Abstract
Objectives
The development of Rheumatoid Arthritis (RA) includes a phase of arthralgia preceding clinical arthritis. The aetiology of symptoms of arthralgia is unclear. Since subclinical joint inflammation is expected to be causally related to pain, we examined associations between subclinical MRI-detected inflammation and pain in patients with arthralgia suspicious for progression to RA.
Methods
Unilateral MRIs of the wrist, MCP(2-5)- and MTP(1-5)-joints of 325 patients who fulfilled the EULAR definition of arthralgia suspicious for progression to RA scored by two readers on subclinical inflammation (synovitis, bone marrow edema (BME) and tenosynovitis). Associations between MRI-detected inflammation and overall pain severity at patient level (measured using the visual analogue scale (VAS)), as well as with local joint tenderness were studied. Analyses were stratified for ACPA.
Results
At patient level, synovitis (β=0.10, p=0.048) and tenosynovitis (β=0.11, p=0.026) associated with the VAS-pain. Of the 1620 imaged joints, 447 (28%) were tender. MRI-detected synovitis associated independently with joint tenderness in all patients (OR 1.74, p<0.001), and in the ACPA-negative stratum (OR 1.96, p<0.001). In the ACPA-positive stratum only BME (osteitis) was independently associated with tenderness (OR 2.39, p=0.005). Sensitivity analyses in patients who developed inflammatory arthritis during follow-up (n=61) revealed similar associations. Subclinical inflammation was present in 51% of tender joints and 39% of non-tender joints.
Conclusions
In patients with arthralgia suspicious for progression to RA, MRI-detected subclinical inflammation is associated with overall pain and local joint tenderness. However, the association is partial, indicating that subclinical inflammation is not the sole explanation of the arthralgia.
Keywords: Magnetic Resonance Imaging (MRI), Pain, Anti-CCP, Rheumatoid Arthritis, Patient Reported Outcomes
Introduction
The development of Rheumatoid Arthritis (RA) can be preceded by several preclinical phases, including a phase of symptoms without clinical synovitis (1). Identification of patients in this early phase is challenging, but presumably important, as very early recognition and treatment of RA might result in better disease outcomes (2–6). A EULAR taskforce recently developed a clinical definition of arthralgia suspicious for progression to RA (7). This serves to homogenize the group of arthralgia patients considered at risk for RA. To achieve optimal prediction of RA development, information on biomarkers needs to be added to this clinical definition of arthralgia.
Although it is known that symptoms in the pre-arthritis phase of arthralgia can be considerable and can lead to functional limitations that are as restrictive as in the arthritis phase (8), the origin of the symptoms in this phase is insufficiently known (9). Previous MRI and ultrasound studies have suggested an association between subclinical MRI-detected inflammation and pain (10–12). However, these studies were small, did not compare ACPA-positive and ACPA-negative arthralgia patients and/or these studies did not evaluate bone marrow edema (BME) and tenosynovitis as features of subclinical inflammation. Consequently, the association between subclinical inflammation and arthralgia remains largely unstudied.
With the ultimate aim to improve the understanding of the aetiology of symptoms in patients with arthralgia suspicious for progression to RA, this large, cross-sectional MRI-study determined whether pain, both at patient and at joint level, can be explained by the presence of subclinical inflammation, evaluating synovitis, BME and tenosynovitis by MRI. Since ACPA-positive and ACPA-negative RA are sub-entities of RA with differences in etiopathology, it was also studied whether these associations were different in ACPA-positive and ACPA-negative patients (13–15). Finally, the patients with arthralgia suspicious for progression to RA that indeed developed inflammatory arthritis (IA) over time were studied separately in a sub analysis, as in these patients the arthralgia was definitely a preceding phase of IA.
Patients & Methods
Patients
All studied patients were included in the Leiden Clinically Suspect Arthralgia (CSA)-cohort that started in April 2012 (16). Patients with CSA have recent-onset (<1-year) arthralgia of the small joints of the hand or feet joints and imminent RA was considered the most likely cause of symptoms based on the clinical expertise of the rheumatologist. Per definition, CSA was not present in case of clinical synovitis or when another more likely explanation for the symptoms (e.g. fibromyalgia or osteoarthritis) was present. Previous use of disease modifying antirheumatic drugs (DMARDs) was an exclusion criterion. Patients were followed for two years or until development of clinical synovitis. No DMARDs were given while patients were in the CSA-cohort. At inclusion, a medical history was taken, a physical examination including a 68 tender joint count (TJC) was performed, lab samples including determination of ACPA (EliA CCP2, Phadia, Nieuwegein, the Netherlands, positive if ≥7 U/mL) were taken, and questionnaires on initial and current symptoms were completed by both the patient and the rheumatologist. In October 2017, MRI-data and clinical data was available for 505 patients. Next, the EULAR-definition for arthralgia suspicious for progression to RA was applied on all patients. This definition is intended to be used on top of the clinical identification of CSA and serves to create a more homogeneous group of patients with an increased risk on RA (7). In line with the definition, the EULAR-definition was applied to all 505 patients with a clinical suspicion of imminent RA. A total of 325 CSA-patients fulfilled the definition (presence of ≥3 parameters) and these patients were studied here (7,17).
