Abstract
Aims/Introduction
Fibroblast growth factor (FGF)19 has been shown to improve glycemic homeostasis and lipid metabolism in animal models. In humans, decreased FGF19 level has been described in diabetes. The present study aimed to investigate the expression of FGF19 in gestational diabetes mellitus (GDM) patients.
Materials and Methods
Samples for measurement were obtained from 20 women with GDM and 25 healthy controls. The messenger ribonucleic acid (mRNA) and protein expression levels of FGF19, FGF21 and co‐receptor β‐klotho (KLB) in the placenta, rectus muscle and subcutaneous fat tissues were quantified by real‐time quantitative polymerase chain reaction, western blot and immunohistochemistry, respectively.
Results
Women with GDM had significantly lower mRNA and protein expressions of FGF19 than control women in the placenta (mRNA 0.33 ± 0.05 vs 0.72 ± 0.09; protein 0.34 ± 0.13 vs 0.85 ± 0.20) and rectus muscle (mRNA 0.83 ± 0.11 vs 1.28 ± 0.19; protein 0.78 ± 0.24 vs 1.23 ± 0.39). However, there were no significant differences between GDM women and controls with respect to the expression levels of FGF21 and β‐klotho in the placenta and rectus muscle. There were almost no detectable FGF19 and FGF21 expressions in subcutaneous fat tissue. Furthermore, β‐klotho expression levels were not different between the GDM and control group in subcutaneous fat.
Conclusions
FGF19 expressions are decreased in the placenta and rectus muscle of women with GDM. This might contribute to the pathophysiology or development of GDM.
Keywords: Fibroblast growth factor 19, Gestational diabetes mellitus, Insulin resistance
Introduction
The endocrine fibroblast growth factor (FGF) subfamily consists of three members, FGF19, FGF21 and FGF23, which have hormone‐like actions1. Human FGF19 is widely expressed in different organs and tissues. As is well known, FGF19 activates the FGF receptor (FGFR) complex containing a so‐called co‐receptor β‐klotho (KLB)2. In the existence of KLB, all of FGFR1c, 2c, 3c and 4 can be activated by both FGF19 and FGF213. FGF19 inhibits bile acid synthesis and induces cell proliferation through the KLB/FGFR4‐dependent pathway2. Furthermore, FGF19 is believed to improve systemic glucose and lipid homeostasis through the FGFR4‐independent pathway, mainly including the KLB/FGFR1c pathway4.
It is remarkable that FGF19 has been supposed to be a potential new drug for metabolic diseases, such as diabetes and obesity. High expression of FGF19 seems to ameliorate the excessive weight gain induced by a high‐fat diet in murine. In the meantime, the metabolic disorders (including insulin resistance and abnormal lipids profile complicated with obesity) are also improved5, 6. Additionally, FGF19 promotes glucose uptake in adipocytes, the same as FGF217. Furthermore, previous studies verified that central FGF19 acted to reduce hypothalamic agouti‐related peptide/neuropeptide Y neuronal activity8, and accelerate insulin‐independent glucose disposal9.
The placenta is a highly active endocrine organ. It produces many hormones and growth factors with endocrine and paracrine effects. Metabolic dysfunction in the placenta might contribute to maternal and fetal adverse outcomes. A recent study showed that the human placenta expression of FGF21 was abnormally elevated in gestational diabetes mellitus (GDM)10. Placental FGF21 might be involved in placental metabolism10. Furthermore, our previous study suggested that serum FGF19 levels were decreased in women with GDM, in contrast to FGF2111. Both circulating FGF19 and FGF21 expressions were closely correlated to insulin resistance11. We speculated that decreased circulating FGF19 expressions might be conducive to the pathophysiology of GDM, whereas elevated circulating FGF21 might be a component of the compensatory reaction to insulin resistance in GDM11. However, it is unclear whether FGF19 is expressed in the placenta and whether the expression changes in GDM patients. In addition, studies of insulin‐sensitive maternal peripheral tissues showed that the defects of the insulin‐signaling pathway resulted in impaired insulin action in adipose tissue and skeletal muscle12. Thus, defects of the insulin‐signaling pathway might induce the pathological insulin resistance in GDM patients. On the basis of the correlation between FGF19 and insulin resistance, we hypothesized that FGF19 expressions would be altered in the insulin‐sensitive tissues of women with GDM.
Furthermore, results from numerous studies showed that FGF19 and FGF21 were both effective for improving lipid and carbohydrate metabolism disorders on multiple organs and tissues13. Notably, serum FGF19 expressions were decreased in overweight/obese and type 2 diabetes patients14. On the contrary, serum FGF21 expressions were found to be elevated in patients with type 2 diabetes or obese patients15. Whether the different expressions between FGF19 and FGF21 are also present in the insulin‐sensitive tissues of women with GDM remain unknown.
Therefore, in the current study, we sought to investigate the expressions of FGF19 and its co‐receptor KLB in the placenta, skeletal muscle and adipose tissue in pregnant women with and without GDM. Meanwhile, we also measured the expression of FGF21 in this study, because we sought to find the different expressions of the two factors.
Methods
Participants, clinical data and sample collection
The study was carried out at the First Affiliated Hospital of Sun Yat‐sen University in Guangzhou, Guangdong, China – a level III tertiary referral university hospital – between October 2014 and December 2015. This analysis is part of an ongoing cohort study. Gravidas who had integrated prenatal data were recruited at the time of screening for GDM. Ethnically Chinese women with a single pregnancy were invited to participant in the present study. The group has been described in our previous study16. In total, after matched for gestational and maternal age, 30 women with GDM and 30 healthy pregnant women were enrolled in this study. This study was approved by the ethics committee of The First Affiliated Hospital of Sun Yat‐sen University, and all participants received written informed consent before they took part in the study. The study was carried out in accordance with the Declaration of Helsinki.
