Supplemental Digital Content is Available in the Text.
Key Words: HIV, PMTCT, ART, attrition, retention and pregnant women
Abstract
Background:
Retention of mothers and infants across the prevention of mother-to-child HIV transmission (PMTCT) continuum remains challenging. We assessed the effectiveness of a lay worker administered combination intervention compared with the standard of care (SOC) on mother–infant attrition.
Methods:
HIV-positive pregnant women starting antenatal care at 10 facilities in western Kenya were randomized using simple randomization to receive individualized health education, retention/adherence support, appointment reminders, and missed visit tracking vs. routine care per guidelines. The primary endpoint was attrition of mother–infant pairs at 6 months postpartum. Attrition was defined as the proportion of mother–infant pairs not retained in the clinic at 6 months postpartum because of mother or infant death or lost to follow-up. Intent-to-treat analysis was used to assess the difference in attrition. This trial is registered with ClinicalTrials.gov; NCT01962220.
Results:
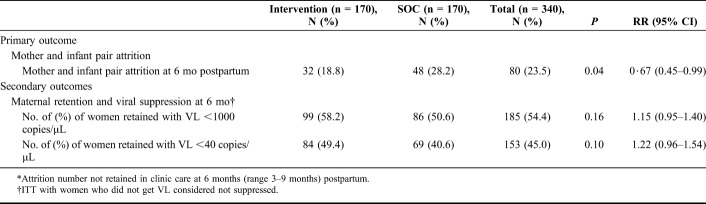
From September 2013 to June 2014, 361 HIV-positive pregnant women were screened, and 340 were randomized to the intervention (n = 170) or SOC (n = 170). Median age at enrollment was 26 years (interquartile range 22–30); median gestational age was 24 weeks (interquartile range 17–28). Overall attrition of mother–infant pairs was 23.5% at 6 months postpartum. Attrition was significantly lower in the intervention arm compared with SOC (18.8% vs. 28.2%, relative risk (RR) = 0.67, 95% confidence interval: 0.45 to 0.99, P = 0.04). Overall, the proportion of mothers who were retained and virally suppressed (<1000 copies/mL) at 6 months postpartum was 54.4%, with no difference between study arms.
Conclusions:
Provision of a combination intervention by lay counselors can decrease attrition along the PMTCT cascade in low-resource settings.
INTRODUCTION
Global efforts to scale up perinatal HIV prevention have met with increasing success, but large numbers of children still acquire HIV through mother-to-child transmission; 160,000 in 2016.1 Antiretroviral coverage for pregnant women reached 76% in 2016, but new concerns have emerged, particularly high attrition (death and loss to follow-up) during pregnancy and the postpartum period.2 Proportionally, more infections are now occurring during the postnatal period when engagement in care dissipates as in Kenya, where the majority of new infant infections now occur during breastfeeding.1 To achieve the UNAIDS elimination of mother-to-child transmission goal and reap the full benefits of PMTCT services, HIV-positive women and their infants need to remain engaged in care throughout the entire period of transmission risk.3
There are well-delineated structural, behavioral, and social factors impacting retention in HIV care; and several evidence-based interventions have proven successful in the general adult population.4,5 Commonly reported barriers to engagement in PMTCT care include transport and childcare costs, long waiting times at clinics, medication side effects, lack of family/partner support, stigma, and nondisclosure of HIV status.6–10 However, interventions to improve retention among pregnant and postpartum women have had mixed results. These include structural interventions such as integrated services, point-of-care CD4 testing; social interventions such as male partner involvement, and use of mentor mothers and lay counselors/community health workers; and behavioral interventions including cash incentives, phone calls or short message services (SMS), and home visits.11–17 Mobile phone–based communications have been associated with improved early postpartum retention; however, male partner involvement, conditional transfers, integrated services, use of community health workers, and peer mentoring have demonstrated some efficacy in some settings but not others.11–18 Furthermore, most studies have assessed a single intervention, rather than examining a combination of interventions, potentially limiting applicability in the field and capacity to address the multiple gaps across the PMTCT cascade.19 Finally, while attrition is acknowledged as a critical outcome in general adult and pediatric populations, few PMTCT studies report attrition, and it has not been measured in any randomized controlled PMTCT intervention studies. There remains an urgent need to determine the most effective strategies to reduce attrition and improve health outcomes for pregnant women and their infants.
The Mother Infant Retention for Health (MIR4Health) study was designed to evaluate the effectiveness of a combination intervention (structural, social, and behavioral) administered by trained lay workers to decrease attrition among HIV-positive pregnant women initiating PMTCT services and their infants through 6 months postpartum in Kenya.
METHODS
The study protocol was approved by the Institutional Review Board of Columbia University Medical Center and the Ethical Review Committee of the Kenya Medical Research Institute.