MRI
A unilateral MRI of the wrist, MCP (2-5)-joints and MTP (1-5)-joints was performed within two weeks after inclusion in the CSA-cohort, using an ONI-MSK-extreme 1.5T extremity MR-scanner (GE, Wisconsin, USA). The MRI was made of the most painful side, or in case of equal symptoms of the dominant side. Detailed information about the scanning protocol has been described previously (16,18) and can be found in the supplementary methods. MRIs were scored on the presence of BME and synovitis in line with the RA MRI scoring (RAMRIS)-method (19–21). Tenosynovitis was scored according to the method described by Haavardsholm et al (22). Tenosynovitis was not assessed in the MTP-joints as no validated scoring system for tenosynovitis exists for these joints. Furthermore, for the first 52 patients imaging of MTP-joints was limited to pre-contrast sequences in the axial direction (see supplementary methods for more details). All MRIs were scored by two trained readers (all ICCs> 0.9, Supplementary table 1). The mean scores of two readers were studied. At patient level the total inflammation score was defined as the sum of the synovitis, tenosynovitis and BME-scores. At joint level the total inflammation score was defined as the sum of the synovitis, tenosynovitis and BME-score in that specific joint (see supplementary methods for more information on possible scores per imaged joint). The MTP-joints were not included in the analyses on joint level because of mentioned limitations of the scan protocol of the feet. In order to study the prevalence of MRI-detected subclinical inflammation, continuous MRI-scores were dichotomized on joint level; subclinical inflammation was considered present if the mean scores of two readers was ≥1 and considered absent in case of a mean scores <1 for either synovitis, tenosynovitis and BME separately. Any type of inflammation was considered present if the mean score of one of the different types of inflammation was ≥1.
Joint pain
Three different measures for pain were studied. The overall severity of joint pain at patient level was measured using a visual analogue scale (VAS, range 0-10) at the time of inclusion. The primary measure for pain at joint level was obtained from the 68-TJC that was assessed by a trained research nurse (RN) at baseline; information on the unilateral MCP- and wrist joints that were imaged was extracted. In addition, as patient-reported pain might differ from tenderness obtained at joint examination, a patient-reported joint count (68 joints) was added to the CSA-protocol in April 2015. Also from the patient-reported joint counts, information on the imaged wrist and MCP-joints was retrieved. Patient-reported pain was available for 156 (48%) patients; missingness was considered to be completely at random.
Statistical analyses
At patient level linear regression was used to study associations between MRI-detected inflammation (independent variable) and the VAS-pain (dependent variable). All types of MRI-detected inflammation were entered separately in univariable analyses. Subsequently these data were entered together in a multivariable analysis as several types of MRI-detected inflammation frequently occur together (Supplementary figure 1). At joint level general estimated equations (GEE) were used to study associations between local MRI-detected inflammation and joint tenderness, as this method allows adjustment for the fact that each patient contributed multiple joints. All GEEs were corrected for age and gender. Also in GEE, different types of MRI-detected inflammation were entered separately and then together in a multivariable model. As the 68-TJC was missing in 1 patient, 324 patients were included in these analyses, all contributing 5 joints (MCP2-5 and wrist), resulting in 1620 studied joints. Analyses were repeated stratified for ACPA (13–15). As a sensitivity analysis, patients that developed inflammatory arthritis (IA) were studied separately as the joint symptoms in these patients were truly a first sign of IA. Until the 1st of December 2017, 61 patients had developed IA during available follow-up (median 2.0-years, range 0.2-2.0-years).
Results
Baseline characteristics
Baseline characteristics of all 325 CSA-patients who fulfilled the EULAR definition for arthralgia suspicious for progression to RA are depicted in Table 1. The majority of patients was female, the mean age was 44, the median 68-TJC at joint examination was 6, and the mean VAS-pain 5.2.
Table 1. Baseline characteristics of patients that fulfilled the EULAR definition of arthralgia suspicious for progression to RA.
| Patients (n=325) | |
|---|---|
| Female gender, n (%) | 239 (74) |
| Age, mean (SD) | 44 (13) |
| Symptom duration weeks, median (IQR) | 18 (9–33) |
| Pain at joint level -68-TJC assessed at physical examination -patient-reported pain (68 joints)* |
6 (3–12) 18 (10–31) |
| Pain in small imaged joints, median (IQR)† -joint tenderness at physical examination -patient-reported pain* |
1 (0–2) 3 (1–4) |
| VAS-pain, mean (SD) | 5.2 (2.1) |
| ESR, median (IQR) | 2 (0–9) |
| ACPA-positive, n (%) | 35 (11) |
| RF-positive, n (%) | 59 (18) |
| ACPA and/or RF-positive, n (%) | 66 (20) |
| MRI-detected features, median (IQR) | |
| Total synovitis score | 1 (0–3) |
| Total BME score | 1 (0–2) |
| Total tenosynovitis score | 0 (0–1.5) |
| Total inflammation score | 2.5 (1.5–6) |
In May 2015 a patient-reported joint count (assessing 68 joints) was added to the CSA-protocol and was collected in 156 consecutive patients.