GDM was diagnosed as long as any of the following blood glucose values met or exceeded the thresholds; that is, fasting glucose ≥5.1 mmol/L, 1‐h result ≥10.0 mmol/L and 2‐h result ≥8.5 mmol/L, using a 75‐g oral glucose tolerance test between the 24th and 28th gestational weeks, following the new diagnostic criteria amended by the Ministry of Health China in 201417. Meanwhile, a fasting blood glucose (FBG) level of 7.0 mmol/L or 2‐h postprandial blood glucose level of 11.1 mmol/L was considered to exclude previously undiagnosed diabetes mellitus at any time, despite the pregnancy stage. The women with GDM were given dietary and physical exercise instruction, and carried out self‐monitoring of blood glucose; insulin treatment was added when glycemic control remained unsatisfactory.
All the participants underwent elective cesarean sections after 36 6/7 weeks of gestation. Breech presentation and/or previous cesarean section were determined to be the indications for cesarean section. On the day of selective cesarean section, maternal peripheral blood samples were drawn from an antecubital vein at the time between 08.00 and 10.00 hours after overnight fasting (at least 8 h, but not more than 14 h). From an abdominal incision, 1‐cm3 samples of rectus muscle and subcutaneous fat tissue were excised. Furthermore, the placenta was collected at cesarean section and a 1‐cm3 sample of placenta was excised from anatomically healthy parts of the placenta. All of the above samples were immediately refrigerated in liquid nitrogen before storage at −80°C. In addition, 1‐cm3 samples of the above tissues were excised and fixed for 48 h in 4% paraformaldehyde and kept in saturated sucrose solution until embedding for immunohistochemistry.
Ribonucleic acid extraction and real‐time polymerase chain reaction
A 100‐mg sample of placenta was used to extract the total ribonucleic acid (RNA) by TRIzol® Reagent following the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). A spectrophotometer (NanoDrop™; Thermo Fisher Scientific, Waltham, MA, USA) was used to quantify the concentrations of RNA. RNA quality and integrity were determined by the A260/A280 ratio and agarose gel electrophoresis. Sequences of the primers used are as follows: FGF19 (GenBank: NM_005117.2), sense: 5′‐CTACAATGTGTACCGATCCGA‐3′, antisense: 5′‐AGAGTGGAAGAAAGCCTCTG‐3′; FGF21 (GenBank: NM_019113.2), sense: 5′‐CTCGCTTCCTGCCACTAC‐3′, antisense: 5′‐GAAGGTCCCACCATGCTC‐3′; KLB (GenBank: NM_175737.3), sense: 5′‐CATGGGTATGGGACAGGTATG‐3′, antisense: 5′‐GGCGGAAATGTGTGTTGTAG‐3′. We converted 1 μg of RNA to complementary deoxyribonucleic acid using the PrimeScript RT reagent Kit With gDNA Eraser (Takara, Dalian, China) based on the manufacturer's instructions. Real‐time polymerase chain reaction (PCR) was carried out in duplicate in a total reaction volume of 20 μL with SYBR Premix Ex TaqTM (Takara) in an ABI 7500 instrument (Applied Biosystems, Foster City, CA, USA). β‐Actin was used to normalize the messenger RNA (mRNA) expression. Additionally, the specificity of the product was evaluated from melting curve analysis. The cycling conditions for reverse transcription PCR were as follows: one cycle at 95°C for 30 s, 40 cycles at 95°C for 5 s and 60°C for 34 s. The expression level was calculated by the 2−▵▵CT method.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blot
Rectus muscles, subcutaneous fat tissues and placentae were lysed with a radioimmunoprecipitation assay buffer (Tris, 1% Triton X‐100, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 150 mmol/L NaCl, l% phenylmethane sulfonyl fluoride [P0013B; Beyotime, Shanghai, China]). Tissues were disrupted by vigorously shaking with a 5‐mm stainless steel bead in a TissueLyser (QIAGEN, Germantown, MD, USA). Lysates were centrifuged for 10 min at 4°C, and the protein content in the supernatant was identified by a bicinchoninic acid assay (P0010; Beyotime). Then, 30 mg of protein was mounted onto the 4–12% gradient NuPAGE Bis‐Tris gel (Life Technologies, Carlsbad, CA, USA), and then transferred onto a polyvinylidene difluoride membrane (Millipore, Burlington, MA, USA). After being sealed with 5% non‐fat dry milk in phosphate‐buffered saline for 1 h, this membrane was incubated overnight with primary antibody for rabbit anti‐FGF19 (No. ab154185; Abcam, Cambridge, UK) at 1:1,000, mouse anti‐FGF21 at 1 μg/mL (No. WH0026291M1; Sigma‐Aldrich, Temecula, CA, USA) or rabbit anti‐KLB at 1 μg/mL (ab106794; Abcam) and mouse anti‐β‐Actin (A5316; Sigma Aldrich) at 1:20,000 in 5% non‐fat dry milk in phosphate‐buffered saline‐Tween 20 at 4°C with agitation. Secondary antibodies, goat anti‐rabbit immunoglobulin G (H + L; dilution 1:10,000; 111‐035‐003; Jackson ImmunoResearch, Baltimore, PA, USA) and goat anti mouse immunoglobulin G horseradish peroxidase (dilution 1:1,500; A0208; Beyotime) were incubated for 1 h at room temperature, and the membranes were incubated with Western Lightning Plus ECL (no. NEL105001EA; PerkinElmer, Waltham, MA, USA). Proteins were tested by the Kodak image station system (Kodak 4000R Pro; Carestream Health, Rochester, NY, USA). Data were corrected for β‐actin expression.
