Abstract
Cardiovascular aging is associated with a decline in the function of the vascular endothelium. Considerable evidence indicates that age-induced impairment of endothelium-dependent vasodilation results from a reduction in the availability of nitric oxide (NO•). NO• can be scavenged by reactive oxygen species (ROS), in particular by superoxide radical (O2•−), and age-related increases in ROS have been demonstrated to contribute to reduced endothelium-dependent vasodilation in numerous large artery preparations. In contrast, emerging data suggest that ROS may play a compensatory role in endothelial function of the aging microvasculature. The primary goal of this review is to discuss reports in the literature which indicate that ROS function as important signaling molecules in the aging microvasculature. Emphasis is placed upon discussion of the emerging roles of hydrogen peroxide (H2O2) and peroxynitrite (ONOO•−) in the aging microcirculation. Overall, existing data in animal models suggest that maintenance in the balance of ROS is critical to successful microvascular aging. The limited work that has been performed to investigate the role of ROS in human microvascular aging is also discussed, and the need for future investigations of ROS signaling in older humans is considered.
Keywords: microvasculature, reactive oxygen species, nitric oxide, hydrogen peroxide, peroxynitrite
INTRODUCTION
Healthy aging, from the microvascular standpoint, is associated with endothelial health and redox balance [23,74], A decline in the function of the endothelium occurs with advancing age. This decline of function manifests as reduced angiogenic capacity, alteration of expression of adhesion molecules that regulate interaction with circulating factors and cells of the immune system, and impaired vasodilatory function. The well-documented loss of endothelium-dependent vasodilation that occurs with advancing age is present in both conduit arteries and resistance arterioles. Animal models have been used to characterize this loss of endothelium-dependent vasodilation and to define the mechanisms that underlie it. The preponderance of data obtained in animal models indicate that age-related endothelial dysfunction of the microcirculation occurs due to decreased availability of NO• [15,60,84,89].
NO• BIOAVAILABILITY IN THE AGED ENDOTHELIUM
Vasodilatory responses that are inhibited by NOS blockade have been reported to decline with age in arterioles from coronary [14,41,42], skeletal muscle [60,84,91,96], cerebral [55], and mesenteric [87] vascular beds. In resistance arteries of skeletal muscle, age-related reduction of NO•-dependent vasodilation is accompanied by reduced expression of eNOS [96]. In contrast, NO•-mediated dilation of soleus muscle resistance arteries declines with advancing age despite an increase in eNOS protein levels [84], Thus, the age-related decline in bioavailability of NO• may be dependent upon numerous factors that regulate both its production and degradation. Parallel findings have been reported in studies of the human microcirculation, obtained indirectly through measures of flow-mediated vasodilation or more directly through study of the skin microcirculation [11,34,66].
The eNOS activity is regulated by availability of substrate and cofactors, by protein‒protein interactions, and by coordinated phosphorylation and dephosphorylation [22,25,31]. In the absence of sufficient levels of the cofactor, tetrahydrobiopterin, uncoupled eNOS can produce O2•−. Degradation of NO• is heavily dependent upon the presence of cellular O2•−, a by-product of cellular respiration, which reacts readily with NO•, eliminating its vasodilatory action [82], Increased production of superoxide ions has been reported to reduce NO• availability in coronary, skeletal muscle, and mesenteric arterioles of aged rats [14,20,56,87,92],
O2•−-DERIVED REACTIVE OXYGEN SPECIES: ROLE IN ENDOTHELIAL SIGNALING WITH ADVANCING AGE