The imaged joints were 4 MCP-joints and 1 wrist per patient, thus 5 joints in total. Missings were as follows: VAS-pain (20), ACPA (2), RF (3), 68-TJC (1), Patient-reported joint pain (169)
SD, standard deviation; IQR, interquartile range; 68-TJC, tender joint count; VAS, visual analogue scale; ESR, erythrocyte sedimentation rate; ACPA, anticitrullinated protein antibody; RF, rheumatoid factor; MRI, magnetic resonance imaging; BME, bone marrow edema; CSA, clinically suspect arthralgia.
Associations between subclinical inflammation and joint pain at patient level
At patient level, synovitis (β=0.10, p=0.048) and tenosynovitis (β=0.11, p=0.026) were significantly associated with the VAS-pain in univariable analysis. A β of 0.10 for synovitis means that a one point increase in the MRI-detected synovitis score was associated with an 0.10 point higher VAS-pain (Table 2). None of these variables were associated with pain independent of the others in multivariable analysis (Table 2).
Table 2. Results of univariable and multivariable linear regression studying the association between MRI-detected inflammation and the VAS-pain at patient level.
| MRI-scores, median (IQR) | Univariable | Multivariable | ||
|---|---|---|---|---|
| β (95%CI) | p-value | β (95%CI) | p-value | |
| Synovitis | 0.10 (0.00–0.19) | 0.048 | 0.08 (-0.06–0.23) | 0.26 |
| BME | -0.01 (-0.12–0.09) | 0.81 | -0.10 (-0.23–0.02) | 0.11 |
| Tenosynovitis | 0.11 (0.01–0.20) | 0.026 | 0.09 (-0.04–0.22) | 0.19 |
| Total inflammation score | 0.03 (-0.01–0.07) | 0.10 | - | - |
MRI, magnetic resonance imaging; IQR, interquartile range; CI, confidence interval; BME, bone marrow edema
Associations between subclinical inflammation and tenderness or pain at joint level
At joint level (n=1620 joints), GEEs adjusted for age and gender were used to study associations between MRI-detected inflammation and joint tenderness. This was done to study if the joints that were tender also showed more subclinical inflammation. The total inflammation score was associated with tenderness at joint examination (OR 1.13, p=0.001, Table 3) in univariable analysis. Further separation by type of MRI-detected inflammation revealed associations with synovitis (OR 1.64, p<0.001), tenosynovitis (OR 1.17, p=0.011), but not with BME (OR 1.08, p=0.58). As the different types of MRI-detected inflammation often occur together (Supplementary figure 1), these were entered together in a multivariable analysis, showing that synovitis remained independently associated with joint tenderness (OR 1.74, p<0.001).
Table 3. Odds ratios for joint tenderness per point increase in MRI-score in the wrist and MCP-joints imaged; analyses were done on joint level.
| MRI-detected features | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95%CI) | p-value | OR (95%CI) | p-value | |
| Joint tenderness as obtained at joint examination | ||||
| Synovitis | 1.64 (1.31–2.06) | <0.001 | 1.74 (1.33–2.29) | <0.001 |
| BME | 1.08 (0.83–1.39) | 0.58 | 0.80 (0.61–1.05) | 0.11 |
| Tenosynovitis | 1.17 (1.04–1.33) | 0.011 | 1.05 (0.92–1.21) | 0.44 |
| Total inflammation score | 1.13 (1.05–1.21) | 0.001 | - | - |
| Patient-reported pain | ||||
| Synovitis | 1.84 (1.26–2.69) | 0.002 | 2.03 (1.32–3.12) | 0.001 |
| BME | 1.05 (0.73–1.50) | 0.80 | 0.72 (0.47–1.10) | 0.12 |
| Tenosynovitis | 1.29 (0.94–1.77) | 0.12 | 1.16 (0.86–1.57) | 0.34 |
| Total inflammation score | 1.18 (0.98–1.42) | 0.082 | - | - |
Results of univariable and multivariable GEEs. All analyses are corrected for age and gender.
Patient-reported joint tenderness was available for 156/325 patients. Missings were at random as a patient-reported 68-TJC was only collected from April 2015 on. Patients included in the CSA-cohort prior to that time were missing.
MRI, magnetic resonance imaging; MCP, metacarpophalangeal; OR, odds ratio; CI, confidence interval; 68-TJC, tender joint count
Next the association between patient-reported pain at joint level and MRI-detected inflammation was assessed and similar findings were obtained; only synovitis remained independently associated with patient-reported joint pain (OR 2.03, p=0.001, Table 3).
