Supplemental Digital Content is available in the text
Keywords: Anticoagulants, critical illness, lung diseases, multiple organ failure, respiratory therapy, ventilator-free days
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation, ARDS, acute respiratory distress syndrome, DIC, disseminated intravascular coagulation, ICU, intensive care unit, JAAM, the Japanese Association for Acute Medicine, J-SEPTIC DIC, Japan Septic Disseminated Intravascular Coagulation, rhTM, recombinant human-soluble thrombomodulin, SIRS, systemic inflammatory response syndrome, SOFA, Sequential Organ Failure Assessment, VFDs, ventilator-free days
ABSTRACT
Background:
Recombinant human-soluble thrombomodulin (rhTM) is a novel class therapeutic agent for managing disseminated intravascular coagulation. The progression of severe respiratory failure may be related to intra-alveolar coagulation/fibrinolytic disorders. We aimed to determine the efficacy of rhTM in treating sepsis patients with severe respiratory failure.
Methods:
We performed a retrospective observational study using an existing dataset collected from 42 intensive care units (ICUs) in Japan. Of 3,195 patients with severe sepsis or septic shock from the dataset, we selected sepsis patients with severe respiratory failure, and compared patient outcomes based on the administration of rhTM (rhTM group and no rhTM group). Propensity score analysis was performed between the two groups. Outcomes of interest were ICU mortality, hospital mortality, and ventilator-free days (VFDs).
Results:
In this study, 1,180 patients (rhTM, n = 356; no rhTM, n = 824) were analyzed. After adjusting for baseline imbalances with propensity score matching, the survival-time analysis revealed a significant difference between the two groups (hazard ratio, 0.654; 95% confidence interval, 0.439–0.974, P = 0.03). ICU mortality was lower in the rhTM group (rhTM: 22.1% [33/149] vs. no rhTM: 36.2% [54/149], P = 0.01). Hospital mortality was also lower in the rhTM group (35.6% [53/149] vs. 49.7% [74/149], P = 0.02). VFDs trended to be higher in the rhTM group than the no rhTM group (12.8 ± 10.1 days vs. 10.6 ± 10.6 days, P = 0.09).
Conclusions:
Administration of rhTM was positively correlated with a reduction in mortality in sepsis patients with severe respiratory failure.
INTRODUCTION
Recombinant human-soluble thrombomodulin (rhTM) is a novel class therapeutic agent for disseminated intravascular coagulation (DIC). Severe sepsis frequently induces DIC, a condition that causes organ damage as a result of microvascular obstruction due to excessive production of thrombin (1). Similar to thrombomodulin, rhTM contains a functional extracellular domain which binds to thrombin and thereby activates protein C and prevents excessive coagulation (2). Recently, various clinical effects of rhTM were investigated in patients with sepsis-induced DIC in a meta-analysis of systematic review and retrospective studies of large sample size (3–7). A meta-analysis of randomized controlled trials showed that rhTM administration was not related to a reduction in mortality in sepsis-induced DIC patients (5). In addition, a multicenter retrospective study, which used the Japanese Diagnosis Procedure Combination inpatient database, showed that rhTM administration was not associated with a reduction in mortality (3, 4). On the contrary, a meta-analysis of retrospective studies showed that rhTM administration was related to reduction in mortality in sepsis-induced DIC patients (5). The Japan Septic Disseminated Intravascular Coagulation (J-SEPTIC DIC) study, which was a multicenter retrospective study conducted at 42 intensive care units (ICUs) in Japan, reported that rhTM administration improved survival outcomes in patients with sepsis-induced DIC (6). A retrospective cohort study suggested that administration of rhTM to sepsis-induced DIC patients reduced mortality among patients at a high risk of death, but not among those at a low risk of death (7). A randomized, double-blind placebo-controlled phase 3 trial (ClinicalTrials.gov Identifier: NCT01598831), in which high-risk patients are included, is ongoing and has yet to report the results.
Severe sepsis is the most common cause of acute respiratory distress syndrome (ARDS) (8, 9). ARDS is caused by alveolar macrophage and neutrophil-activated inflammatory cytokines (10). Tissue factors released by the alveolar macrophages and neutrophils generate thrombin in an extrinsic coagulation cascade pathway (11, 12). Analysis of bronchoalveolar lavage fluid from patients with pneumonia and ARDS showed increased activation of coagulation and inhibition of fibrinolysis, correlating with inflammation severity (13). Furthermore, intra-alveolar coagulation/fibrinolytic disorders might contribute to the progression of lung injuries (14–16). Intra-alveolar coagulation/fibrinolytic disorders in severe respiratory failure may more accurately be termed “intra-alveolar DIC.” It was reported that antithrombotic drugs might have a beneficial role for patients with ARDS (17, 18).