Immunohistochemistry
The formalin‐fixed, paraffin‐embedded tissues were cut into 4‐mm thickness, then loaded on the Superfrost slides (FD NeuroTechnologies Inc., Columbia, MD, USA), and incubated for 1 h at 70°C. The slices were first deparaffinized with xylene, and then rehydrated by a series of graded ethanol washes. Slides were heated in citrate buffer (100 mmol/L, pH 6.0) for 10 min, and further incubated for 20 min to retrieve antigen, and afterwards incubated in 3% hydrogen peroxide in methanol to inhibit the activity of endogenous peroxidases. The sections were incubated for 1 h in 1:100 dilution of primary antibody (FGF19 [No. ab89271; Abcam, Hong Kong], FGF21 [No. WH0026291M1; Sigma‐Aldrich) and KLB (No. HPA021136; Sigma‐Aldrich) diluted in 1% BSA/Tris‐buffered saline. Secondary staining at a dilution of 1:2,500 was then added for a period of 30 min at room temperature. DAB (No. GK500705; Dako, Glostrup, Denmark) was added for 10 min at room temperature. Sections were finally stained with Dako hematoxylin, mounted and dried ready for viewing.
The immunoreactive score, produced by the intensity of staining and mean percentage of positive cells, was assessed by two independent researchers. We counted 100 cells in each area, calculated the intensity of staining and the percentage of positive cells. The intensity of staining was ranked as follows: weak, 1; moderate, 2; and intense, 3. The percentage of positive cells was scored as follows: ≤5%, 0; 6–25%, 1; 26–50%, 3; 51–75%, 4; and ≥75%, 5. Immunoreactive scores ≤2 points were considered as negative, and otherwise as positive: 2–5 points, (+); 5–9 points, (++); and ≥9 points, (+++).
FGF19 and FGF21 enzyme‐linked immunosorbent assay
On the basis of the manufacturer's instructions, the serum concentration of FGF19 and 21 was detected by a sandwich enzyme‐linked immune sorbent assay (FGF19/FGF21 Quantikine® ELISA kit, Cat. No. DF1900/No. DF2100; R&D Systems, Minneapolis, MN, USA). The standard curve range of this assay was 15.6–1,000 pg/mL and 31.3–2,000 pg/mL, respectively. All of the above samples were repeated twice, and the mean value of the two measurements was used for analyses.
Statistical analysis
The data were analyzed by the Statistical Package for Social Science (SPSS) 20.0 (SPSS Inc., Chicago, IL, USA). Continuous variables with normal distribution were expressed as mean ± standard deviation, skewed data as the median with interquartile range and categorical variables as frequencies. The Kolmogorov–Smirnov test was used to detect normality of distribution. Intergroup differences were compared by Student's unpaired t‐test or the Mann–Whitney U‐test for continuous variables, and the χ2‐test for categorical variables. A P‐value <0.05 was used to determine the statistical significance.
Results
In the present study, 30 women with GDM and 30 healthy pregnant women, matched for gestational and maternal age, were recruited. However, 33.3% (10/30) and 16.7% (5/30) of pregnant women in the GDM and control groups were lost to follow up, respectively. A total of 10 women with GDM were excluded for the following reasons: vaginal delivery (n = 3), delivery in other hospitals (n = 3) and personal reasons; for example, withdrawing from the study (n = 4). In contrast, five healthy pregnant women were excluded due to vaginal delivery (n = 2), delivery in other hospitals (n = 1) and personal reasons (n = 2). In the end, 20 women in the GDM group and 25 women in the control group were finally analyzed in the present study. The baseline clinical characteristics of the two groups (control, GDM) are shown in Table 1. Parameters between the two groups were similar, because the participants were matched for gestational and maternal age. In addition, differences between women with GDM and the control group regarding gravidity, parity, blood pressure and FBG in the first trimester were not statistically significant. By contrast, FBG, 1‐ and 2‐h glucose values during the 75‐g oral glucose tolerance test, hemoglobin A1c at the time of oral glucose tolerance test, and prepregnancy and prepartum body mass index were significantly higher in women with GDM as compared with the control group (P < 0.05). The women with GDM were given dietary and physical exercise instruction, and carried out self‐monitoring of blood glucose at fasting and 2 h after each meal four times a day. Insulin treatment had been added when FBG levels were persistently >5.3 mmol/L, or 2‐h levels were persistently >6.7 mmol/L. Among 20 women with GDM, just two participants received insulin therapy. The proportion on insulin therapy was 10%. After receiving insulin treatment, both of them achieved the target glucose levels. In contrast, neonatal birthweight and placental weight were higher in the GDM group than those in the control group (P < 0.05). The prevalence of large for gestational age in the GDM group was 10%, whereas in the control group it was 0%. However, the difference between the groups had no statistical significance.
Table 1.