Study Design and Participants
The study design was previously described.20 In brief, MIR4Health was an individual-randomized trial comparing the standard of care (SOC) for PMTCT to the study combination intervention among pregnant HIV-positive women and their infants at 10 health facilities (HF) in Kisumu and Siaya Counties, Kenya (Bondo District Hospital (DH), Ahero Sub-DH (SDH), Ambira SDH, Masogo SDH, Ukwala Health Center, Nyakach DH, Madiany DH, Agulu SDH, Siaya DH, and Jaramogi Oginga Odinga Teaching and Referral Hospital). The primary objective was to evaluate the effectiveness of our combination intervention as compared with SOC on attrition among mother–infant pairs at 6 months postpartum. HIV-positive pregnant women were recruited from maternal child health (MCH) clinics at 10 HF supported by ICAP at Columbia University through funding from the President's Emergency Fund for AIDS Relief (PEPFAR) and the Centers for Disease Control and Prevention.21.
Women were eligible if they were aged older than 16 years, HIV-positive, able to provide informed consent in English or Luo, and owned or had access to a cell phone. Initially, we included only women who were newly diagnosed HIV-positive at the first antenatal visit. However, to reach study accrual in the expected timeframe, we revised the eligibility criteria to include women who were previously diagnosed with HIV. We excluded women with obstetric conditions requiring referral to another facility for specialized care, and those intending to relocate before 6 months postpartum. Clinic staff referred eligible women to onsite study staff for potential enrollment, and all participants provided written informed consent.
Randomization and Masking
Participants were assigned to the intervention or SOC at the enrollment visit using simple randomization. Numbers were generated by the study coordinator, placed in sequentially numbered opaque envelopes, and used in ascending order. Study staff opened envelopes, assigned participants to each study arm after consent had been signed, and enrollment procedures and baseline assessments were completed. Recruitment continued until the target study enrollment for each arm, across all study sites, was met. Participants and study staff were not masked to randomization group.
SOC Services
Routine antenatal, delivery, postpartum, and PMTCT care was provided to participants as per Kenyan national guidelines by health facility MCH staff. Antenatal care (ANC) included at least 4 visits during pregnancy depending on gestational age at enrollment; after delivery, mothers and infants received monthly follow-up in the MCH clinic together, for the first 6 months of life. All participants received group health education during ANC visits and had the option to enroll in monthly support group led by facility staff. Option A [antiretroviral treatment (ART) for women with CD4 <350 cells/µL or WHO 3/4, and zidovudine (AZT) after 14 weeks of gestation for women not eligible for ART] was provided at study commencement and changed to option B+ in August 2014, at which time enrollment was complete and follow-up was ongoing; women who received AZT were switched, by clinic staff to ART. As part of routine clinic procedures, women who missed an appointment were to have telephonic follow-up within 1 week, followed by a home visit for those not reached by phone.
Combination Intervention
In addition to SOC services, participants who randomized to the intervention arm were assigned a lay counselor, called a “Mama Mshauri,” at enrollment. The Mama Mshauri provided the following: (1) individualized PMTCT health education using a standardized flip chart during home and clinic visits; (2) retention and adherence support; (3) phone and SMS appointment reminders; (4) and follow-up and tracking for missed clinic visits. Mama Mshauri assisted with expediting service provision, enhancing communication between participants and health providers, assisting participants to identify and problem-solve barriers to retention and adherence, and providing psychosocial support and counseling.
Study Measures and Data Collection
All participants attended up to 5 study visits scheduled to coincide with, but conducted separately from, ANC and PMTCT/HIV visits. Study visits were planned to coincide with ANC visit in the first, second, and third trimesters and 6 weeks and 6 months postpartum. At each study visit, research assistants administered questionnaires, covering a range of topics including HIV knowledge, medication beliefs, breastfeeding practices, family planning intention, depression screening, violence, abuse, and stigma. Participants received Kenyan Shilling 400/5 USD per study visit. Blood was drawn for HIV-1 RNA viral load (VL) at the third (32–40 weeks of gestation) and fifth study visits (6 months postpartum); dried blood samples from infants for DNA polymerase chain reaction (PCR) testing were collected at study visits 4 (6 weeks postpartum) for routine early infant diagnosis and 5 (6 months postpartum) for study-specific PCR testing. Study staff contacted all participants who missed a study visit through telephone for rescheduling. Those unable to reschedule were asked to complete a short questionnaire by phone. Women who missed the final study visit were contacted by phone and visited at home to ascertain outcomes. Maternal study follow-up ended if there was a pregnancy loss or infant death.
Maternal blood samples for VL were batched and tested at the KEMRI CDC laboratory after study completion using the COBAS AmpliPrep/COBAS TaqMan HIV-1 Test. Samples with VL <40 copies/µL were reported as undetectable. For study analysis, we also reported on clients with VL <1000 copies/µL.22 Infant DNA PCR was performed at the KEMRI CDC laboratory, and results were provided to study participants within 21 days of blood draw. Infants were considered HIV infected if DNA PCR was reported as positive. Routinely collected data from ANC, maternity, HIV care, and HIV-exposed infant (HEI) care were abstracted into a customized DHIS2 study database while data from the study questionnaires were entered into a Lime Survey database. To ensure accuracy and completeness, study staff undertook data quality assurance activities to validate data entered and to check for data entry errors.