Prevalence of subclinical inflammation in tender and in non-tender joints
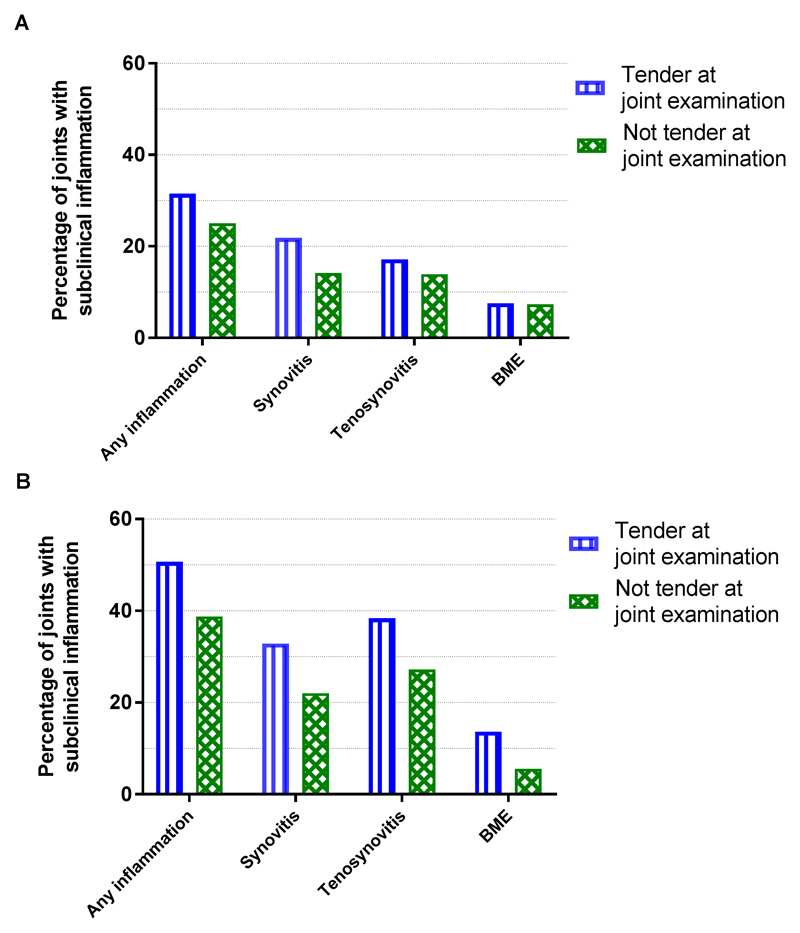
The data obtained so far revealed associations between subclinical inflammation and pain in arthralgia patients at risk for RA. Next, in order to determine how often subclinical inflammation was present in tender and in non-tender joints, the prevalence of subclinical inflammation was assessed after dichotomizing the continuous MRI-scores. Any type of subclinical inflammation was present in 434/1620 (27%) of all studied joints. The prevalence was higher in tender joints 141/447 (32%) joints than in non-tender joints 293/1173 (25%). All types of MRI-detected inflammation separately were more frequent in tender joints than in non-tender joints (22% versus 14% for synovitis, 17% versus 14% for tenosynovitis and 8% versus 7% for BME, Figure 1A).
Figure 1. Prevalence of subclinical MRI-detected inflammation in tender and in non-tender joints in all patients who fulfilled the EULAR definition of arthralgia suspicious for progression to RA at presentation (A) and in the subgroup that developed IA during follow-up (B).
MRI, magnetic resonance imaging; IA, inflammatory arthritis; BME, bone marrow edema.
Analyses stratified by ACPA-status
ACPA-positive CSA patients had a median 68-TJC of 5 and a mean VAS-pain of 5.6; in ACPA-negative patients this was 6 and 5.2 respectively. Analyses on joint level were repeated, stratified by ACPA-status. In univariable GEE analysis, the total inflammation score at joint level was associated with tenderness (as assessed at physical examination of joints) both in ACPA-positive (OR 1.28, p=0.010) and in ACPA-negative CSA (OR 1.12, p=0.006) (Table 4). Within ACPA-positive patients both tenosynovitis (OR 1.36, p=0.041) and BME (OR 2.47, p=0.001) were associated with tenderness in univariable analysis. In multivariable analysis only BME remained independently associated with tenderness (OR 2.39, p=0.005). Within ACPA-negative CSA both synovitis (OR 1.72, p<0.001) and tenosynovitis (OR 1.18, p=0.026) were associated with tenderness in univariable analysis, and synovitis showed an independent association in multivariable analysis (OR 1.96, p<0.001) (Table 4).