Baseline clinical characteristics and biochemical parameters of two groups
| GDM (n = 20) | NGT (n = 25) | P‐value | |
|---|---|---|---|
| Maternal parameters | |||
| Age (years) | 29.16 ± 3.43 | 28.72 ± 3.11 | 0.583 |
| Gravidity | 1.93 ± 1.22 | 1.72 ± 0.96 | 0.548 |
| Parity | 1.21 ± 0.51 | 1.13 ± 0.47 | 0.624 |
| Prepregnancy BMI (kg/m2) | 22.02 ± 3.03 | 20.03 ± 2.68 | <0.01* |
| Prepartum BMI (kg/m2) | 27.69 ± 3.23 | 26.67 ± 3.31 | 0.017* |
| FBG in 1st trimester (mmol/L) | 4.37 ± 0.63 | 4.28 ± 0.27 | 0.124 |
| OGTT fast (mmol/L) | 4.66 ± 0.53 | 4.29 ± 0.41 | <0.01* |
| OGTT 1‐h (mmol/L) | 10.03 ± 1.33 | 7.13 ± 1.32 | <0.01* |
| OGTT 2‐h (mmol/L) | 8.93 ± 1.25 | 6.63 ± 1.12 | <0.01* |
| HbA1c (%) | 5.13 ± 0.47 | 4.86 ± 0.32 | <0.01* |
| (mmol/mol) | 3.26 ± 0.51 | 2.96 ± 0.35 | <0.01* |
| SBP (mmHg) | 119 ± 12 | 114 ± 11 | 0.834 |
| DBP (mmHg) | 73 ± 9 | 76 ± 8 | 0.656 |
| Serum FGF19 before delivery (pg/mL) | 61.76 (44.76–83.43) | 72.39 (49.39–113.52) | 0.108 |
| Serum FGF21 before delivery (pg/mL) | 187.35 (62.44–345.50) | 128.10 (49.11–287.33) | 0.096 |
| Fetal parameters | |||
| Gestational age (weeks) | 38.91 ± 1.03 | 39.12 ± 1.01 | 0.595 |
| Sex | |||
| Male, n (%) | 9 (45) | 12 (48) | 0.382 |
| Female, n (%) | 11 (55) | 13 (52) | 0.306 |
| Birthweight (kg) | 3.41 ± 0.35 | 3.17 ± 0.33 | 0.045* |
| Birth length (cm) | 50.00 ± 1.55 | 49.77 ± 1.91 | 0.913 |
| LGA, n (%) | 2 (10) | 0 (0) | 0.121 |
| Placental weight (kg) | 0.65 ± 0.06 | 0.54 ± 0.05 | 0.026* |
Data are expressed as mean ± standard deviation or median (interquartile range), as appropriate. Categorical variables are expressed as number (percentage). P‐values are presented for comparison between women with gestational diabetes mellitus (GDM) and healthy pregnant women. *P < 0.05. BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; FGF, fibroblast growth factor; HbA1c, hemoglobin A1c; LGA, large for gestational age; NGT, normal glucose tolerance; OGTT, oral glucose tolerance test; SBP, systolic blood pressure.
mRNA expression
Women with GDM had significantly lower median mRNA expressions of FGF19 than women in the control group in the placenta (0.33 ± 0.05 vs 0.72 ± 0.09, P < 0.01) and rectus muscle (0.83 ± 0.11 vs 1.28 ± 0.19, P < 0.01; Figure 1a). There were no significant differences in the mRNA expressions of FGF21 and KLB in the placenta and rectus muscle (P > 0.05; Figure 1b,c).
Figure 1.
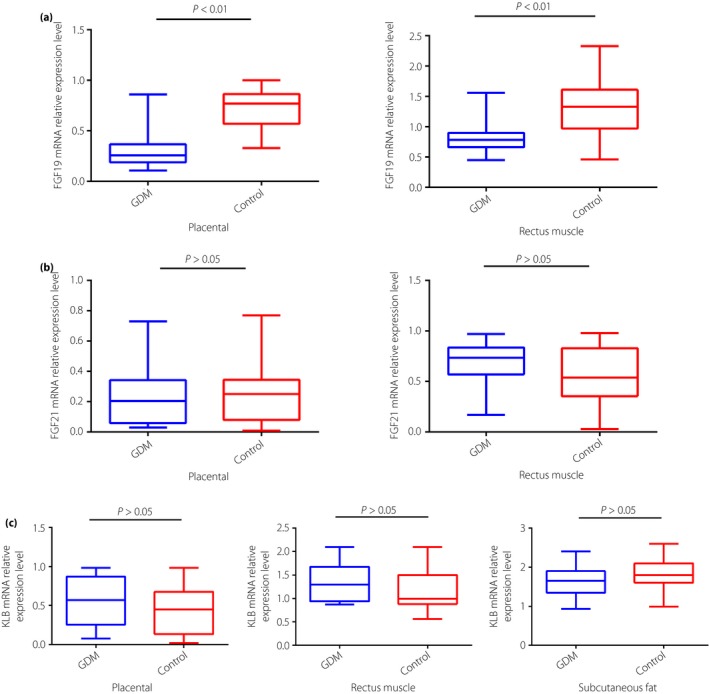
(a) Fibroblast growth factor (FGF)19 and (b) FGF21 messenger ribonucleic acid (mRNA) relative expressions in gestational diabetes mellitus (GDM) and control placenta and rectus muscle tissues, and (c) β‐klotho (KLB) mRNA relative expression in GDM and control placenta, rectus muscle and subcutaneous fat tissues by reverse transcription polymerase chain reaction. Blue boxes, GDM pregnancies (n = 20); red boxes, control pregnancies (n = 25).
Subcutaneous fat mRNA expressions of FGF19 and FGF21 were not detectable in the present study. In addition, KLB mRNA expression levels were not different between the GDM and control groups in subcutaneous fat (P > 0.05; Figure 1c).
Protein expression
The results of western blot showed that FGF19 protein expressions in the placenta (0.34 ± 0.13 vs 0.85 ± 0.20, P < 0.01) and rectus muscle (0.78 ± 0.24 vs 1.23 ± 0.39, P < 0.01) were lower in women with GDM than those in women in the control (Figure 2a). There were no significant differences in the protein expressions of FGF21 and KLB in the placenta and rectus muscle (P > 0.05; Figure 2b,c).
Figure 2.