Although there are many studies that examined the effect of rhTM on septic-induced DIC patients (3, 5–7), only two studies on sepsis patients with severe respiratory failure, such as ARDS, have so far been published with mixed results (4, 19). Thus, we conducted the present study to analyze the effect of rhTM administration on sepsis patients with severe respiratory failure, regardless of the complication of DIC. We accessed an existing dataset from a multicenter, retrospective observational study and used propensity score analysis to ensure balance in the treatment and nontreatment groups.
PATIENTS AND METHODS
Study design and setting
This study was a retrospective observational study using the dataset from the J-SEPTIC DIC study. The J-SEPTIC DIC study reviewed information from consecutive patients admitted to 42 ICUs from 40 institutions throughout Japan. The study design was approved by the Institutional Review Board at each hospital (see Table S1), and the requirement for informed consent was waived because of the retrospective study design. The present study analyzed information from the anonymized database of the J-SEPTIC DIC study, which was registered in the University Hospital Medical Information Network Individual Case Data Repository (UMIN-CTR000012543, http://www.umin.ac.jp/icdr/index-j.html).
The J-SEPTIC DIC study included patients with severe sepsis or septic shock who were admitted to a target ICU between January 1, 2011 and December 31, 2013. Severe sepsis was defined using the International Sepsis Definitions Conference criteria (20). Patients were excluded if they were less than 16 years old, or developed either severe sepsis or septic shock after admission to the ICU.
Patient selection and outcomes
Patient information in the J-SEPTIC DIC study dataset was extracted from the electronic medical records of the participating hospitals. This information included patient characteristics, therapeutic interventions, and ICU characteristics. The data accessed for our study were age; sex; body weight; admission route to the ICU; comorbidities; preexisting coagulation abnormality; Acute Physiology and Chronic Health Evaluation (APACHE) II score (21); Sequential Organ Failure Assessment (SOFA) score (22); initial systemic inflammatory response syndrome (SIRS) score (23); primary infection site; blood culture results; microorganisms responsible for sepsis; daily results of laboratory tests; lactate levels; medications, including anti-DIC drugs, other anticoagulants, immunoglobulins, and low-dose steroids; transfusion volumes and bleeding complications during the first week in ICU; therapeutic interventions, including surgery at the infection site, renal replacement therapy, polymyxin B direct hemoperfusion, extracorporeal membrane oxygenation, and intra-aortic balloon pumping; and outcomes until ICU discharge. Comorbidity information was assembled using the APACHE II-weighted comorbidity score. Use of medications and therapeutic interventions, laboratory tests, transfusion volumes, and bleeding complications were recorded up to 7 days after ICU admission. SOFA score, SIRS score, and lactate levels were recorded on days 1, 3, and 7 after ICU admission.
For the present study, we selected 1,180 sepsis patients from the J-SEPTIC DIC dataset who had severe respiratory failure, defined as an initial respiratory SOFA score at least 3. These patients were then divided into two groups: the rhTM group (received rhTM) and the no rhTM group (did not receive rhTM). Among patients with DIC, rhTM was administered at the discretion of the attending physician, and there was no predefined protocol regarding rhTM use. Typically, 380 U/kg/d was administered intravenously to patients with DIC, but without severe renal dysfunction. In patients with DIC and severe renal dysfunction, the dosage of rhTM was 130 U/kg/d. Administration of rhTM usually continued for 6 days or until the DIC improved. DIC improvement was determined by each attending physician. The attended physicians determined DIC improvement based on the Japanese Association for Acute Medicine (JAAM)'s DIC diagnostic criteria. DIC scores were calculated using the JAAM's DIC scoring algorithm (24). Missing values were scored as 0. Patients were classified as having DIC if they had no preexisting hemostatic disorders and a DIC score at least 4 on a single day during the first week after ICU admission (on days 1, 3, or 7).
Outcomes of interest were ICU mortality, hospital mortality, and ventilator-free days (VFDs). The VFDs were calculated by subtracting the number of days of assisted breathing from 28 days. If a patient was discharged within 28 days of ICU admission, VFDs were calculated by subtracting the length of their ICU stay from 28 days. This is because no information was available after their discharge from the ICU. For example, if a patient with ventilator was discharged at 14 days, VFDs of the patient was 14. Patients who died within 28 days of admission were assigned 0 VFDs.