Although many studies of the aging vasculature indicate that increased production of ROS contributes to endothelial dysfunction, both O2•− and O2•−-derived ROS exhibit vasoactive properties. As early as 1996, Wei et al. [95] demonstrated that superoxide and reactive species derived from superoxide relaxed cat cerebral vessels. Cellular O2•− is regulated by SOD, which catalyzes the dismutation of O2•− into H2O2. H2O2 has also been reported to produce membrane hyperpolarization of vascular smooth muscle, leading to reduced calcium entry through voltage-gated calcium channels, and subsequent vasorelaxation of arteries in various vascular beds [54,58]. Furthermore, H2O2 regulates eNOS protein expression and activity [32,90]. In addition, ONOO•−, formed from the reaction of O2•− with NO•, may cause relaxation through two mechanisms: (1) generation of NO• and activation of guanylate cyclase in smooth muscle [43,63,64,71], and (2) hyperpolarization of smooth muscle [43,65]. Although the vasoactive and signaling properties of these ROS have been well-documented, relatively little work has been performed to determine whether or not these molecules can compensate for an age-related decline in NO•-mediated vasodilation. In particular, clinical studies have only begun to consider two important possibilities regarding the role of ROS in the loss and/or maintenance of endothelium-dependent vasodilation that occurs with advancing age. The first possibility that deserves consideration is that tight regulation of the balance of ROS is more critical to preservation of endothelium-dependent function in the aged vasculature than the absolute levels of any specific molecule or enzyme. The second possibility that warrants investigation is that ROS can act as vasodilatory signaling molecules that compensate for an age-induced reduction in NO• signaling. Although such compensatory signaling may be less efficient than vasodilation mediation by authentic NO•, elimination of these compensatory pathways may prove detrimental in an aged vasculature where NO• production is reduced.
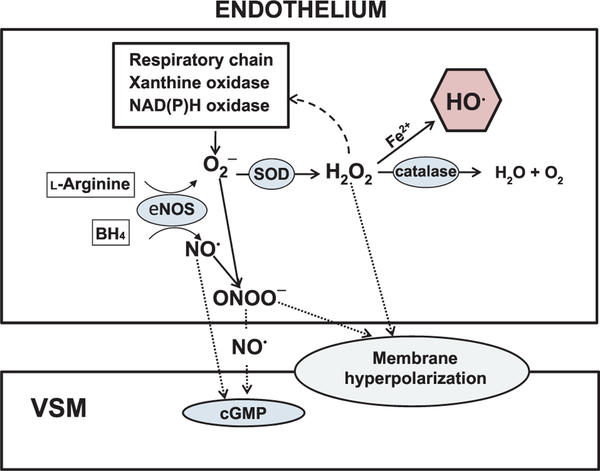
Work performed in animal models provides limited evidence that a balance in ROS signaling is critical to successful cardiovascular aging. Although it is clear that overproduction of ROS can lead to endothelial dysfunction in the microvasculature [14], evidence also exists to indicate that regulated production of both H2O2 and ONOO•− can contribute to endothelium-dependent vasodilation in the aged vasculature [39,40], which may be linked to SOD activity through at least three vasodilatory pathways. As shown in Figure 1, dismutation of O2•− could (1) increase levels of vasodilatory NO•, (2) increase levels of vasodilatory H2O2, and (3) reduce levels of vasodilatory ONOO•−. Dismutation of O2•− could also indirectly alter vasoactive signaling pathways by (1) increasing levels of highly reactive hydroxyl radical HO• if the rate of dismutation of O2•− into H2O2 exceeds that rate of conversion of H2O2 to H2O by catalase or glutathione peroxideases, or (2) reducing levels of ONOO•− that act as donors of NO•. Thus, tight regulation of 02•− is necessary for maintenance of optimal endothelium-dependent function, and age-related alterations in the balance of activity between eNOS, SOD, and catalase are likely contributors to age-induced endothelial dysfunction. Alternatively, because age-induced changes in vascular signaling occur over an extended time course, alterations in the relative activity of SOD and catalase could compensate for reduced eNOS-mediated production of authentic NO•. For example, in coronary arterioles from old and young female rats, treatment with either the SOD mimetic, Tempol (Sigma, St. Louis, MO, USA), or with catalase reduced flow-induced vasodilation and eliminated age-related differences in the maximal response to flow [39]. Treatment with the Cu/Zn SOD inhibitor, diethyldithiocarbamate, enhanced flow-induced vasodilation in arterioles from both young and old rats but did not eliminate age-related differences in flow-induced vasodilation. These findings suggest that with age, the dependence on H2O2-mediated vasodilation increases in coronary arterioles, although an ONOO•− component of the dilation persists. In contrast, in skeletal muscle arterioles from rats, H2O2-mediated vasodilation to flow decreases with age [40,85].