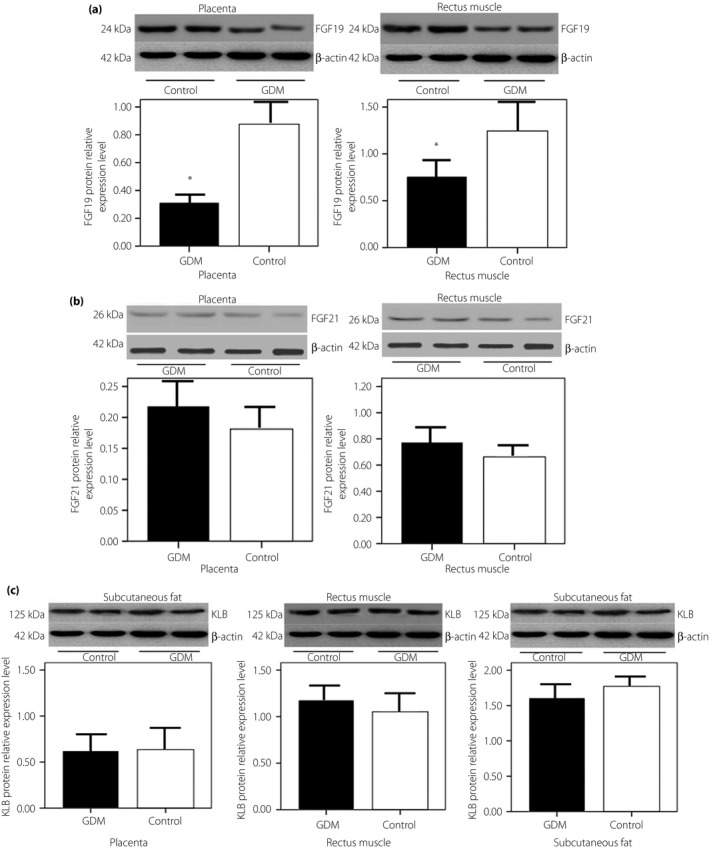
(a) Fibroblast growth factor (FGF)19 and (b) FGF21 protein relative expressions in gestational diabetes mellitus (GDM) and control placenta and rectus muscle tissues, and (c) β‐klotho (KLB) protein relative expression in GDM and control placenta, rectus muscle and subcutaneous fat tissues by western blot. Representative western blot shows FGF19 (24 kDa), FGF21 (26 kDa) and KLB (125 kDa) protein expression, and the loading control β‐Actin (42 kDa). Densitometry analysis of FGF19 expression normalized to β‐Actin in the placenta and rectus muscle tissues from GDM (n = 20) and control (n = 25) pregnancies. *Significant difference P < 0.01. Each bar represents median levels. Black bars, GDM pregnancies; white bars, control pregnancies.
There were almost no detectable FGF19 and FGF21 protein expressions in subcutaneous fat tissue. Furthermore, KLB protein expression levels were not different between the GDM and control groups in subcutaneous fat (P > 0.05; Figure 2c).
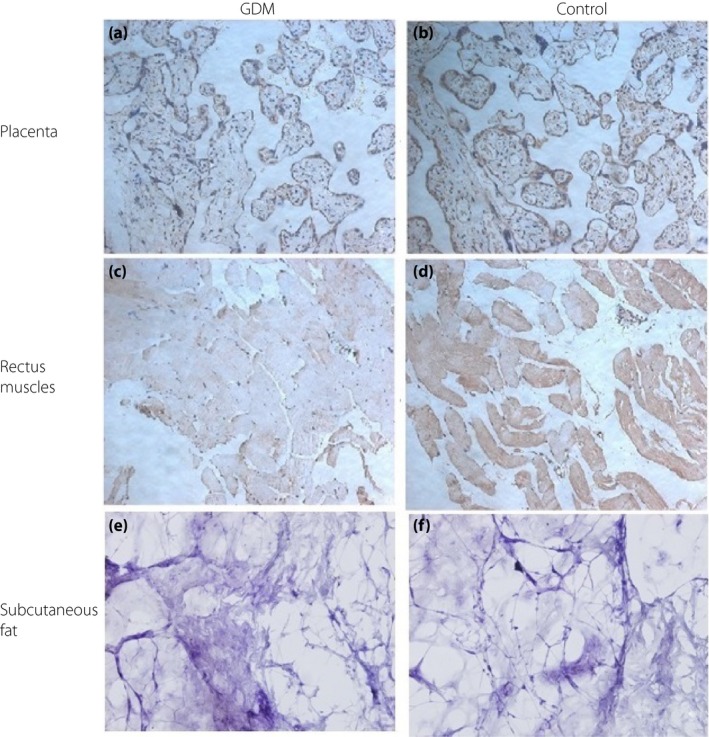
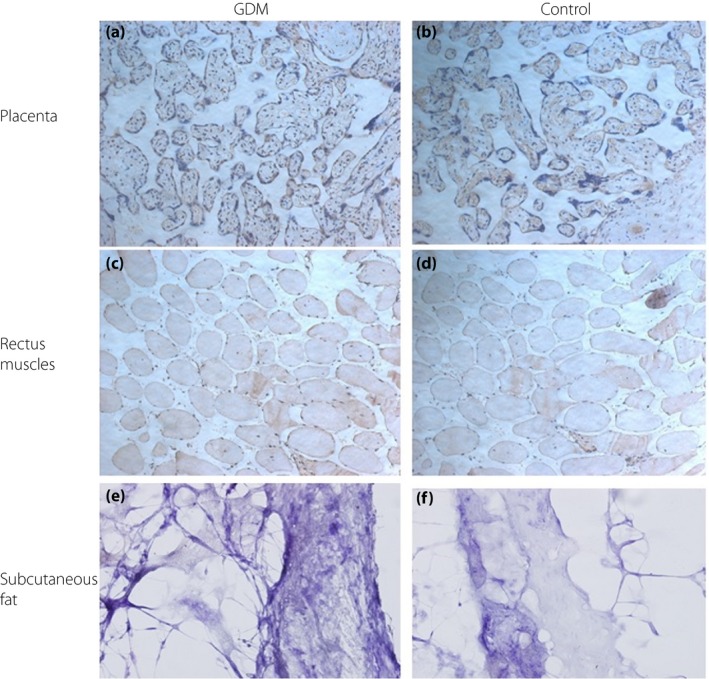
Immunohistochemistry showed similar results to those of western blot (Figures 3, 4, 5). However, subcutaneous fat tissues were easily dissolved in paraffin section, so the expression of FGF19, FGF21 and KLB in the subcutaneous fat was negative.