Study Outcomes
The primary outcome, mother–infant attrition, was defined as the proportion of mother–infant pairs not retained in the clinic at 6 months postpartum because of mother or infant death, or lost to follow-up (LTFU). LTFU was defined as no documented clinic attendance at 6 months postpartum in the 3 months prior or after the 6-month scheduled visit. Maternal clinic attendance was measured through attendance at an ANC or HIV care visit, and infant attendance through attendance at HEI visit, as documented in medical records and registers. We calculated retention among mother–infant pairs at 6 months postpartum to compare outcomes with other reports in the literature. We defined retention as the complement to attrition (percent attrition + percent retention = 100%).23 Women reported to have transferred to another HF were verified through phone call, and these subjects were classified as retained in outcome measures. Secondary outcomes included maternal viral suppression at 6 months postpartum, proportion retained and virally suppressed at 6 months postpartum, and measurements of PMTCT service uptake, exclusive breastfeeding, and infant HIV testing at 6 weeks and 6 months.
Statistical Analysis
The study aimed to recruit and randomize 340 HIV-positive pregnant women, 170 in each arm, to detect a 25% relative decrease in attrition with power of 80% at a significance level of 0·05 assuming 40% attrition in the SOC arm (based on historical ICAP data from Kenya). We summarized enrollment characteristics with mean values and proportions and used intent-to-treat (ITT) analysis to compare the risk of attrition between the intervention and SOC arms for mother–infant pairs using Rao–Scott likelihood ratio χ2 test. We report on the combined endpoint of retained and virally suppressed at 6 months postpartum using ITT with women who did not have a VL considered not suppressed. Infant attrition at 6 months postpartum, breastfeeding duration, and infant HIV testing were calculated separately because this was dependent on a live birth. To detect factors that may have modified the effect of the intervention, we also stratified attrition of mother–infant pairs at 6 months by HIV status at enrollment (known HIV-positive or newly identified HIV-positive), maternal age, gestational age, and HIV disclosure to partner at study enrollment. We used Breslow–Day tests for homogeneity to detect statistically significant interactions to examine whether the effect of the intervention varied depending on patient demographics and clinical characteristics. Relative risks and 95% confidence intervals were calculated for the overall comparison and the stratified analyses. Absolute risk differences were also calculated by subtracting the attrition in the intervention arm from the SOC arm. All statistical analyses were performed using SAS 9·4, Cary, NC.
RESULTS
Between September 1, 2013, and June 30, 2014, 361 pregnant HIV-positive pregnant women were assessed for eligibility; 340 were enrolled and randomized. Follow-up was completed by September 30, 2015. Table 1 shows the demographic characteristics at enrollment. The median age was 26 years [interquartile range (IQR) 22–30]; 234 (75.5%) were married, and nearly half were employed; median gestational age at enrollment was 24 weeks (IQR 17–28); and 197 (62.5%) reported the current pregnancy as unintended. Overall, 106 women (31.2%) were diagnosed HIV-positive before current pregnancy and 234 (68.8%) newly tested positive. Majority of the women were WHO stage I, 141 (41.8%) or stage II, 153 (45.4%), and only 26 (7.7%) were stage III. Among 286 (84.1%) women with documented CD4 count, median CD4 was 427 cells/mm3 (IQR, 274–601), and 103 (36%) had CD4 less than 350 cells/mm3. One hundred sixty-seven women (58.2%) reported being on AZT; 106 (36.9%) on ART with 2 nucleoside reverse transcriptase inhibitors with efavirenz or nevirapine; antiretroviral regimen was not available for 53 (15.6%) women.
TABLE 1.