Figure 1.
Depiction of NO•- and ROS-mediated signaling in the endothelium and vascular smooth muscle. In addition to causing direct activation of cGMP in vascular smooth muscle, NO• can react with O2•− to form ONOO•−. ONOO•− can then act as a donor of NO• to activate cGMP. O2•− from multiple sources and can either combine with NO• to form ONOO•− or can be dismutated to H2O2 by SOD. Cell-permeable H2O2 can produce hyperpolarization of vascular smooth muscle. H2O2 that is not enzymatically converted into H2O and O2 by catalase can react with Fe2+ to produce highly reactive HO•.
The source of ROS that act as signaling molecules in the aged microvascular endothelium has not been definitively determined; however, recent reports indicate that an imbalance of ROS is a critical contributor to age-induced endothelial dysfunction in rodents [40,78,92], Trott et al [92] reported that either inhibition of NAD(P)H oxidase or scavenging of O2•− improved endothelial function in skeletal muscle feed arteries of aged rats. These results imply that either overproduction of 02•− or inadequate scavenging of O2•− contributes to endothelial dysfunction with age. In contrast, scavenging of endogenous O2•− by addition of exogenous SOD reduced endothelium-dependent vasodilation in arteries from young rats [92], Similarly, scavenging of O2•− with Tempol impaired flow-mediated vasodilation in coronary arterioles from young but not old rats, indicating that the contribution of this ROS to endothelium-dependent vasodilation changes with age [40]. In coronary arterioles from old rats, endogenous SOD protein increased significantly but this increase in SOD was not paralleled by a rise in catalase protein, resulting in an imbalance of these antioxidant enzymes and overproduction of H2O2 [40]. These results suggest that balanced activity of antioxidant enzymes is necessary for maintenance of endothelial function with advancing age.
Recent work also indicates that successful maintenance of endothelial function is critically dependent upon the ability to maintain antioxidant defense mechanisms [45,93,94], Relocation of SOD-1 to the endothelial mitochondria has been reported to function as a compensatory mechanism that counters increased ROS production in the aged aorta [45], eNOS upregulation in large arteries has also been reported to parallel the age-related increase in 02•− that occurs in rat aorta [46], NAD(P)H oxidase- derived ROS may act as intercellular regulators of the redox-sensitive transcription factors HIF-lα and Nrf2, and their target genes including NQOl, γ-glutamylcysteine synthetase, and HO-1 [94], In aortic endothelial cells, advanced glycation end products evoke ROS generation and activate Nrf2-dependent expression of HO-1 and NQOl, providing evidence of adaptive Nrf-2-mediated protection against oxidative stress in diabetes [33], Increased ROS production by the mitochondria, xanthine oxidase, and uncoupled eNOS may also activate these transcription factors leading to upregulation of antioxidant enzymes; however, with age the responsiveness of redox- sensitive transcription factors wanes in the aorta and carotid arteries [93,94], Together, these findings suggest that an age-related decline in the ability to activate endogenous antioxidant mechanisms contributes to increased endothelial inflammation and apoptosis in large arteries. Future work will be needed to determine whether or not the function of endogenous antioxidant defense mechanisms declines in the microvascular endothelium with advancing age. The impact of an age-related decline in endogenous antioxidant mechanisms on angiogenesis, endothelium- dependent vasodilation, and microvascular permeability remains to be assessed in the microvasculature.