Figure 3.
Fibroblast growth factor (FGF)19 protein expressions by immunohistochemistry. Representative immunohistochemistry of FGF19 in the placenta and rectus muscle from women with gestational diabetes mellitus (GDM) (left column) and healthy pregnant women (control; right column). (a) GDM, placenta, FGF19 weak positive (+); (b) control, placenta, FGF19 strong positive (+++); (c) GDM, rectus muscle, FGF19 weak positive (+); (d) control, rectus muscle, FGF19 moderate positive (++); (e) GDM, subcutaneous fat, FGF19 negative (–); and (f) control, subcutaneous fat, FGF19 negative (–).
Figure 4.
Fibroblast growth factor (FGF)21 protein expressions by immunohistochemistry. Representative immunohistochemistry of FGF21 in the placenta, and rectus muscle from women with gestational diabetes mellitus (GDM; left column) and healthy pregnant women (control; right column). (a) GDM, placenta, FGF21 moderate positive (++); (b) control, placenta, FGF21 moderate positive (++); (c) GDM, rectus muscle, FGF21 weak positive (+); (d) control, rectus muscle, FGF21 weak positive (+); (e) GDM, subcutaneous fat, FGF21 negative (–); and (f) control, subcutaneous fat, FGF21 negative (–).
Figure 5.

β‐Klotho (KLB) protein expressions by immunohistochemistry. Representative immunohistochemistry of fibroblast growth factor (FGF)21 in placenta, rectus muscle and subcutaneous fat tissues from women with gestational diabetes mellitus (GDM; left column) and healthy pregnant women (control; right column). (a) GDM, placenta, KLB moderate positive (++); (b) control, placenta, KLB moderate positive (++); (c) GDM, rectus muscle, KLB weak positive (+); (d) control, rectus muscle, KLB weak positive (+); (e) GDM, subcutaneous fat, KLB negative (–); and (f) control, subcutaneous fat, KLB negative (–).
Serum levels
Maternal serum FGF19 and FGF21 levels were measured at the end of the third trimester. There were no significant differences between women with GDM and women in the control group in serum levels of both FGF19 and FGF21 (Table 1).
Discussion
In the current study, to our best knowledge, the presence of FGF19 mRNA and protein were both determined for the first time in pregnant women. FGF19 mRNA and protein determined by PCR, western blot and immunohistochemistry were significantly decreased in GDM placental and rectus muscle tissues. This finding is in accordance with our previous report that circulating FGF19 levels during the second trimester were reduced in GDM patients11. However, in the present study, there were no significant differences in serum levels of FGF19 at the time of delivery between women with GDM and women in the control group. Although it can be explained, at least in part, by the mediating effect of strict glycemic control during the third trimester in the clinical setting, the paradoxical findings warranted further exploration.
The placenta, a specific endocrine organ in pregnancy, can secrete many hormones and cytokines to regulate the maternal metabolism and fetal growth. Previous studies showed that there were abnormal expressions of some proteins in the placenta of women with GDM that re associated with insulin resistance18, 19, 20. Colomiere et al.21 reported that post‐receptor defects were present in the insulin signaling pathway in the placenta from women complicated by GDM or obesity. Our present study showed that the expression levels of FGF19 mRNA and protein in the placenta were significantly lower in women with GDM than those in women in the control group. Given that FGF19 is strongly associated with insulin resistance; the current finding might provide more evidence to support that endogenous expression levels of insulin‐related signaling components in the human term placenta are altered in GDM. In contrast, skeletal muscle is another important secretory organ. As the skeletal muscle accounts for approximate 75% of whole body insulin‐stimulated glucose uptake, defects in this tissue could result in insulin resistance12. In this insulin‐sensitive maternal peripheral tissue, a previous study showed defects in the insulin signaling pathway, which could be involved in the pathophysiology and development of insulin resistance associated with GDM12. Transgenic mice have shown the ectopic expression of FGF19 in skeletal muscle6. The present study found the expression of FGF19 in the skeletal muscle of pregnant woman. Simultaneously, we reported the expression levels of FGF19 mRNA and protein in skeletal muscle were significantly lower in GDM women. We cannot identify the mechanism of the different expressions of FGF19 in GDM. However, FGF19 has been implicated in maintaining glucose homeostasis. Thus, it can be speculated that the abnormally decreased expressions of FGF19 in GDM might involve in the development of GDM or GDM‐associated complications. Previous functional studies showed that FGF19 combined with FGFR‐KLB complex, activating an insulin‐independent endocrine pathway to mediate different metabolic effects13. In the liver, FGF19 maintains glucose homeostasis by inhibiting gluconeogenesis through the repression of the cyclic adenosine monophosphate response element binding protein/peroxisome proliferator activated receptor γ coactivator 1‐α and stimulating glycogen synthesis through the suppression of glycogen synthase kinase 313. In adipose tissue, FGF19 increases energy expenditure and insulin sensitivity, and reduces bodyweight and circulating glucose levels13. In the brain, FGF19 decreases the activity of agouti‐related peptide/neuropeptide Y, resulting in increased glucose disposal and also improving the glycemic homeostasis8, 9. Furthermore, FGF19 appears to inhibit fatty acid synthesis by suppression of lipogenic genes22. FGF19 represses the sterol regulatory element‐binding protein‐1c activity by increasing the signal transducers and activators of transcription 3, and decreasing the peroxisome proliferator activated receptor γ coactivator 1‐α activity. Sterol regulatory element‐binding protein‐1c is a critical transcriptional activator of lipogenic genes. In addition, FGF19 stimulates the small heterodimer partner, which is a transcriptional repressor that inhibits the expression of lipogenic enzyme through sterol regulatory element‐binding protein‐1c‐independent mechanisms22. If these actions of FGF19 are similar in the placenta and skeletal muscles, the decrease of its expression in GDM might weaken the beneficial effects on glucose homeostasis, so as to lead to the occurrence of GDM. Furthermore, elevated free fatty acid levels were strongly correlated with fetal growth in pregnancies of women with GDM23. This might reflect a reduced effect of FGF19 on inhibiting fatty acid synthesis.