Demographic Characteristics of Women (n = 340) at Study Enrollment*
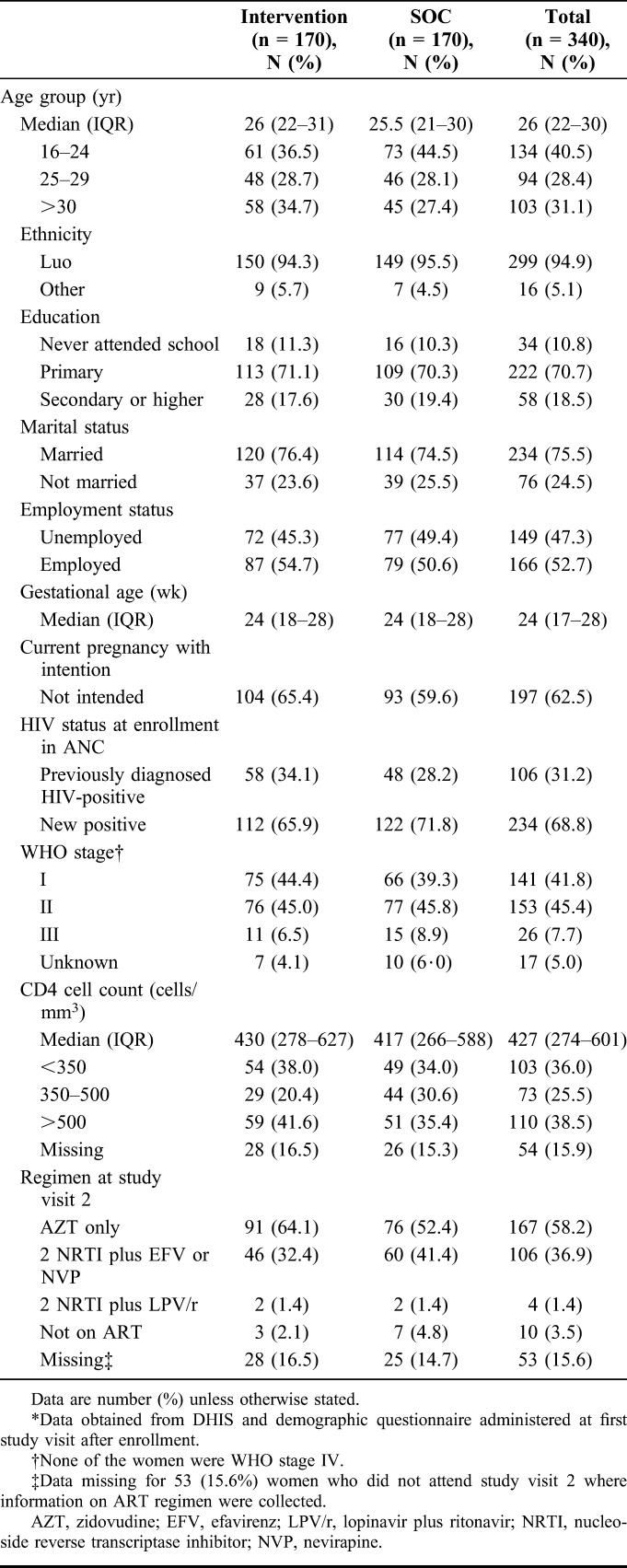
Figure 1 shows the consort diagram for the study; 340 women were randomized with 170 assigned to each study arm. During follow-up, these women had a total of 271 known live births, 142 in the intervention arm, and 129 in the SOC arm. Overall, 70 (20.5%) women were LTFU [27 (7.9%) intervention vs. 43 (12.6%) SOC]. Among women LTFU, there were 29 known pregnancy losses (12 intervention vs. 17 SOC) corresponding to 41.4% of LTFU cases. There were 9 infant deaths (5 intervention vs. 4 SOC) and 18 verified transfers (8 intervention vs. 10 SOC). Including 18 verified transfers, 260 mother–infant pairs were in care at 6 months postpartum (138 intervention vs. 122 SOC).
FIGURE 1.
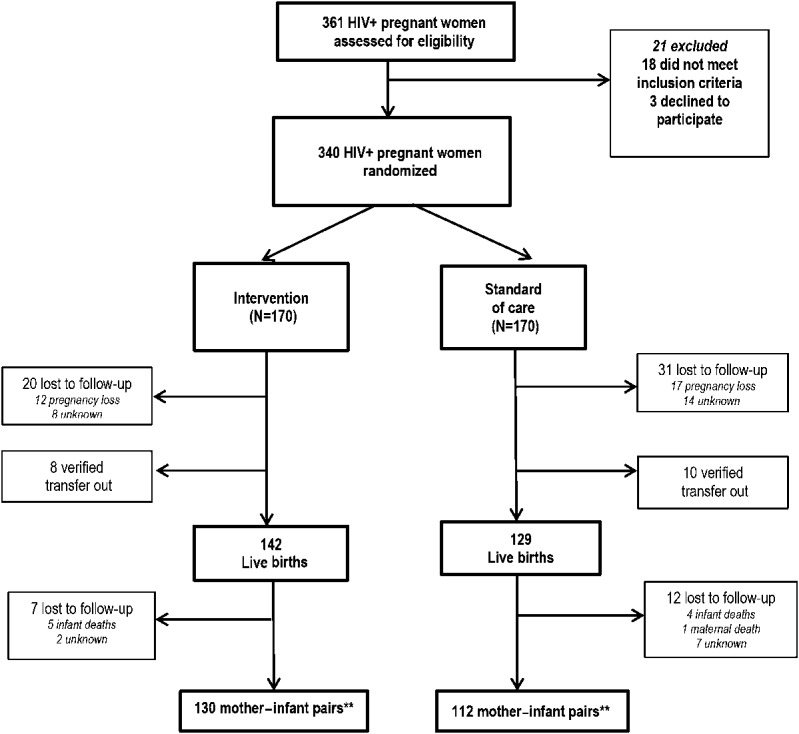
Study profile. **Eighteen verified transfers; 8 in the intervention arm and 10 in the SOC arm. These mother–infant pairs were included as retained in the subsequent calculations of mother–infant attrition. Total mother–infant pairs at 6 months including verified transfers: 260; combination intervention 138 and SOC 122.