Table 4. Odds ratios for joint tenderness (at joint examination) per point increase in MRI-score in the wrist and MCP-joints, analyses done on joint level were stratified by ACPA-status.
| MRI-detected features | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95%CI) | p-value | OR (95%CI) | p-value | |
| ACPA-positive | ||||
| Synovitis | 1.45 (0.71–2.96) | 0.31 | 0.83 (0.44–1.58) | 0.57 |
| BME | 2.47 (1.42–4.31) | 0.001 | 2.39 (1.31–4.36) | 0.005 |
| Tenosynovitis | 1.36 (1.01–1.83) | 0.041 | 1.22 (0.95–1.55) | 0.11 |
| Total inflammation score | 1.28 (1.06–1.54) | 0.010 | - | - |
| ACPA-negative | ||||
| Synovitis | 1.72 (1.33–2.21) | <0.001 | 1.96 (1.45–2.66) | <0.001 |
| BME | 0.97 (0.74–1.26) | 0.79 | 0.67 (0.50–0.90) | 0.008 |
| Tenosynovitis | 1.18 (1.02–1.36) | 0.026 | 1.06 (0.90–1.25) | 0.47 |
| Total inflammation score | 1.12 (1.03–1.21) | 0.006 | - | - |
Results of univariable and multivariable GEEs with joint tenderness as the outcome. All analyses are corrected for age and gender.
MRI, magnetic resonance imaging; MCP, metacarpophalangeal; OR, odds ratio; CI, confidence interval; ACPA, anticitrullinated protein antibody
Sub analysis in patients who developed IA during follow-up
Finally analyses were repeated in 61 patients who developed IA during follow-up. At patient level, synovitis tended to be associated with the VAS-pain in univariable analysis (β=0.18, p=0.053) (Supplementary table 2). A total of 305 joints were studied of which 73 (24%) were tender. The total inflammation score was associated with joint tenderness (OR 1.27, p=0.001, Table 5). The different types of MRI-detected inflammation separately were also associated with tenderness (synovitis: OR 1.97, p=0.003; tenosynovitis: OR 1.35, p=0.019; and BME: OR 2.21, p=0.004, Table 5); though statistical significance was lost in multivariable analysis (Table 5). Forty-two percent of the small joints imaged had subclinical inflammation; it was present in 51% of the tender joints and in 39% of the non-tender joints. All types of MRI-detected inflammation were more prevalent in tender joints than in non-tender joints (Figure 1B).
Table 5. Odds ratios for joint tenderness per point increase in MRI-score in the wrist and MCP-joints; analyses were done on joint level in patients who developed IA during follow-up.
| MRI-detected features | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95%CI) | p-value | OR (95%CI) | p-value | |
| Synovitis | 1.97 (1.26–3.06) | 0.003 | 1.50 (0.93–2.43) | 0.095 |
| BME | 2.21 (1.29–3.77) | 0.004 | 1.58 (0.85–2.97) | 0.15 |
| Tenosynovitis | 1.35 (1.05–1.75) | 0.019 | 1.11 (0.91–1.34) | 0.31 |
| Total inflammation score | 1.27 (1.10–1.46) | 0.001 | - | - |
Results of univariable and multivariable GEEs with joint tenderness as reported by a trained research nurse was taken as the outcome. All analyses are corrected for age and gender.
MRI, magnetic resonance imaging; MCP, metacarpophalangeal; IA, inflammatory arthritis OR, odds ratio; CI, confidence interval
Discussion
In this large cross-sectional MRI-study we aimed to improve our understanding about the aetiology of symptoms in patients with arthralgia suspicious for progression to RA. We observed that subclinical MRI-detected inflammation, synovitis in particular, is associated with pain, both at patient and at joint level. In addition, different associations were observed in ACPA-positive and ACPA-negative patients. Whereas BME (osteitis) was independently associated with joint tenderness in ACPA-positive CSA, synovitis was independently associated with tenderness in ACPA-negative CSA, suggesting different pathways for pain in both subtypes.
This study also demonstrated that subclinical inflammation was absent in a large proportion of tender joints; this concerned 49% of the joints imaged at first presentation with CSA in patients that did progress to IA over time. This indicates that the presence of subclinical inflammation cannot fully explain the aetiology of joint symptoms in these patients. However, this finding is not different from that obtained in the phase of early arthritis, as a previous study on patients presenting with arthritis of recent-onset showed that 51-59% of tender hand or foot joints did not show MRI-detected inflammation (23).
To our best knowledge, this is the first study to reveal that associations between subclinical inflammation and joint pain are different for ACPA-positive and ACPA-negative patients. Furthermore, because of the large number of patients and available MRIs (n=325) it was possible to correct for the combined presence of synovitis, tenosynovitis and BME in multivariable analyses. Previously two small MRI-studies have been performed (10,11) and observed a univariable association between joint tenderness and subclinical synovitis in ACPA-negative CSA, which is in line with our results. A large ultrasound study also observed an association between subclinical synovitis and tenderness in seropositive arthralgia (12), but a downside of ultrasound is that it cannot visualise BME. Also tenosynovitis was not assessed in this study. Thus, the finding that joint pain is partly explained by subclinical synovitis is in line with previous findings, although more thoroughly explored in the present study. In contrast the finding that in ACPA-positive arthralgia BME (osteitis) is associated with joint tenderness independent of synovitis and tenosynovitis is novel.