In the present study, we failed to find significant differences between GDM women and controls in regard to the expression levels of FGF21 and KLB mRNA, and protein in the placenta or rectus muscle. Dekker et al.10 have investigated the placental expression of FGF21, KLB, and the transcription factors peroxisome proliferator activated receptor α and peroxisome proliferator activated receptor γ in 20 pregnant women with and 18 normal pregnant women without GDM. They showed that late pregnancy serum levels of FGF21 were not different between normoglycemic women and women with GDM. Furthermore, FGF21 was shown to be expressed in endothelial cells, stromal cells and syncytiotrophoblasts in the placenta. The aforementioned findings are in line with those of the present study. However, they reported that FGF21 mRNA and protein levels, and KLB mRNA expression were all significantly increased in placentae from women with GDM compared with normoglycemic women. They speculated that FGF21 could affect placental metabolism. The results are contradictory with the findings from the present study. In Dekker's study, screening for GDM was carried out with a two‐step procedure, and the diagnosis of GDM was according to the criteria of the Australasian Diabetes In Pregnancy guidelines. Thus, the glycemic levels in the women with GDM from Dekker's study were obviously higher than those of the participants in the present study. In addition, the prepregnancy body mass indexes of the women with GDM in Dekker's study were also significantly higher than those in the present study. These might be the reasons that partially explain the paradoxical results. Nevertheless, Hojman et al.24 studied the expression of FGF21 in muscle and plasma after acute insulin stimulation in young healthy adults during a hyperinsulinemic‐euglycemic clamp. They found that insulin stimulated muscular expression of FGF21 and increased plasma FGF2124. Furthermore, muscular FGF21 expression was associated with hyperinsulinemia. They considered that in the muscles, FGF21 expression seemed to be regulated by the insulin‐serine/threonine kinase (Akt) signaling pathway24. Intriguingly, their study showed that the association between muscle FGF21 and insulin was only present in men, whereas not in women. They speculated the reason might be based on the sex‐dependent Akt‐signaling pathway24. Estrogen stimulates the phosphorylation of Akt and nuclear translocation. A human myocardial study showed that nuclear localization of phosphor‐Akt was increased in young women25. This increased activity of Akt in women might explain the large muscular expression of FGF21 in women, and the relative lack of associations with insulin levels24. This conclusion might explain the lack of difference in the muscular FGF21 expression in the present study.
The identification of mouse KLB was first reported in 2000, which encodes a type I membrane protein with high resemblance to klotho26. FGFR activity is increased by the presence of the co‐receptor‐KLB, widely expressed in the liver, brain, pancreas, adipose tissue and so on of rodents27. In contrast to Dekker's study10, we did not find significant differences between GDM women and controls in regard to the expression levels of KLB mRNA and protein in the placenta or rectus muscle. According to this result, we speculate that the expression of KLB in the placenta or skeletal muscular tissue might not be influenced by the insulin resistance state during GDM. The expression distribution of KLB might be wide and complicated in humans. The precise contribution of KLB to the placenta and systemic metabolism in pregnant women warrants further exploration.
In the present study, we measured the expressions of FGF19 and FGF21 in subcutaneous fat tissue. Unfortunately, there was an absence of detectable FGF19 and FGF21 in subcutaneous fat tissue. This finding is contradictory to what we expected and the previous study15. In the present study, we did not isolate the preadipocytes and adipocytes from the subcutaneous adipose tissues. In addition, the biopsy samples of the adipose tissues might be too small. Thus, there were fewer adipocytes ingredients, besides lipids droplets. These defects of the experiment might contribute to the unexpected result. However, it is not clear whether the FGF19 and FGF21 are abundantly expressed in situ in human subcutaneous fat tissues. This problem requires further exploration.
There exist several limitations in the current study. The study design was cross‐sectional, reduced FGF19 expression could only be defined as a marker of GDM. We did not address the cause–effect relationship between FGF19 and metabolic disorders in GDM. However, FGF19 has been shown to have beneficial metabolic effects on glucose homeostasis. The abnormal expressions of FGF19 in GDM could be involved in the pathophysiology of GDM and downstream regulation of fetal growth. In addition, we are aware that the FGF19 result was based on relatively small sample numbers. Larger cohort studies are required to provide further reliable results. Furthermore, prospective studies in different ethnic groups should help address whether decreased FGF19 expression is causally associated with the occurrence and development of GDM and its complications or simply in response to the insulin resistance state in GDM.