SIGNALING ROLES OF H2O2 IN THE AGING ENDOTHELIUM
In contrast to O2•−, H2O2 is not a free radical (i.e., unpaired electrons on an open shell configuration), making it less reactive, more stable and longer lasting [2], These properties and the ability of H2O2 to diffuse across cell membranes allow it to play an important signaling role. H2O2 is primarily produced by the dismutation of O2•− by SOD, but can also be formed by the spontaneous dismutation of O2•−, or directly by the action of enzymes such as xanthine oxidase, glucose oxidase [7], and NADPH oxidase [17,51,72,76]. H2O2 is found in both physiological and pathophysiological states. In aging, H2O2 production is increased [13,48] possibly due to age-related increases in mitochondrial H2O2 generation [79–81] and eNOS dependent O2•− generation [4],
H2O2 does not inactivate NO• and in conditions of oxidant stress, H2O2 may act as a compensatory mechanism to maintain NO• bioavailability. H2O2 has been shown to cause a potent dose-dependent increase in NO• production [9], upregulate eNOS expression [8,19], and to enhance eNOS function by promoting eNOS phosphorylation and eNOS dephosphorylation at Thr-495 [90]. Recently, Martin-Garrido et al [50] demonstrated th–at H2O2 enhances vascular relaxation to NO by stabilizing sGCβ1 mRNA through HuR, increasing the expression of sGCβ1 and thus increasing cGMP formation. However, Gerassimou et al. [27] showed that higher concentrations of H2O2 downregulated sGCα1 mRNA indicating that the levels of H2O2 may dictate its action.
The impact of H2O2 on vascular function is complex depending on the species, vascular bed and experimental protocol (exogenous vs. endogenous H2O2, localization, and concentrations). Several studies have proposed that H2O2 is an EDHF [52,53,58,59,77]. H2O2 produces vasorelaxation in various murine, porcine, and human vessels via either endothelium-dependent or endothelium-independent mechanisms [3,5,6,24,37,44,47,75,98,99] but in some studies H2O2 causes vasoconstriction [26,38,47,68,73,83,100]. H2O2 is required for flow-induced increases of NO• [40] and flow-mediated dilation [58]. Overexpression of NAD(P)H oxidase in transgenic mice predominately increases H2O2 levels and exerts beneficial effects on vasodilator function and blood pressure due to H2O2 production [72], In coronary ischemia/reperfiision injury endogenous H2O2 contributes in vivo to coronary vasodilation to compensate for the loss of NO• and plays a cardioprotective role, particularly in microvessels [97].
H2O2 that functions in endothelial signaling may be derived from several sources, depending on physiological conditions. In skeletal muscle arterioles exposed to intraluminal flow, both age and exercise training increased eNOS- derived O2•− signaling; this elevation in eNOS-derived O2•− was accompanied by an increase in catalase-sensitive vasodilation, suggesting that eNOS-derived O2•− constituted the source of vasodilatory H2O2 [78]. In contrast, in skeletal muscle arterioles from both young and old rats, stimulation with acetylcholine produces catalase-sensitive vasodilation that is abolished by treatment with either apocynin or an inhibitor of gp91phox (Sindler, A.L., Muller-Delp, J.M, unpublished observations). In cerebral arterioles of aged rats, both p67phox and gp91phox proteins increased, with accompanying impairment of endothelial function, suggesting that NAD(P)H-derived O2•− is not transformed to vasodilatory H2O2 [55]. In the aged myocardium, H2O2 is generated by the electron transport chain of myocytes, and because it is freely diffusible, produces metabolic vasodilation of coronary arterioles [48]. Thus, the cellular sources of H2O2 vary between arterioles from distinct vascular beds. In future work, identifying the sources of ROS generation may provide insight into therapeutic targets for prevention and/or remediation of age- related vascular dysfunction.