While the relation between BME and erosive progression has been widely established (24–26), the association between BME and pain in ACPA-positive arthralgia has not been reported before. As the bone marrow is not innervated, it remains to be determined how to explain this finding. A histologic study in 11 RA-patients revealed an increased number of osteoclasts in bone samples in which MRI-detected BME was present, showing that BME in RA truly is inflammation (therefore called osteitis) (27). Although histologic studies in CSA have not been performed, BME in CSA might also be considered as osteitis. Recent mice-data have suggested that ACPAs can bind osteoclasts and stimulate CXCL-1 and IL-8 release and that this in turn can produce pain by activating sensory neurons. (28) If such ACPA-dependent pathways for pain play a role in humans during the development of RA requires further investigation. Surprisingly, within ACPA-negative CSA, BME was inversely associated with joint tenderness in multivariable analysis; possibly collinearity contributed to this effect. Furthermore, in line with previous studies on the etiopathology of RA, our results on subclinical inflammation and symptoms in patients with CSA emphasize the notion that ACPA-positive and ACPA-negative RA are different subsets of disease with differences in underlying biologic mechanisms (14,15).
Thus far differences between ACPA-positive and ACPA-negative RA have been shown for genetic and environmental risk factors and differences in the synovial infiltrate (13,14,29). In the symptomatic pre-arthritis phase, some clinical differences have been shown recently (15). In addition, this study shows that joint tenderness is associated with different inflammatory features in ACPA-positive and negative arthralgia. Further studies are needed to evaluate whether there are also histological differences in the joint in this symptomatic pre-arthritis phase.
Although this study was cross-sectional in nature, a strength is the fact that we were able to perform a sensitivity analysis in patients who developed arthritis during follow-up, as (in retrospect) these patients truly were in a pre-arthritis phase. Results in this subgroup were similar to those in the whole population of CSA-patients studied. A single difference is that univariable analysis in this subgroup showed a significant association for BME, which was not the case in the whole group. Presumably this is explained by the higher prevalence of ACPA-positivity in the subgroup (30 vs. 11%). Importantly, part of the patients that did not progress to IA had a limited follow-up duration. Therefore the nature of the data did not allow to compare the patients that progressed to IA compared to those that did not progress; as misclassification might be present in the latter group. Likewise, the current percentage of IA development (19%) may be underestimated.
There are different ways to define the presence of subclinical inflammation. In this study, subclinical inflammation was considered present if the mean score of two readers on either synovitis, tenosynovitis or BME on a specific joint location was ≥1. Thus the definition used ignored the fact that some low-graded subclinical inflammation is also present in symptom-free controls, especially at higher age (30). Because we observed that subclinical inflammation was present in non-tender joints, we wondered whether this inflammation was similar to that observed in symptom-free controls. Repeating the analyses using a definition of subclinical inflammation that incorporated findings of the normal population as described previously (31) did not change the results (Supplementary figure 2).
There are limitations to the present study. While we can reveal associations between pain and subclinical inflammation, we cannot prove causality based on our current data. In addition, although the total study population was relatively large, patient numbers in subgroups were small, possibly leading to lack of power in multivariable analyses. For example, in the subgroup of patients who developed IA the effect sizes of the associations between subclinical inflammation and pain were larger than that in the total group of patients, but confidence intervals were wider and p-values higher in multivariable analysis. Therefore, larger studies are needed in patients who developed IA during follow-up. Furthermore, longitudinal MRI-data were not available. This would be of interest for future studies in order to study whether changes in subclinical inflammation co-occur with changes in pain and vice versa. Finally, we did not include the MTP-joints in our analyses on joint level, mostly because of limitations in our MRI-scanning protocol for the feet in part of the patients (32). Exploring if the association between foot pain or local tenderness and MRI detected subclinical inflammation is different from the observed associations in the hand joints is also subject for future research.
In sum, this cross-sectional MRI-study in patients with arthralgia suspicious for progression to RA revealed associations between pain and subclinical inflammation. This association was strongest for synovitis in the total group and ACPA-negative patients, while in the subset of ACPA-positive arthralgia patients, BME (osteitis) was independently associated with joint pain. However, as subclinical inflammation was absent in part of the tender joints, the aetiology of joint symptoms in the symptomatic phase preceding RA-development cannot fully be explained by the presence of subclinical inflammation.
Supplementary Material
Acknowledgements
The authors would like to thank HW van Van Steenbergen HW, L Mangnus, WP Nieuwenhuis, AC Boer and DM Boeters for their help with scoring of all MRIs included in this study.
Funding
This work was supported by a Vidi-grant of the Netherlands Organisation for Scientific Research and has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Starting grant, agreement No 714312).
Footnotes
Authorship Criteria
LEB performed the statistical analysis, interpreted the data and drafted the manuscript. RMtB contributed to data acquisition, helped interpret the data and revised the manuscript. AHMvdHvM helped design the study, participated in data acquisition and interpretation of the data and contributed to drafting the manuscript. All authors read and approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Patient Consent
Obtained.