Overall, mother–infant attrition at 6 months postpartum was 23.5% (Table 2). Attrition of mother–infant pairs was significantly lower at 6 months postpartum in the intervention arm compared with SOC (18.8% vs. 28.2%, P = 0.04). The risk of attrition for mother–infant pairs at 6 months postpartum was 33% lower in the intervention arm compared with the SOC arm [RR = 0.67, 95% confidence interval (CI): 0.45 to 0.99, P = 0.04]; the absolute risk reduction was 9.4% (95% CI: 0.5% to 18.4%). Complications resulting in pregnancy loss accounted for 36% (29/80) of overall attrition (see Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B226). We calculated the combined outcome of retained and virally suppressed at 6 months postpartum for all women; 99 (58.2%) women in the intervention arm and 86 (50.6%) in SOC were retained and suppressed to <1000 copies/µL, (RR 1.15, 95% CI: 0.95 to 1·40).
TABLE 2.
Comparison of Attrition* at 6 Months Postpartum Between Intervention and SOC Arm, ITT Analysis
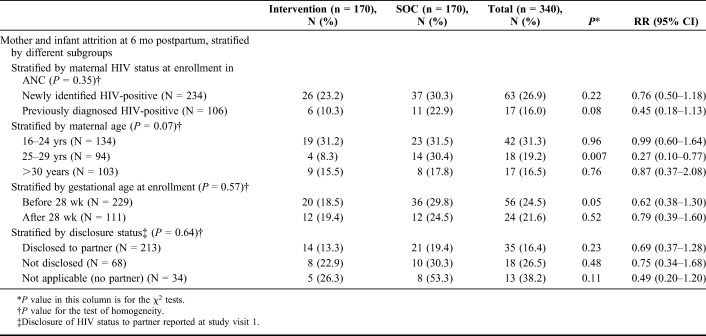
When stratified by HIV status (diagnosed HIV-positive before current pregnancy or newly identified HIV-positive), gestational age at enrollment, and partner disclosure, attrition was not significantly different among women in the intervention arm in all strata (Table 3). Maternal age seemed to be an effect measure modifier; although the intervention did not seem to be significantly associated with attrition among the youngest and oldest age groups, for women aged 25–29 years, there was a 22% reduction in mother–infant attrition risk in the intervention arm, 8.3%, compared with 30.4% in the SOC arm (RR 0.27, 95% CI: 0.10 to 0.77). After adjusting for demographic and clinical variables for the overall cohort, none of the factors were associated with mother–infant attrition (see Table 2, Supplemental Digital Content, http://links.lww.com/QAI/B226).
TABLE 3.
Stratified Analysis of Mother–Infant Attrition at 6 Months Postpartum
Amongst the 216 women on ART at the 6-month visit, 176 (81.5%) had VL <1000 copies/µL. We stratified viral suppression by duration of ART; 23 (79.3%) of 29 women who reported being on ART for less than 3 months had VL <1000 copies/µL; 48 (84.2%) of 57 on ART for 3–6 months; 40 (87.0%) of 46 on ART for 6–9 months; and 65 (77.4%) of 84 on ART greater than 9 months. Similar trends were observed for VL <40 copies/mL (see Table 3, Supplemental Digital Content, http://links.lww.com/QAI/B226).
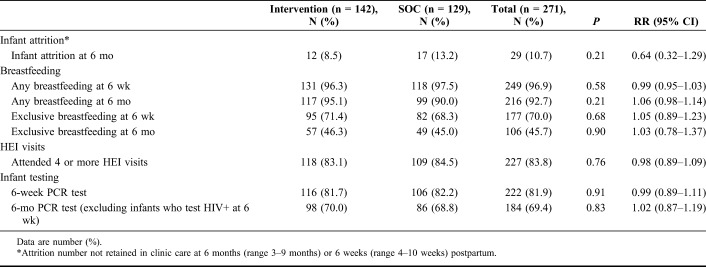
There were 271 live births, to 142 and 129 women randomized to the intervention and SOC arms, respectively. Almost all mothers reported breastfeeding, 249 (96.9%) and 216 (92.7%) at 6 weeks and 6 months, respectively (Table 4). Rates of exclusive breastfeeding were considerably lower, 177 (70.0%) and 106 (45.7%) at 6 weeks and 6 months, respectively, with no difference by study arms. A total of 222 infants (81.9%) had HIV PCR test at 6 weeks, and among infants with a negative result, 184 (69.4%) retested at 6 months test with no difference by study arm. Nine infants (3%) had positive PCR results, 3 in the intervention arm and 6 in the SOC arm (P = 0.25) (see Table 4, Supplemental Digital Content, http://links.lww.com/QAI/B226). Of these 9 infants, 2 died, 7 initiated ART, and 6 were still engaged in care at the study close.