ROLE OF CYTOTOXIC HYDROXYL RADICAL IN THE AGING ENDOTHELIUM
SOD reduces oxidant stress by dismutating O2•− into H2O2; however, in the presence of catalytic transition metals, SOD can rapidly form HO• [67]. H2O2 generates HO• through metal-catalyzed reactions, such as the Fenton reaction as follows: H2O2 + Fe2+ → Fe3+ + HO• + OH−. The formation of HO• is further promoted by the presence of O2•−, which reacts with Fe3+ to produce Fe2+ through the Haber-Weiss reaction [29,70]. The net effect of SOD is the dismutation of O2•− to produce either the vasodilatory H2O2, or in the presence of Fe2+, HO•. This production of HO• may occur more readily if the production of H2O2 exceeds the enzymatic capacity of endogenous catalase or peroxidases. In coronary arterioles from both young and old rats, addition of the SOD-mimetic, Tempol, reduced flow-induced vasodilation, and addition of the iron chelating agent, deferoxamine, reversed this Tempol-mediated inhibition of endothelium-dependent dilation [40]. These results suggest that treatment with exogenous SOD may drive overproduction of H2O2 and promote formation of HO* in the endothelium. Deferoxamine alone reversed impairment of flow-induced vasodilation in coronary arterioles from old rats, but had no effect on arterioles from young rats [40], suggesting that flow stimulates production of HO• in arterioles from old but not young rats. Similarly, deferoxamine reversed Tempol-induced reduction of flow- induced vasodilation in skeletal muscle of old rats [78]. Together these data suggest that although H2O2 may function as an important endothelium-dependent vasodilator, production of H2O2 that exceeds the buffering capacity of the endothelium can impair endothelial function, and this is likely due to excess production of HO•. The age-related increase in production of HO• could result from (1) an age-associated decrease in the activities of catalase and/or peroxidases in the endothelium, (2) an age-induced increase in the activity of SOD isoforms, or (3) increased accumulation of Fe2+ in the aged endothelium. It is also possible that accumulation of Fe2+ is accompanied by a relative imbalance in the activities of SOD and catalase.
AGE-INDUCED ALTERATIONS OF NO• BIOAVAILABILITY IN HUMANS
Several in vivo models have been used to study vascular aging in humans. Doppler methods for determination of cutaneous blood flow and blood flow in large/medium size upper body arteries are the most commonly employed models [1,11,28,36]. In general, these models have assessed the participation of NO• in vascular reactivity using NOS inhibition (i.e., l-NAME or l-NNMA). Interestingly, these studies have shown conflicting results, which could be associated with differences in the vascular beds being studied and differences in the stimuli employed to trigger vasodilation, e.g., acetylcholine vs. cuff occlusion methods.
Both Green et al. [28] and Casey et al. [11] have shown an age-dependent decrease in NO•-mediated forearm blood flow during exercise. In contrast, Holowatz et al. [34,35] have shown an increase in NO•-dependent, cutaneous vasodilation in the elderly. Despite these conflicting results, all these studies concluded that reduced NO• bioavailability would be the principal cause of age-related impairment of vascular reactivity [11,34,35].
Compensatory vasodilation that occurs in response to a stressor such as hypoxic exercise is blunted in aged subjects [10,11]. Casey et al. [11] reported that eNOS inhibition reduced the vascular response to hypoxemic exercise in young but not in old subjects, suggesting that the age- related reduction of this vasodilatory response occurred as a result of impaired NO• signaling. The hypoxic stress produced greater vasodilation in young subjects; however, there were no differences in forearm blood flow and vascular conductance between old and young subjects [11]. These results could indicate that healthy aging involves arterial remodeling, such as increased brachial diameter [16,18,66], thereby providing a compensatory mechanism for the impairment of NO• signaling. Similar observations have been shown using the skin blood flow model [34], Although NO•-dependent cutaneous vasodilation was impaired in the elderly, there was no significant difference in the reflex cutaneous vasodilation threshold between old and young subjects [34], Unfortunately, due to the relative nature of Laser-Doppler probes, cutaneous raw blood flow cannot be used to assess age-related structural changes, lArginine supplementation and arginase inhibition improve thermoregulatory cutaneous vasodilation in the elderly, confirming the NO•-dependency of this age-related alteration in vascular reactivity [35].