Statistical analysis
Patients were assigned a propensity score for the administration of rhTM. Confounders were chosen for their potential association with the outcomes of interest based on existing clinical knowledge. Propensity to administer rhTM was estimated using a logistic regression model. To estimate the propensity score, we selected clinically relevant covariates: age; sex; body weight; preexisting hemostatic disorders; APACHE II score; initial SOFA score of each organ system (except coagulation); initial SIRS score; initial DIC score; respiratory infection; microorganisms responsible for the sepsis; blood culture results; initial laboratory tests (white blood cell count, platelet count, hemoglobin level, prothrombin time–international normalized ratio); anti-DIC drugs (antithrombin, protease inhibitor, heparins); other drugs (anticoagulants, immunoglobulins, low-dose steroids); surgery at the infection site; renal replacement therapy; and polymyxin B direct hemoperfusion. Propensity scores were matched using a caliper width one-fifth of the SD (0.187), which was calculated by logit transformation of the propensity score. Data were described in terms of means ± SDs or medians [interquartile range], as appropriate. The standardized difference was used to measure the covariate balance. In the matched groups, unadjusted comparisons were performed using the Mann–Whitney U test, Fisher exact test, and chi-squared test, as appropriate. Kaplan–Meier curves for the estimated probability of survival as a function of time from ICU admission were compared in the matched groups using the log-rank test and a Cox regression model.
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan). This is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) in which the R software is modified to include statistical functions frequently used in biostatistics (25).
RESULTS
Of the 3,195 patients with severe sepsis or septic shock included in the J-SEPTIC DIC study, 1,180 patients met the entry criteria for the present study. Of these, 356 (30.2%) were administered rhTM (Fig. 1). When comparing the unmatched groups, patients in the rhTM group had more severe conditions and more frequent treatment interventions than those in the no rhTM group. Propensity score matching created 149 matched pairs, after which the characteristics of the two groups were considerably more balanced (Table 1).
Fig. 1.
Patient flow diagram.
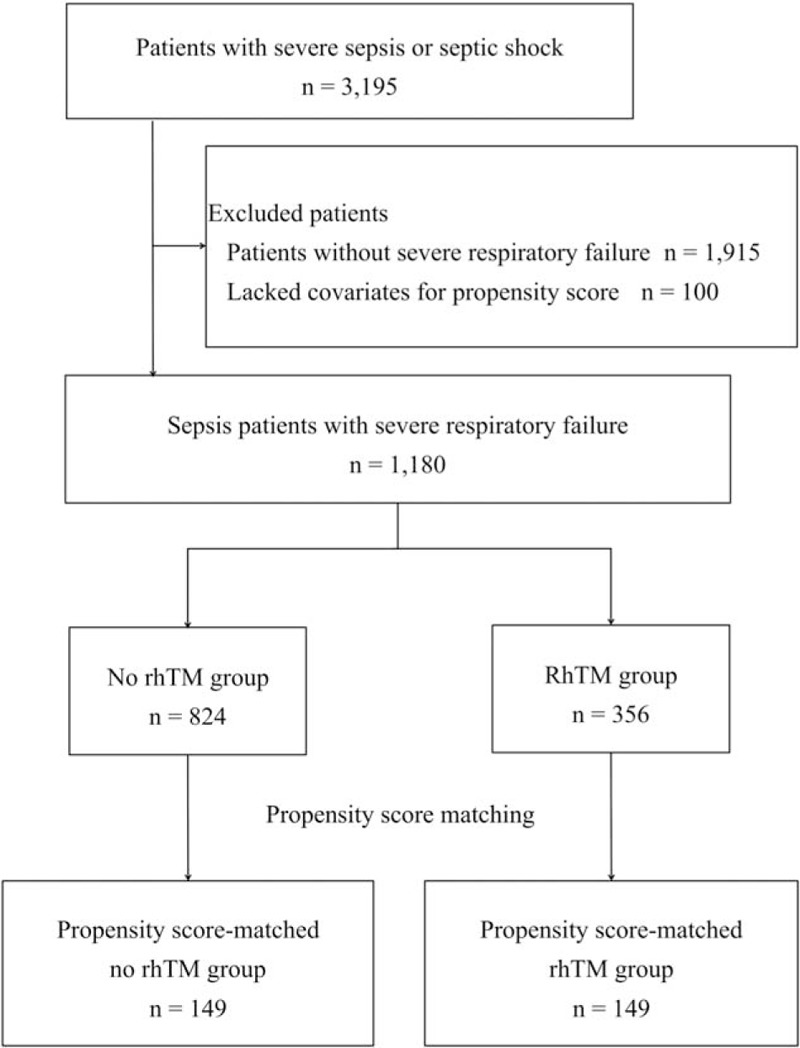
Severe respiratory failure is defined as an initial respiratory SOFA score at least 3. rhTM, recombinant human-soluble thrombomodulin.