TABLE 4.
Impact of Intervention on Infant Attrition and Uptake of Selected PMTCT Services Among Live Births (n = 271)
DISCUSSION
The MIR4Health study demonstrated that a combination intervention administered by lay counselors targeting barriers across the antenatal and postpartum PMTCT/HIV care cascade resulted in a 33% reduction in the risk of attrition among mother–infant pairs at 6 months postpartum. These interventions, provided entirely by lay workers, addressed well-described social, structural, and behavioral barriers to effective engagement in PMTCT services.20 In Kenya, where PMTCT services are relatively mature, attrition at 6 months postpartum was significantly lower among mother–infant pairs randomized to the intervention (18.8%), compared with the SOC (28.2%).
The choice of the primary outcome, attrition of mother–infant pair, underscores the importance of both members of the PMTCT dyad. Although attrition has been reported in studies of adults in HIV services, there are few PMTCT studies that measured attrition, and no randomized PMTCT trials measuring attrition.24–28 We chose to report on attrition of the mother–infant dyad because it accounts for the cumulative impact of all of the relevant events across the PMTCT cascade. Of note, all women with known pregnancy losses were subsequently LTFU from HIV care; this accounted for 41.4% of LTFU outcomes and 36% of total attrition. These findings highlight the importance of targeting this group of women if we are to improve retention of women on ART.
We hypothesized that by combining interventions, we could target multiple barriers to engagement along the PMTCT cascade, social (peer mentoring, individualized health education at home and during clinic visits), behavioral (phone calls and SMS), and structural (enhanced referrals and patient escort) challenges threatening a woman's ability to remain in long-term PMTCT services. Our retention (81.2%) of mother–infant pairs at 6 months postpartum in the intervention arm is within the range for studies that have evaluated single interventions, most for a much shorter duration of time.10,11,19,29–31 We found only 3 studies that have evaluated combination interventions, integrated clinics and male involvement in Nigeria, task shifting and home visits by peer counselors in Uganda, and integrated mother–infant pair clinics and SMS reminders for community health care workers in Malawi.32–34 In the 2 latter studies, the interventions were not superior to the SOC, probably because of high uptake of services in both the groups. Although our effect size was similar to what is reported in the literature with single interventions, we have shown that a combination package provided by lay counselors is effective in improving mother–infant retention. This is important because PMTCT programs have to cater to pregnant women with varied needs and challenges that can change over the course of the antenatal and postpartum period.
The proportion of women with VL < 1000 copies/µL (81.5%) at 6 months postpartum among retained women who reported being on ART was similar between the groups, but when we looked at the combined outcome of viral suppression and retention, only 53.5% of the total cohort of women had VL <1000 copies/µL, and somewhat higher in the intervention group (58.2%) compared with the SOC arm (50.6%, P = 0.16). These findings may help explain the shift in timing of new infant infections to the postpartum period and underscore the critical importance of retaining mothers on treatment after delivery.
While we found an important impact on attrition, we did not find a difference in PMTCT service uptake between the 2 groups, which may be attributed to high uptake of services early on in the PMTCT cascade in Kenya.35 Overall breastfeeding was high with 92.7% reporting any breastfeeding at 6 months; however, less than half of women were exclusively breastfeeding.36 The rate of perinatal HIV transmission by 6 months postpartum was low in this cohort with only 3% of the live births having acquired HIV at 6 months.37 However, we could not account for possible infections in the 9 infants who died or those who were not retained.
The scale up of HIV services has also led to the formal recognition of the lay worker cadre.22 Our study was designed to maximize the capacity of the lay workers by giving them a toolkit of interventions that have been shown to improve retention. Our results show that lay counselors who are trained, mentored, and provided with the necessary tools and job aids can improve PMTCT service delivery and outcomes, similar to findings from other studies.15,19 Our study adds to the growing literature that lay workers can assume a multifaceted set of activities including sending phone and SMS reminders, patient facilitation, and comprehensive health education both during the antenatal and postpartum period.
The study had several strengths. The use of a combination intervention by lay health workers targeting social, behavioral, and structural barriers is the first of its kind in the PMTCT setting. Our combined outcome of mother–infant attrition highlights the importance of the mother–infant dyad in PMTCT programming and also underscores the contribution of early pregnancy losses to attrition outcomes.