Although the aforementioned studies suggest that NO• availability is impaired in the elderly, a recent study [21] has shown that cellular signaling downstream of NO•, i.e., activation of cAMP and cGMP, is preserved in smooth muscle cells of older subjects. Therefore, we could speculate that NO• production is blunted in the elderly, whereas NO• bioavailability is not decreased. Vascular structural changes observed in the elderly [16,18,66] may also impact NO•-dependent vasodilation. Increased basal and submaximal blood flow through larger vessels may compensate for impaired reactivity and a decrease in the shear stress- induced endothelial NO• production. This “new” healthy vascular status in the elderly could be associated with a new endothelial redox status in which NO• production is not the primary determinant of endothelium-dependent- vasodilation.
ROLE OF H2O2 IN ENDOTHELIAL FUNCTION IN HUMANS
Although some reports describe H2O2 as an EDHF in humans [53,58], others have offered conflicting evidence regarding the role of H2O2 in mediating endothelium- dependent vasodilation [12,30,32,44,53,57,62,69]. Hamilton et al. [30] reported that NO•/prostanoid-independent relaxation of human radial arteries to carbachol was resistant to treatment with either SOD or catalase, suggesting that this EDHF-like component of the endothelium-dependent response to carbachol was not mediated by H2O2. It is important to note that these authors assessed only the contribution of H2O2 that originated from O2•−. In contrast, Nacitarhan et al. [62] studied internal thoracic artery rings and found that authentic H2O2 produced dose-dependent relaxations that were blunted by 4-aminopyridine, a voltage-dependent potassium channel blocker. These contradictory results may reflect differences in the vascular beds and vasodilatory stimuli being studied. Using a similar approach, Conklin et al. [12] assessed vasoreactivity to H2O2 in rings from human radial arteries, internal mammary arteries, and saphenous veins. Although the responses differed between vessels, a vasorelaxant effect of H2O2 was observed, especially in radial arteries and internal mammary arteries. Interestingly, these authors suggested that H2O2 generation occurs at the vascular smooth muscle cell plasma membrane rather than in the endothelium [12].
In coronary arterioles from heart failure patients [44,58], flow-induced vasodilation is inhibited by catalase and by inhibitors of potassium channels, providing evidence that H2O2 functions as an EDHF in this vascular bed. Similar observations have been made in other human microvascular beds [32,53,69]. For example, Matoba et al. [53] found that H2O2 is a primary EDHF in human mesenteric resistance arteries and Phillips et al. [69] observed that H2O2 could replace NO• as the primary vasodilatory agent in microvessels from human visceral fat. Interestingly, Hatoum et al. [32] observed that H2O2 is released by the vascular endothelium of human submucosal intestinal microvessels, but that it does not act as EDHF in these vessels; on the contrary, it produces vasoconstriction in denuded vessels. Overall these results indicate that H2O2 functions as an EDHF in human arterioles; however, the net vasoactive effect of H2O2 may depend on the vascular bed and the health status of the patients being studied [32].
In a recent study of the human cutaneous microcirculation, Medow et al [57], showed that H2O2 scavenging with Ebselen (Sigma, St. Louis, MO, USA) reduced cutaneous vasodilation to heat in healthy young subjects. These results provide evidence that H2O2 contributes to control of local blood flow in vivo and emphasize the need for further studies to establish the mechanisms of H2O2 generation and action in the human microcirculation in vivo. Moreover, it would be interesting to use this in vivo model to study the role of H2O2 in regulation of cutaneous blood flow in elderly subjects.
Although numerous studies have now implicated a role for H2O2 in regulation of vascular resistance in humans, virtually nothing is known regarding the effects of age on H2O2 signaling in the microcirculation of humans. The work of Miura et al. suggests that H2O2 functions as a significant endothelium-dependent vasodilator in coronary arterioles from heart failure patients [57], a disease that is more prevalent in elderly populations. It is possible that H2O2 compensates for a loss of NO•-mediated vasodilation in elderly humans. Alternatively, if dysregulation of H2O2 production/degradation occurs with age, damage to either the endothelium or the vascular smooth muscle could ensue and contribute to age-induced vascular dysfunction. Further studies in human subjects are needed to assess the effects of age on (1) regulation of vascular H2O2 production/scavenging, and (2) H2O2 signaling in both the endothelium and vascular smooth muscle.