In conclusion, we find that the expression levels of FGF19 mRNA, and protein in the placenta and rectus muscle are significantly lower in women with GDM than those in healthy pregnant women. However, there are no significant differences between women with GDM and healthy pregnant women as to the expression levels of FGF21 and KLB mRNA, and protein in the placenta and rectus muscle. In view of FGF19 being shown to improve glucose homeostasis and lipid metabolism, the abnormal lower levels of FGF19 in GDM might reduce its beneficial effects, contributing to the pathophysiology or development of GDM.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
We thank the National Natural Science Foundation of China (Grant No: 81571452), and Science and Technology Program of Guangdong, China (Grant No: 201707010107) for financial support.
J Diabetes Investig 2019; 10: 171–181
References
- 1. Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev 2012; 26: 312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang J, Li HT, Fang QC, et al Role of fibroblast growth factor 19 in maintaining nutrient homeostasis and disease. Biomed Environ Sci 2014; 27: 319–324. [DOI] [PubMed] [Google Scholar]
- 3. Kurosu H, Choi M, Ogawa Y, et al Tissue‐specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity ofFGF19 and FGF21. J Biol Chem 2007; 282: 26687–26695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu AL, Coulter S, Liddle C, et al FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4‐dependent and independent pathways. PLoS One 2011; 6: e17868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fu L, John LM, Adams SH, et al Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin deficient diabetes. Endocrinology 2004; 145: 2594–2603. [DOI] [PubMed] [Google Scholar]
- 6. Tomlinson E, Fu L, John L, et al Transgenic mice expressing human fibroblast growth factor‐19 display increased metabolic rate and decreased adiposity. Endocrinology 2002; 143: 1741–1747. [DOI] [PubMed] [Google Scholar]
- 7. Adams AC, Coskun T, Rovira AR, et al Fundamentals of FGF19 & FGF21 action in vitro and in vivo. PLoS One 2012; 7: e38438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marcelin G, Jo YH, Li X, et al Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol Metab 2013; 3: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morton GJ, Matsen ME, Bracy DP, et al FGF19 action in the brain induces insulin‐independent glucose lowering. J Clin Invest 2013; 123: 4799–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dekker Nitert M, Barrett HL, Kubala MH, et al Increased placental expression of fibroblast growth factor 21 in gestational diabetes mellitus. J Clin Endocrinol Metab 2014; 99: E591–E598. [DOI] [PubMed] [Google Scholar]
- 11. Wang D, Zhu W, Li J, et al Serum concentrations of fibroblast growth factors 19 and 21 in women with gestational diabetes mellitus: association with insulin resistance, adiponectin, and polycystic ovary syndrome history. PLoS One 2013; 8: e81190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karlsson HK, Zierath JR. Insulin signaling and glucose transport in insulin resistant human skeletal muscle. Cell Biochem Biophys 2007; 48: 103–113. [DOI] [PubMed] [Google Scholar]
- 13. Izaguirre M, Gil MJ, Monreal I, et al The role and potential therapeutic implications of the fibroblast growth factors in energy balance and type 2 diabetes. Curr Diab Rep 2017; 17: 43. [DOI] [PubMed] [Google Scholar]
- 14. Mráz M, Lacinová Z, Kaválková P, et al Serum concentrations of fibroblast growth factor 19 in patients with obesity and type 2 diabetes mellitus: the influence of acute hyperinsulinemia, very‐low calorie diet and PPAR‐α agonist treatment. Physiol Res 2011; 60: 627–636. [DOI] [PubMed] [Google Scholar]
- 15. Zhang X, Yeung DC, Karpisek M, et al Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008; 57: 1246–1253. [DOI] [PubMed] [Google Scholar]
- 16. Wang D, Xu S, Chen H, et al The associations between triglyceride to high‐density lipoprotein cholesterol ratios and the risks of gestational diabetes mellitus and large‐for‐gestational‐age infant. Clin Endocrinol (Oxf) 2015; 83: 490–497. [DOI] [PubMed] [Google Scholar]
- 17. Yang HX. Diagnostic criteria for gestational diabetes mellitus (WS 331‐2011). Chin Med J (Engl) 2012; 125: 1212–1213 (Chinese). [PubMed] [Google Scholar]
- 18. Uzelac PS, Li X, Lin J, et al Dysregulation of leptin and testosterone production and their receptor expression in the human placenta with gestational diabetes mellitus. Placenta 2010; 31: 581–588. [DOI] [PubMed] [Google Scholar]
- 19. Ma Y, Cheng Y, Wang J, et al The changes of visfatin in serum and its expression in fat and placental tissue in pregnant women with gestational diabetes. Diabetes Res Clin Pract 2010; 90: 60–65. [DOI] [PubMed] [Google Scholar]
- 20. Chen H, Chen H, Wu Y, et al Adiponectin exerts antiproliferative effect on human placenta via modulation of the JNK/c‐Jun pathway. Int J Clin Exp Pathol 2014; 7: 2894–2904. [PMC free article] [PubMed] [Google Scholar]
- 21. Colomiere M, Permezel M, Riley C, et al Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur J Endocrinol 2009; 160: 567–578. [DOI] [PubMed] [Google Scholar]
- 22. Bhatnagar S, Damron HA, Hillgartner FB. Fibroblast growth factor‐19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem 2009; 284: 10023–10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schaefer‐Graf UM, Graf K, Kulbacka I, et al Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008; 31: 1858–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hojman P, Pedersen M, Nielsen AR, et al Fibroblast growth factor‐21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes 2009; 58: 2797–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camper‐Kirby D, Welch S, Walker A, et al Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res 2001; 88: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 26. Ito S, Kinoshita S, Shiraishi N, et al Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev 2000; 98: 115–119. [DOI] [PubMed] [Google Scholar]
- 27. Fon Tacer K, Bookout AL, Ding X, et al Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 2010; 24: 2050–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]