Ethics Approval
The study protocol of the CSA-study was approved by the local medical ethical committee of the Leiden University Medical Center.
References
- 1.Gerlag DM, Raza K, van Baarsen LGM, Brouwer E, Buckley CD, Burmester GR, et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann Rheum Dis. 2012;71:638–641. doi: 10.1136/annrheumdis-2011-200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Linden MPM, le Cessie S, Raza K, van der Woude D, Knevel R, Huizinga TWJ, et al. Long-term impact of delay in assessment of patients with early arthritis. Arthritis Rheum. 2010;62:3537–3546. doi: 10.1002/art.27692. [DOI] [PubMed] [Google Scholar]
- 3.Van Nies JAB, Tsonaka R, Gaujoux-Viala C, Fautrel B, van der Helm-van Mil AHM. Evaluating relationships between symptom duration and persistence of rheumatoid arthritis: does a window of opportunity exist? Results on the Leiden Early Arthritis Clinic and ESPOIR cohorts. Ann Rheum Dis. 2015;74:806–812. doi: 10.1136/annrheumdis-2014-206047. [DOI] [PubMed] [Google Scholar]
- 4.van der Woude D, Young A, Jayakumar K, Mertens BJ, Toes REM, van der Heijde D, et al. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug–free remission in rheumatoid arthritis: Results from two large early arthritis cohorts. Arthritis Rheum. 2009;60:2262–2271. doi: 10.1002/art.24661. [DOI] [PubMed] [Google Scholar]
- 5.Deane KD. Can rheumatoid arthritis be prevented? Best Pract Res Clin Rheumatol. 2013;27:467–485. doi: 10.1016/j.berh.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Steenbergen HW, da Silva JAP, Huizinga TWJ, van der Helm-van Mil AHM. Preventing progression from arthralgia to arthritis: targeting the right patients. [Accessed December 18, 2017];Nat Rev Rheumatol. 2017 doi: 10.1038/nrrheum.2017.185. Available at: https://www.nature.com/articles/nrrheum.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Steenbergen HW, Aletaha D, Beaart-van de Voorde LJJ, Brouwer E, Codreanu C, Combe B, et al. EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-209846. annrheumdis-2016-209846. [DOI] [PubMed] [Google Scholar]
- 8.ten Brinck RM, van Steenbergen HW, Mangnus L, Burgers LE, Reijnierse M, Huizinga TW, et al. Functional limitations in the phase of clinically suspect arthralgia are as serious as in early clinical arthritis; a longitudinal study. RMD Open. 2017;3:e000419. doi: 10.1136/rmdopen-2016-000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jutley GS, Latif ZP, Raza K. Symptoms in individuals at risk of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2017;31:59–70. doi: 10.1016/j.berh.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Krabben A, Stomp W, van der Heijde D, van Nies JAB, Bloem JL, Huizinga TWJ, et al. MRI of hand and foot joints of patients with anticitrullinated peptide antibody positive arthralgia without clinical arthritis. Ann Rheum Dis. 2013;72:1540–1544. doi: 10.1136/annrheumdis-2012-202628. [DOI] [PubMed] [Google Scholar]
- 11.van Steenbergen HW, van Nies JAB, Huizinga TWJ, Reijnierse M, van der Helm-van Mil AHM. Subclinical inflammation on MRI of hand and foot of anticitrullinated peptide antibody–negative arthralgia patients at risk for rheumatoid arthritis. Arthritis Res Ther. 2014;16:R92. doi: 10.1186/ar4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Stadt LA, Bos WH, Meursinge Reynders M, Wieringa H, Turkstra F, van der Laken CJ, et al. The value of ultrasonography in predicting arthritis in auto-antibody positive arthralgia patients: a prospective cohort study. Arthritis Res Ther. 2010;12:R98. doi: 10.1186/ar3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padyukov L, Seielstad M, Ong RTH, Ding B, Rönnelid J, Seddighzadeh M, et al. A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann Rheum Dis. 2010;70:259–265. doi: 10.1136/ard.2009.126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klareskog L, Stolt P, Lundberg K, Källberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 15.Burgers LE, van Steenbergen HW, ten Brinck RM, Huizinga TW, van der Helm-van Mil AHM. Differences in the symptomatic phase preceding ACPA-positive and ACPA-negative RA: a longitudinal study in arthralgia during progression to clinical arthritis. Ann Rheum Dis. 2017 doi: 10.1136/annrheumdis-2017-211325. annrheumdis-2017-211325. [DOI] [PubMed] [Google Scholar]
- 16.van Steenbergen HW, van Nies JAB, Huizinga TWJ, Bloem JL, Reijnierse M, van der Helm-van Mil AHM. Characterising arthralgia in the preclinical phase of rheumatoid arthritis using MRI. Ann Rheum Dis. 2015;74:1225–1232. doi: 10.1136/annrheumdis-2014-205522. [DOI] [PubMed] [Google Scholar]