Table 1.
Baseline characteristics of sepsis patients with severe respiratory failure
| Variate | Unmatched | Matched | ||||
| No rhTM | RhTM | No rhTM | RhTM | |||
| n | 824 | 356 | Standardized difference, % | 149 | 149 | Standardized difference, % |
| Age, y | 70.4 ± 13.9 | 68.6 ± 13.2 | −1.0 | 69.9 ± 13.8 | 69.7 ± 13.5 | −0.1 |
| Male sex | 530 (64.3%) | 223 (62.6%) | −3.5 | 90 (60.4%) | 97 (65.1%) | 9.7 |
| Weight, kg | 57.9 ± 14.8 | 57.3 ± 13.7 | −0.27 | 55.2 ± 13.1 | 58.0 ± 14.3 | 1.5 |
| Preexisting hemostatic disorders | 156 (18.9%) | 62 (17.4%) | −3.9 | 29 (19.5%) | 27 (18.1%) | −3.6 |
| APACHEII score | 25 [19–31] | 27 [21–32] | 1.3 | 27 [21–34] | 25 [19–31] | −2.2 |
| Initial total SOFA score | 11 [8–14] | 13 [10–15] | 12.0 | 11 [9–14] | 11 [8–13] | −5.6 |
| Central nervous system | 2 [1–3] | 2 [1–3] | 1.4 | 2 [1–3] | 2 [1–3] | −5.8 |
| Renal | 1 [0–3] | 3 [1–3] | 21.0 | 1 [0–3] | 1 [0–3] | −1.9 |
| Respiratory | 3 [3–4] | 3 [3–4] | −25.0 | 3 [3–4] | 3 [3–4] | −12.5 |
| Coagulation | 1 [0–2] | 2 [0–3] | 33.7 | 1 [0–2] | 1 [0–2] | −14.6 |
| Cardiovascular | 3 [1–4] | 4 [3–4] | 23.0 | 3 [2–4] | 3 [2–4] | −12.0 |
| Liver | 0 [0–1] | 0 [0–2] | 26.8 | 0 [0–1] | 0 [0–1] | −5.5 |
| Initial SIRS score | 3 [3–4] | 3 [3–4] | 10.2 | 3 [3–4] | 3 [3–4] | 7.1 |
| Initial JAAM DIC score | 4 [2–5] | 5 [4–7] | 28.3 | 5 [3–6] | 4 [3–6] | −3.7 |
| Respiratory SOFA score | ||||||
| Score 3 | 531 (64.4%) | 249 (69.9%) | 11.7 | 101 (67.8%) | 105 (70.5%) | 5.8 |
| Score 4 | 293 (35.6%) | 107 (30.1%) | −11.7 | 48 (32.2%) | 44 (29.5%) | −5.8 |
| Presence of lung infection | 350 (42.5%) | 126 (35.4%) | −14.6 | 66 (44.3%) | 60 (40.3%) | −8.1 |
| Blood culture results | ||||||
| Positive | 323 (39.2%) | 176 (49.4%) | 20.7 | 57 (38.3%) | 64 (43.0%) | 9.6 |
| Negative | 455 (55.2%) | 168 (47.2%) | −16.1 | 83 (55.7%) | 79 (53.0%) | −5.3 |
| No test | 46 (5.6%) | 12 (3.4%) | −10.7 | 9 (6.0%) | 6 (4.0%) | −9.2 |
| Microorganisms responsible for the sepsis | ||||||
| Gram negative rods | 261 (31.7%) | 138 (38.8%) | 14.9 | 44 (29.5%) | 45 (30.2%) | 1.5 |
| Gram positive cocci | 199 (24.2%) | 96 (27.0%) | 6.5 | 31 (20.8%) | 35 (23.5%) | 6.4 |
| Fungus | 14 (1.7%) | 6 (1.7%) | −0.1 | 6 (4.0%) | 4 (2.7%) | −7.4 |
| Virus | 6 (0.7%) | 2 (0.6%) | −2.1 | 2 (1.3%) | 1 (0.7%) | −6.7 |
| Mixed infection | 126 (15.3%) | 50 (14.0%) | −3.5 | 27 (18.1%) | 31 (20.8%) | 6.7 |
| Others | 24 (2.9%) | 4 (1.1%) | −12.8 | 2 (1.3%) | 4 (2.7%) | 9.5 |
| Unknown | 194 (23.5%) | 60 (16.9%) | −16.7 | 37 (24.8%) | 29 (19.5%) | −13.0 |
| White blood cell count, 109/L | 10.4 [4.0–16.1] | 9.0 [2.7–16.9] | 0.03 | 9.4 [2.6–16.1] | 9.6 [2.8–15.3] | −0.16 |
| Hemoglobin, g/L | 10.7 [8.9–12.6] | 10.6 [9.1–12.1] | −2.7 | 9.0 [7.5–10.8] | 10.6 [9.3–12.2] | 19.7 |