The study also has several limitations. Participants had to own or have access to a cell phone and agree to home visits, which could limit generalizability. The change in national PMTCT guidelines from option A–B+ also resulted in some programmatic changes, which may have affected patient care–seeking and outcomes, and affected our effect size (decrease in effect size). Finally, the study was not powered to assess the effect of individual interventions included in the combination strategy, and thus, it is not possible to determine the individual contribution of each component in decreasing attrition and the particular populations that may benefit from each one.
In conclusion, the study demonstrated a combination intervention administered by lay workers reduced attrition among pregnant and postpartum women and their infants.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the mothers and their infants who participated in the study, the MIR4Health study coordinators, research assistants, data managers, and ICAP staff in Kenya who supported recruitment, and study implementation. The authors are also grateful to the MCH/PMTCT managers at Bondo District Hospital, Ahero Sub District Hospital, Ambira Sub District Hospital, Masogo Sub District Hospital, Ukwala Health Center, Nyakach District Hospital, Madiany District Hospital, Agulu Sub District Hospital, Siaya District Hospital, and Jaramogi Oginga Odinga Teaching and Referral Hospital.
Footnotes
Supported by National Institutes of Health (NIH), Award Number: RO1 HD075163-01 and the President's Emergency Fund for AIDS Relief. NIH approved this study, which complies with NIH policies on human subjects.
Trial registration: This trial is registered with ClinicalTrials. gov; NCT0196222. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.UNAIDS. Ending AIDS. Progress Towards the 90-90-90 Targets. Geneva, Switzerland: UNAIDS; 2017. [Google Scholar]
- 2.Chi BH, Adler MR, Bolu O, et al. Progress, challenges, and new opportunities for the prevention of mother-to-child transmission of HIV under the US President's Emergency Plan for AIDS Relief. J Acquir Immune Defic Syndr. 2012;60(suppl 3):S78–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNairy ML, Teasdale CA, El-Sadr WM, et al. Mother and child both matter: reconceptualizing the prevention of mother-to-child transmission care continuum. Curr Opin HIV AIDS. 2015;10:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keane J, Pharr JR, Buttner MP, et al. Interventions to reduce loss to follow-up during all stages of the HIV care continuum in sub-Saharan Africa: a systematic review. AIDS Behav. 2017;21:1745–1754. [DOI] [PubMed] [Google Scholar]
- 5.Okeke NL, Ostermann J, Thielman NM. Enhancing linkage and retention in HIV care: a review of interventions for highly resourced and resource-poor settings. Curr HIV/AIDS Rep. 2014;11:376–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gourlay A, Birdthistle I, Mburu G, et al. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16:18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colvin CJ, Konopka S, Chalker JC, et al. A systematic review of health system barriers and enablers for antiretroviral therapy (ART) for HIV-infected pregnant and postpartum women. PLoS One. 2014;9:e108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clouse K, Pettifor A, Shearer K, et al. Loss to follow-up before and after delivery among women testing HIV-positive during pregnancy in Johannesburg, South Africa. Trop Med Int Health. 2013;18:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler MG, Lampe MA, Jamieson DJ, et al. Reducing the risk of mother-to-child human immunodeficiency virus transmission: past successes, current progress and challenges, and future directions. Am J Obstet Gynecol. 2007;197(3 suppl):S3–S9. [DOI] [PubMed] [Google Scholar]
- 10.Duff P, Kipp W, Wild TC, et al. Barriers to accessing highly active antiretroviral therapy by HIV-positive women attending an antenatal clinic in a regional hospital in western Uganda. J Int AIDS Soc. 2010;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambia J, Mandala J. A systematic review of interventions to improve prevention of mother-to-child HIV transmission service delivery and promote retention. J Int AIDS Soc. 2016;19:20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geldsetzer P, Yapa HM, Vaikath M, et al. A systematic review of interventions to improve postpartum retention of women in PMTCT and ART care. J Int AIDS Soc. 2016;19:20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz SR, Clouse K, Yende N, et al. Acceptability and feasibility of a mobile phone-based case management intervention to retain mothers and infants from an option B+ program in postpartum HIV care. Matern Child Health J. 2015;19:2029–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shroufi A, Mafara E, Saint-Sauveur JF, et al. Mother to mother (M2M) peer support for women in prevention of mother to child transmission (PMTCT) programmes: a qualitative study. PLoS One. 2013;8:e64717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcos Y, Phelps B, Bachman G. Community strategies that improve care and retention along the prevention of mother-to-child transmission of HIV cascade: a review. J Int AIDS Soc. 2012;15:17394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turan JM, Onono M, Steinfeld RL, et al. Implementation and operational research: effects of antenatal care and HIV treatment integration on elements of the PMTCT cascade: results from the SHAIP cluster-randomized controlled trial in Kenya. J Acquir Immune Defic Syndr. 