- 17.Burgers LE, Siljehult F, ten Brinck RM, van Steenbergen HW, Landewé RBM, Rantapää-Dahlqvist S, et al. Validation of the EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Rheumatol Oxf Engl. 2017;56:2123–2128. doi: 10.1093/rheumatology/kex324. [DOI] [PubMed] [Google Scholar]
- 18.Nieuwenhuis WP, van Steenbergen HW, Stomp W, Stijnen T, Huizinga TWJ, Bloem JL, et al. The course of bone marrow edema in early undifferentiated and rheumatoid arthritis; a longitudinal MRI study on bone level. Arthritis Rheumatol. 2015 doi: 10.1002/art.39550. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 19.Østergaard M, Edmonds J, McQueen F, Peterfy C, Lassere M, Ejbjerg B, et al. An introduction to the EULAR–OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis. 2005;64:i3–i7. doi: 10.1136/ard.2004.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Østergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–1386. [PubMed] [Google Scholar]
- 21.Baan H, Bezooijen R, Avenarius JKA, Dubbeldam R, Drossaers-Bakker WK, van de Laar MAFJ. Magnetic Resonance Imaging of the Rheumatic Foot According to the RAMRIS System Is Reliable. J Rheumatol. 2011;38:1003–1008. doi: 10.3899/jrheum.100906. [DOI] [PubMed] [Google Scholar]
- 22.Haavardsholm EA, Østergaard M, Ejbjerg BJ, Kvan NP, Kvien TK. Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: reliability in a multireader longitudinal study. Ann Rheum Dis. 2007;66:1216–1220. doi: 10.1136/ard.2006.068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krabben A, Stomp W, Huizinga TWJ, van der Heijde D, Bloem JL, Reijnierse M, et al. Concordance between inflammation at physical examination and on MRI in patients with early arthritis. Ann Rheum Dis. 2015;74:506–512. doi: 10.1136/annrheumdis-2013-204005. [DOI] [PubMed] [Google Scholar]
- 24.McQueen FM. Bone marrow edema and osteitis in rheumatoid arthritis: the imaging perspective. Arthritis Res Ther. 2012;14:224. doi: 10.1186/ar4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hetland ML, Ejbjerg B, Hørslev-Petersen K, Jacobsen S, Vestergaard A, Jurik AG, et al. MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA) Ann Rheum Dis. 2009;68:384–390. doi: 10.1136/ard.2008.088245. [DOI] [PubMed] [Google Scholar]
- 26.Krabben A, Stomp W, van Nies JAB, Huizinga TWJ, van der Heijde D, Bloem JL, et al. MRI-detected subclinical joint inflammation is associated with radiographic progression. Ann Rheum Dis. 2014;73:2034–2037. doi: 10.1136/annrheumdis-2014-205208. [DOI] [PubMed] [Google Scholar]
- 27.Dalbeth N, Smith T, Gray S, Doyle A, Antill P, Lobo M, et al. Cellular characterisation of magnetic resonance imaging bone oedema in rheumatoid arthritis; implications for pathogenesis of erosive disease. Ann Rheum Dis. 2009;68:279–282. doi: 10.1136/ard.2008.096024. [DOI] [PubMed] [Google Scholar]
- 28.Wigerblad G, Bas DB, Fernades-Cerqueira C, Krishnamurthy A, Nandakumar KS, Rogoz K, et al. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine-dependent mechanism. Ann Rheum Dis. 2016;75:730–738. doi: 10.1136/annrheumdis-2015-208094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Oosterhout M, Bajema I, Levarht EWN, Toes REM, Huizinga TWJ, van Laar JM. Differences in synovial tissue infiltrates between anti–cyclic citrullinated peptide–positive rheumatoid arthritis and anti–cyclic citrullinated peptide–negative rheumatoid arthritis. Arthritis Rheum. 2008;58:53–60. doi: 10.1002/art.23148. [DOI] [PubMed] [Google Scholar]
- 30.Mangnus L, van Steenbergen HW, Reijnierse M, van der Helm-van Mil AHM. Magnetic Resonance Imaging-Detected Features of Inflammation and Erosions in Symptom-Free Persons From the General Population. Arthritis Rheumatol Hoboken NJ. 2016;68:2593–2602. doi: 10.1002/art.39749. [DOI] [PubMed] [Google Scholar]
- 31.Boer AC, Burgers LE, Mangnus L, ten Brinck RM, Nieuwenhuis WP, van Steenbergen HW, et al. Using a reference when defining an abnormal MRI reduces false-positive MRI results-a longitudinal study in two cohorts at risk for rheumatoid arthritis. Rheumatol Oxf Engl. 2017;56:1700–1706. doi: 10.1093/rheumatology/kex235. [DOI] [PubMed] [Google Scholar]
- 32.Østergaard M, Peterfy CG, Bird P, Gandjbakhch F, Glinatsi D, Eshed I, et al. The OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging (MRI) Scoring System: Updated Recommendations by the OMERACT MRI in Arthritis Working Group. J Rheumatol. 2017;44:1706–1712. doi: 10.3899/jrheum.161433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.