| Platelet count, 109/L | 136.5 [77.0–212.5] | 88.5 [46.0–146.0] | −0.49 | 99.0 [52.0–187.0] | 124.0 [78.0–183.0] | 0.15 |
| PT-INR | 1.3 [1.2–1.6] | 1.4 [1.2–1.7] | 7.8 | 1.4 [1.2–1.7] | 1.3 [1.2–1.5] | −13.0 |
| Medications | ||||||
| Antithrombin III | 209 (25.4%) | 208 (58.4%) | 71.0 | 31 (20.8%) | 42 (28.2%) | 17.3 |
| Protease inhibitor | 124 (15.0%) | 61 (17.1%) | 5.7 | 23 (15.4%) | 29 (19.5%) | 10.8 |
| Heparins | 41 (5.0%) | 15 (4.2%) | −3.8 | 5 (3.4%) | 12 (8.1%) | 20.3 |
| Other anticoagulant | 336 (40.8%) | 208 (58.4%) | 35.8 | 68 (45.6%) | 66 (44.3%) | 2.6 |
| Immunoglobulins | 232 (28.2%) | 185 (52.0%) | 50.1 | 29 (19.5%) | 51 (34.2%) | 33.6 |
| Low-dose steroids | 222 (26.9%) | 164 (46.1%) | 40.7 | 43 (28.9%) | 45 (30.2%) | 2.8 |
| Therapeutic interventions | ||||||
| Surgical interventions at the infection site | 266 (32.3%) | 140 (39.3%) | 14.6 | 46 (30.9%) | 53 (35.6%) | 10.0 |
| RRT | 253 (30.7%) | 202 (56.7%) | 54.3 | 55 (36.9%) | 48 (32.2%) | −9.9 |
| PMX-DHP | 154 (18.7%) | 129 (36.2%) | 40.0 | 25 (16.8%) | 33 (22.1%) | 13.4 |
APACHE indicates Acute Physiology and Chronic Health Evaluation; DIC, disseminated intravascular coagulation; ICU, intensive care unit; JAAM, the Japanese Association for Acute Medicine; PMX-DHP, Polymyxin B-direct hemoperfusion; PT-INR, prothrombin time–international normalized ratio; rhTM, recombinant human soluble thrombomodulin; RRT, renal replacement therapy; SIRS, systemic inflammatory response syndrome; SOFA, Sequential Organ Failure Assessment.
Data are presented as n (%), mean ± SD, or median [interquartile range].
Both ICU mortality and hospital mortality were significantly lower in the matched rhTM group than the matched no rhTM group (Table 2). Survival-time analysis revealed a significant difference between the propensity score matched treatment and no rhTM group (hazard ratio, 0.654; 95% confidence interval, 0.439–0.974, P = 0.03) (Fig. 2). VFDs trended to be higher in the matched rhTM group than the matched no rhTM group (matched rhTM group: 12.8 ± 10.1 days vs. matched no rhTM group: 10.6 ± 10.6 days, P = 0.09) (Table 2). Adverse events related to bleeding and transfusion volumes were not statistically different between the two matched groups during the first 7 days after admission to the ICU (Table 3).
Table 2.
Outcomes in the matched no rhTM and rhTM groups
| No rhTM | RhTM | ||
| n | 149 | 149 | P |
| Ventilator free days, d | 10.6 ± 10.6 | 12.8 ± 10.1 | 0.09 |
| Hospital mortality | 74 (49.7%) | 53 (35.6%) | 0.02 |
| ICU mortality | 54 (36.2%) | 33 (22.1%) | 0.01 |
ICU indicates intensive care unit; rhTM, recombinant human soluble thrombomodulin.
P values were calculated using the Mann-Whitney U test and Chi-squared test.
Data are presented as n (%), mean ± SD.
Fig. 2.
Survival-time analysis for patients in the matched no rhTM and rhTM groups.