2015;69:e172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Lettow M, Bedell R, Mayuni I, et al. Towards elimination of mother-to-child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (option B+) 2014. J Int AIDS Soc. 2014;17:18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nance N, Pendo P, Masanja J, et al. Short-term effectiveness of a community health worker intervention for HIV-infected pregnant women in Tanzania to improve treatment adherence and retention in care: a cluster-randomized trial. PLoS One. 2017;12:e0181919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rollins NC, Essajee SM, Bellare N, et al. Improving retention in care among pregnant women and mothers living with HIV: lessons from INSPIRE and implications for future WHO guidance and monitoring. J Acquir Immune Defic Syndr. 2017;75:S111–S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fayorsey RN, Chege D, Wang C, et al. Mother infant retention for health (MIR4Health): study design, Adaptations, and challenges with PMTCT implementation Science research. J Acquir Immune Defic Syndr. 2016;72:S137–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PEPFAR. PEPFAR 3.0 Controlling the Epidemic: Delivering on the Promise of an AIDS-free Generation; 2014. Available at: https://www.pepfar.gov/documents/organization/234744.pdf. Accessed January 19, 2018. [Google Scholar]
- 22.WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva, Switzerland: WHO; 2016. [PubMed] [Google Scholar]
- 23.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elul B, Saito S, Chung H, et al. Attrition from human immunodeficiency virus treatment programs in Africa: a longitudinal ecological analysis using data from 307 144 patients initiating antiretroviral therapy between 2005 and 2010. Clin Infect Dis. 2017;64:1309–1316. [DOI] [PubMed] [Google Scholar]
- 25.Myrtil MP, Puttkammer N, Gloyd S, et al. ART attrition across health facilities implementing option B+ in Haiti. J Int Assoc Provid AIDS Care. 2018;17:2325958218774037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domercant JW, Puttkammer N, Young P, et al. Attrition from antiretroviral treatment services among pregnant and non-pregnant patients following adoption of Option B+ in Haiti. Glob Health Action. 2017;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puttkammer N, Domerçant JW, Adler M, et al. ART attrition and risk factors among Option B+ patients in Haiti: a retrospective cohort study. PLoS One. 2017;12:e0173123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipira L, Kemp C, Domercant JW, et al. The role of service readiness and health care facility factors in attrition from Option B+ in Haiti: a joint examination of electronic medical records and service provision assessment survey data. Int Health. 2018;10:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odeny TA, Bukusi EA, Cohen CR, et al. Texting improves testing: a randomized trial of two-way SMS to increase postpartum prevention of mother-to-child transmission retention and infant HIV testing. AIDS (London, England). 2014;28:2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kebaya L, Nduati R, Wamalwa D, et al. Efficacy of mobile phone use on adherence to nevirapine prophylaxis and retention in care among HIV-exposed infants in PMTCT: a randomized control trial. Arch Dis Child. 2014;99(suppl 2):A329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fincchario-Kesseler S, Gautney BJ, Khamadi S, et al. If you text them they will come; using the HIV tracking system to improve early infant diagnosis quality and retention in Kenya. AIDS. 2014;28:S313–S321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aliyu MH, Blevins M, Audet CM, et al. Integrated prevention of mother-to-child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north-central Nigeria: a cluster-randomised controlled trial. Lancet HIV. 2016;3:e202–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiweewa FM, Wabwire D, Nakibuuka J, et al. Noninferiority of a task-shifting HIV care and treatment model using peer counselors and nurses among Ugandan women initiated on ART: evidence from a randomized trial. J Acquir Immune Defic Syndr. 2013;63:e125–e32. [DOI] [PubMed] [Google Scholar]
- 34.Mwapasa V, Joseph J, Tchereni T, et al. Impact of mother-infant pair clinics and short-text messaging service (SMS) reminders on retention of HIV-infected women and HIV-exposed infants in eMTCT care in Malawi: a cluster randomized trial. J Acquir Immune Defic Syndr. 2017;75(suppl 2):S123–S131. [DOI] [PubMed] [Google Scholar]
- 35.Kenya National Bureau of Statistics, Ministry of Health/Kenya, National AIDS Control Council/Kenya, Kenya Medical Research Institute, National Council for Population and Development/Kenya, and ICF International. Kenya Demographic and Health Survey 2014. Rockville, MD: Kenya National Bureau of Statistics, Ministry of Health/Kenya, National AIDS Control Council/Kenya, Kenya Medical Research Institute, National Council for Population and Development/Kenya, and ICF International; Available at: http://dhsprogram.com/publications/publication-FR308-DHS-Final-Reports.cfm#sthash.zKSEf7Gu.dpuf. Accessed January 19, 2018. [Google Scholar]
- 36.Cherutich P, Kim AA, Kellogg TA, et al. Detectable HIV viral load in Kenya: data from a population-based survey. PLoS One. 2016;11:e0154318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.UNAIDS. Count Down to Zero. Global Plan towards the Elimination of New HIV Infections Among Children and Keeping Their Mothers Alive. Geneva, Switzerland: UNAIDS; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.