0164354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod 1998;3:13–22. [DOI] [PubMed] [Google Scholar]
- 42.Shirakawa S, Tsuchiya S, Tsutsumi Y, Kotorii T, Uchimura N, Sakamoto T, Yamada S. Time course of saliva and serum melatonin levels after ingestion of melatonin. Psychiatry Clin Neurosci 1998;52:266–7. [DOI] [PubMed] [Google Scholar]
- 43.Sundberg I, Ramklint M, Stridsberg M, Papadopoulos FC, Ekselius L, Cunningham JL. Salivary melatonin in relation to depressive symptom severity in young adults. PLoS One 2016;11:e0152814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunningham JL, Zanzi M, Willebrand M, Ekselius L, Ramklint M. No regrets: Young adult patients in psychiatry report positive reactions to biobank participation. BMC Psychiatry 2017;17:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svanborg P, Asberg M. A new self-rating scale for depression and anxiety states based on the Comprehensive Psychopathological Rating Scale. Acta Psychiatr Scand 1994;89:21–8. [DOI] [PubMed] [Google Scholar]
- 46.Mattila-Evenden M, Svanborg P, Gustavsson P, Asberg M. Determinants of self-rating and expert rating concordance in psychiatric out-patients, using the affective subscales of the CPRS. Acta Psychiatr Scand 1996;94:386–96. [DOI] [PubMed] [Google Scholar]
- 47.Cunningham JL, Wernroth L, von Knorring L, Berglund L, Ekselius L. Agreement between physicians' and patients' ratings on the Montgomery-Åsberg Depression Rating Scale. J Affect Disord 2011;135:148–53. [DOI] [PubMed] [Google Scholar]
- 48.Wiklund IK, Fullerton S, Hawkey CJ, Jones RH, Longstreth GF, Mayer EA, Peacock RA, Wilson IK, Naesdal J. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol 2003;38:947–54. [DOI] [PubMed] [Google Scholar]
- 49.Merle A, Delagrange P, Renard P, Lesieur D, Cuber JC, Roche M, Pellissier S. Effect of melatonin on motility pattern of small intestine in rats and its inhibition by melatonin receptor antagonist S 22153. J Pineal Res 2000;29:116–24. [DOI] [PubMed] [Google Scholar]
- 50.Sommansson A, Saudi WS, Nylander O, Sjöblom M. Melatonin inhibits alcohol-induced increases in duodenal mucosal permeability in rats in vivo. Am J Physiol Gastrointest Liver Physiol 2013;305:G95–105. [DOI] [PubMed] [Google Scholar]
- 51.Harlow HJ, Weekley BL. Effect of melatonin on the force of spontaneous contractions of in vitro rat small and large intestine. J Pineal Res 1986;3:277–84. [DOI] [PubMed] [Google Scholar]
- 52.Storr M, Schusdziarra V, Allescher HD. Inhibition of small conductance K+ -channels attenuated melatonin-induced relaxation of serotonin-contracted rat gastric fundus. Can J Physiol Pharmacol 2000;78:799–806. [PubMed] [Google Scholar]
- 53.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000;47:804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry 2016;21:738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chojnacki C, Walecka-Kapica E, Lokiec K, Pawlowicz M, Winczyk K, Chojnacki J, Klupinska G. Influence of melatonin on symptoms of irritable bowel syndrome in postmenopausal women. Endokrynol Pol 2013;64:114–20. [PubMed] [Google Scholar]
- 56.Mickle A, Sood M, Zhang Z, Shahmohammadi G, Sengupta JN, Miranda A. Antinociceptive effects of melatonin in a rat model of post-inflammatory visceral hyperalgesia: a centrally mediated process. Pain 2010;149:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonouali-Pellissier S. Melatonin is involved in cholecystokinin-induced changes of ileal motility in rats. J Pineal Res 1994;17:79–85. [DOI] [PubMed] [Google Scholar]
- 58.Souetre E, Salvati E, Belugou JL, Pringuey D, Candito M, Krebs B, Ardisson JL, Darcourt G. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res 1989;28:263–78. [DOI] [PubMed] [Google Scholar]
- 59.Mendlewicz J, Branchey L, Weinberg U, Branchey M, Linkowski P, Weitzman ED. The 24 hour pattern of plasma melatonin in depressed patients before and after treatment. Commun Psychopharmacol 1980;4:49–55. [PubMed] [Google Scholar]
- 60.Claustrat B, Chazot G, Brun J, Jordan D, Sassolas G. A chronobiological study of melatonin and cortisol secretion in depressed subjects: plasma melatonin, a biochemical marker in major depression. Biol Psychiatry 1984;19:1215–28. [PubMed] [Google Scholar]
- 61.Bouwmans ME, Bos EH, Booij SH, van Faassen M, Oldehinkel AJ, de Jonge P. Intra- and inter-individual variability of longitudinal daytime melatonin secretion patterns in depressed and non-depressed individuals. Chronobiol Int 2015;32:441–6. [DOI] [PubMed] [Google Scholar]
- 62.Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. J Clin Sleep Med 2008;4:66–9. [PMC free article] [PubMed] [Google Scholar]
- 63.Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev 2005;9:11–24. [DOI] [PubMed] [Google Scholar]
- 64.Radogna F, Diederich M, Ghibelli L. Melatonin: a pleiotropic molecule regulating inflammation. Biochem Pharmacol 2010;80:1844–52. [DOI] [PubMed] [Google Scholar]
- 65.Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res 2015;59:403–19. [DOI] [PubMed] [Google Scholar]
- 66.Anderson G, Maes M. Local melatonin regulates inflammation resolution: a common factor in neurodegenerative, psychiatric and systemic inflammatory disorders. CNS Neurol Disord Drug Targets 2014;13:817–27. [DOI] [PubMed] [Google Scholar]
- 67.Martin-Subero M, Anderson G, Kanchanatawan B, Berk M, Maes M. Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut-brain pathways. CNS Spectr 2016;21:184–98. [DOI] [PubMed] [Google Scholar]
- 68.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res 2011;51:1–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