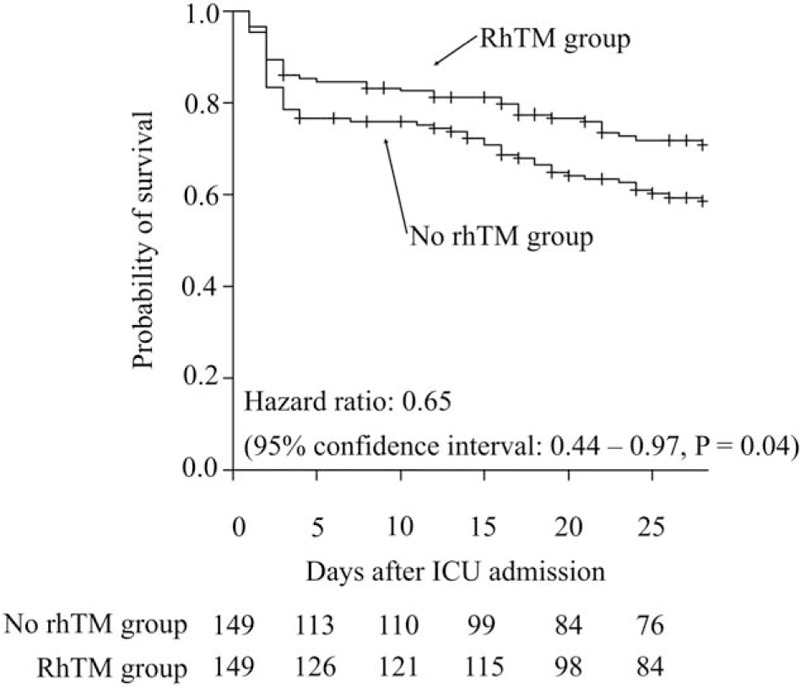
The probability of survival is higher in the rhTM group than the no rhTM group. rhTM, recombinant human-soluble thrombomodulin.
Table 3.
Bleeding complications and transfusion amounts in the matched no rhTM and rhTM groups
| No rhTM | RhTM | ||
| n | 149 | 149 | P |
| Bleeding complications during 7 d after ICU admission | |||
| Bleeding requiring transfusion | 18 (12.1%) | 13 (8.7%) | 0.45 |
| Bleeding requiring a therapeutic intervention | 1 (0.7%) | 1 (0.7%) | 1.00 |
| Intracranial bleeding | 1 (0.7%) | 0 (0.0%) | 1.00 |
| Death by bleeding | 0 (0.0%) | 1 (0.7%) | 1.00 |
| Amount of transfusion during 7 d after ICU admission | |||
| Red blood cell concentration, units | 2.0 [0.0–6.0] | 2.0 [0.0–4.0] | 0.17 |
| Fresh frozen plasma, units | 0.0 [0.0–5.0] | 0.0 [0.0–4.0] | 0.53 |
| Platelet concentration, units | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.48 |
ICU indicates intensive care unit; rhTM, recombinant human soluble thrombomodulin.
P values were calculated using the Mann–Whitney U test and Fisher exact test.
Data are presented as n (%), median [interquartile range].
DISCUSSION
This study involved propensity score matching analysis of a selection of patients from the database of a multicenter, retrospective observational study of 42 ICUs throughout Japan. We identified that administration of rhTM was related to a reduced mortality in sepsis patients with severe respiratory failure, regardless of the complication of DIC. VFDs trended to be higher in the matched rhTM group than the matched no rhTM group. In addition, bleeding complications were not increased by the administration of rhTM.
Some studies did not show any beneficial effects of rhTM on sepsis-induced DIC patients (3, 4, 26). Patients included in these studies were presumed to be at a lower-risk of death (27). In a previous randomized, double-blind placebo-controlled phase 2b study, although no survival benefit of rhTM was observed in patients with both sepsis and suspected DIC, a survival benefit was observed in more severe patients (28). Furthermore, other studies reported that benefits of rhTM were easier to observe in more severe patients (5, 7).
In retrospective studies, administration of rhTM was associated with improvement in respiratory dysfunction associated with sepsis-induced DIC (29–31). In sepsis-induced DIC patients, rhTM administration showed a tendency toward a decrease in respiratory SOFA scores in previous historic cohort studies (29, 30). In sepsis-induced DIC patients, administration of rhTM increased in the number of VFDs when compared with a control in a propensity score analysis (31). Though improvement in respiratory dysfunction by rhTM administration was expected, the results of each study for patients with severe respiratory failure were contradictory (4, 19). In one recent retrospective study, rhTM administration was not associated with an increase in the VFDs of sepsis-induced DIC patients with severe pneumonia (4). In another retrospective study that included a small sample size, rhTM administration was associated with improvement of mortality in patients with both DIC and ARDS though respiratory outcomes were not investigated (19). Several animal studies suggested the mechanisms for the effect of rhTM on ARDS (32–35). Inhibition of pulmonary vascular injury by rhTM was related to protein C activation, which had anti-inflammatory, rather than anticoagulant effects (32, 33). One anti-inflammatory mechanism is the inhibition of the inflammatory pathway. Levels of inflammatory cytokines and proinflammatory high-mobility group box 1 protein were decreased by rhTM (34). Another mechanism is the activation of the anti-inflammatory pathway. The proliferation of regulatory T cells and the expression of anti-inflammatory cytokines were increased by rhTM (35). The respiratory outcome of rhTM administration has never been studied in a group with severe respiratory failure, defined as having a PaO2/FiO2 ratio at most 200 and receiving respiratory support, regardless of the complication of DIC. Our study suggests that rhTM administration may be associated with beneficial respiratory effects in sepsis patients with severe respiratory failure of non-ARDS. Because statistical power and respiratory system-related outcomes other than VFDs are lacking, further research is needed to determine the role of rhTM in sepsis patients with severe respiratory failure.
Our study had some limitations. First, we could not identify the exact timing of therapeutic interventions. However, therapeutic interventions, including the administration of rhTM, usually occurred at the time of ICU admission, and other therapeutic interventions were not affected by rhTM administration. Therefore, we considered it acceptable to use the therapeutic interventions to estimate the propensity score. Second, the dose and duration of therapeutic interventions, including rhTM, could not be identified. However, we assumed that patients received the dose and duration approved in Japan. Third, we evaluated the severity of respiratory failure using the respiratory SOFA score. More detailed information (e.g., ventilator settings, lung injury scores) may be needed for an accurate evaluation. Finally, we could not follow up patients after discharge from the treating hospital.
CONCLUSIONS
Using propensity analysis in a large cohort, we identified that administration of rhTM was related to a reduced mortality in sepsis patients with severe respiratory failure, regardless of the complication of DIC. To understand the clinical implications of this, future studies of patients with severe respiratory failure, such as septic ARDS, in prospective multicenter randomized trials should be conducted.
Supplementary Material
Footnotes
Author's contributions: SY is principal author and takes responsibility for the integrity of the data and the accuracy of the data analysis. MS and MH contributed to the study concept and design; SY, MS, AH, AT, NK, and TT contributed to data collection; SY, MS, MH, and KO contributed to data analysis. All authors contributed to writing and revising the manuscript and approved the final manuscript.
All authors received no funding for this work from any organization.
The authors report no conflicts of interest.
REFERENCES
- 1.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol 145 1:24–33, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Mohri M, Sugimoto E, Sata M, Asano T. The inhibitory effect of recombinant human soluble thrombomodulin on initiation and extension of coagulation—a comparison with other anticoagulants. Thromb Haemost 82 6:1687–1693, 1999. [PubMed] [Google Scholar]
- 3.Tagami T, Matsui H, Fushimi K, Yasunaga H. Use of recombinant human soluble thrombomodulin in patients with sepsis-induced disseminated intravascular coagulation after intestinal perforation. Front Med (Lausanne) 2:7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Recombinant human soluble thrombomodulin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost 13 1:31–40, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Yamakawa K, Aihara M, Ogura H, Yuhara H, Hamasaki T, Shimazu T. Recombinant human soluble thrombomodulin in severe sepsis: a systematic review and meta-analysis. J Thromb Haemost 13 4:508–519, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Hayakawa M, Yamakawa K, Saito S, Uchino S, Kudo D, Iizuka Y, Sanui M, Takimoto K, Mayumi T, Ono K. Recombinant human soluble thrombomodulin and mortality in sepsis-induced disseminated intravascular coagulation. A multicentre retrospective study. Thromb Haemost 115 6:1157–1166, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura J, Yamakawa K, Ogura H, Umemura Y, Takahashi H, Morikawa M, Inoue Y, Fujimi S, Tanaka H, Hamasaki T, et al. Benefit profile of recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Crit Care 19:78, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315 8:788–800, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Zampieri FG, Mazza B. Mechanical ventilation in sepsis: a reappraisal. Shock 47 1S Suppl. 1:41–46, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med 17 (3–4):293–307, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz MJ, Haitsma JJ, Zhang H, Slutsky AS. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia—a review. Crit Care Med 34 3:871–877, 2006. [PubMed] [Google Scholar]
- 12.Glas GJ, Van Der Sluijs KF, Schultz MJ, Hofstra JJ, Van Der Poll T, Levi M. Bronchoalveolar hemostasis in lung injury and acute respiratory distress syndrome. J Thromb Haemost 11 1:17–25, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Gunther A, Mosavi P, Heinemann S, Ruppert C, Muth H, Markart P, Grimminger F, Walmrath D, Temmesfeld-Wollbruck B, Seeger W. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med 161 (2 Pt. 1):454–462, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Abraham E. Coagulation abnormalities in acute lung injury and sepsis. Am J Respir Cell Mol Biol 22 4:401–404, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Gando S, Kameue T, Matsuda N, Sawamura A, Hayakawa M, Kato H. Systemic inflammation and disseminated intravascular coagulation in early stage of ALI and ARDS: role of neutrophil and endothelial activation. Inflammation 28 4:237–244, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med 35 8:1821–1828, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyoshi S, Ito R, Katayama H, Dote K, Aibiki M, Hamada H, Okura T, Higaki J. Combination therapy with sivelestat and recombinant human soluble thrombomodulin for ARDS and DIC patients. Drug Des Devel Ther 8:1211–1219, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panka BA, de Grooth HJ, Spoelstra-de Man AM, Looney MR, Tuinman PR. Prevention or treatment of ARDS with aspirin: a review of preclinical models and meta-analysis of clinical studies. Shock 47 1:13–21, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uba T, Nishi K, Umegaki T, Ohashi N, Kusaka Y, Umegaki O, Nishi S. The influence of human soluble recombinant thrombomodulin on in-hospital mortality in patients with acute respiratory distress syndrome and disseminated intravascular coagulation: a retrospective multicenter study. J Intensive Crit Care 3 3:40, 2017. [Google Scholar]
- 20.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31 4:1250–1256, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 13 10:818–829, 1985. [PubMed] [Google Scholar]
- 22.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26 11:1793–1800, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101 6:1644–1655, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, Mayumi T, Murata A, Ikeda T, Ishikura H, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med 34 3:625–631, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Kanda Y. Investigation of the freely available easy-to-use software ’EZR’ for medical statistics. Bone Marrow Transplant 48 3:452–458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagiwara A, Tanaka N, Uemura T, Matsuda W, Kimura A. Can recombinant human thrombomodulin increase survival among patients with severe septic-induced disseminated intravascular coagulation: a single-centre, open-label, randomised controlled trial. BMJ Open 6 12:e012850, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wada H, Aota T, Matsumoto T, Suzuki K, Imai H, Katayama N. Antithrombin or thrombomodulin administration in severe pneumonia patients with sepsis and disseminated intravascular coagulation: comment on two papers. J Thromb Haemost 13 4:684–685, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Vincent JL, Ramesh MK, Ernest D, LaRosa SP, Pachl J, Aikawa N, Hoste E, Levy H, Hirman J, Levi M, et al. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med 41 9:2069–2079, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Yamakawa K, Fujimi S, Mohri T, Matsuda H, Nakamori Y, Hirose T, Tasaki O, Ogura H, Kuwagata Y, Hamasaki T, et al. Treatment effects of recombinant human soluble thrombomodulin in patients with severe sepsis: a historical control study. Crit Care 15 3:R123, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa Y, Yamakawa K, Ogura H, Kiguchi T, Mohri T, Nakamori Y, Kuwagata Y, Shimazu T, Hamasaki T, Fujimi S. Recombinant human soluble thrombomodulin improves mortality and respiratory dysfunction in patients with severe sepsis. J Trauma Acute Care Surg 72 5:1150–1157, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Yamakawa K, Ogura H, Fujimi S, Morikawa M, Ogawa Y, Mohri T, Nakamori Y, Inoue Y, Kuwagata Y, Tanaka H, et al. Recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Intensive Care Med 39 4:644–652, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchiba M, Okajima K, Murakami K, Nawa K, Okabe H, Takatsuki K. Recombinant human soluble thrombomodulin reduces endotoxin-induced pulmonary vascular injury via protein C activation in rats. Thromb Haemost 74 5:1265–1270, 1995. [PubMed] [Google Scholar]
- 33.Uchiba M, Okajima K, Murakami K, Johno M, Okabe H, Takatsuki K. Recombinant thrombomodulin prevents endotoxin-induced lung injury in rats by inhibiting leukocyte activation. Am J Physiol 271 (3 Pt. 1):L470–L475, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Hagiwara S, Iwasaka H, Matsumoto S, Hasegawa A, Yasuda N, Noguchi T. In vivo and in vitro effects of the anticoagulant, thrombomodulin, on the inflammatory response in rodent models. Shock 33 3:282–288, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Kudo D, Toyama M, Aoyagi T, Akahori Y, Yamamoto H, Ishii K, Kanno E, Maruyama R, Kaku M, Kushimoto S, et al. Involvement of high mobility group box 1 and the therapeutic effect of recombinant thrombomodulin in a mouse model of severe acute respiratory distress syndrome. Clin Exp Immunol 173 2:276–287, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