ROLE OF ONOO•− IN ENDOTHELIAL FUNCTION OF ELDERLY PATIENTS
Although increased oxidative stress in the endothelial cell can result in increased production of ONOO•− (Figure 1), an increase in ONOO•− does not necessarily decrease NO• bioavailability. In fact, ONOO•− could become a NO• donor when NO• production is impaired [63,88] and an increased endothelial ONOO•− is associated with aging [46], which could establish a “new” redox status. This is in agreement with animal studies [63,78,92] in which ROS have been reported to play a significant role as signaling molecules in this “new” healthy vascular endothelium. In their recent study, Medow et al [57] also showed that O2•− scavenging with Tempol produced a decrease in skin blood flow in healthy young subjects [57]. If these results, added to those obtained with H2O2, mimic those obtained in young rats [78,92], it would be interesting to determine the effects of Tempol and/or Ebselen on skin blood flow in elderly subjects.
Although these models have answered several important questions, they are not designed to study peripheral muscle or myocardial microvascular beds, which are more difficult to study in vivo in humans. One way to study the coronary microvasculature in vivo in humans is by studying refractory angina. Refractory angina is normally observed in patients with coronary artery disease that do not respond to antiangina treatment [61]. Moreover, an increase in nitrate dosage, normally a sublingual NO• donor (e.g., nitroglycerine), does not improve chest pain. Interestingly, there is a negative association between the use of nitrates and outcomes in the elderly when compared with younger patients [86] and, although nitrates are commonly prescribed drugs, they do not reduce mortality in aged patients [49]. There are multiple mechanisms that could explain this nitrate intolerance [61]. It is assumed that, in some patients, adding extrinsic NO• to an oxidatively stressed vessel would increase ONOO•− production resulting in a further decrease of NO• bioavailability; however, in the elderly coronary artery disease patient adding extrinsic NO• could disrupt the “new” vascular redox status, limiting ONOO•− as an NO• donor. Currently, these hypotheses are speculative, and there is ample opportunity for new studies investigating the role of NO• and ONOO•− in the coronary microcirculation of patients with refractory angina.
PERSPECTIVE
The effectiveness of therapeutic interventions in elderly patients relies upon comprehensive knowledge of the alterations in vascular control mechanisms that occur with advancing age. In the microcirculation of aged animals, increasing evidence indicates that ROS function as important signaling molecules in both the endothelium and vascular smooth muscle. Therapies directed at scavenging or removal of these reactive species could have deleterious consequences, particularly if vascular control becomes increasingly dependent upon these reactive species with advancing age. In patients, future studies need to focus on determining how age affects the balance between oxidant production and antioxidant enzymes. In addition, future studies are needed to determine whether or not ROS signaling is critical to maintenance of vascular control mechanisms in healthy, successful aging.
Abbreviations used:
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- EDHF
endothelium- derived hyperpolarizing factor
- eNOS
endothelial nitric oxide synthase
- H2O2
hydrogen peroxide
- HIF-lα
hypoxia-inducible factor-lα
- HO
hydroxyl radical
- HO-1
heme oxygenase-1
- L-NAME
L-nitro-arginine methyl ester
- L-NNMA
L-NG- monomethyl arginine
- NAD(P)H
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NOS
nitric oxide synthase
- NQOl
NAD(P)H:quinone reductase 1
- Nrfi2
nuclear factor (erythroid-derived 2)-related factor-2
- O2
superoxide
- ONOO
peroxynitrite
- sGC(β)1
soluble guanylate cyclase (β)1 subunit
- SOD
superoxide dismutase
Biographies

Christiaan Leeuwenburgh received his PhD from the University of Illinois, Urbana-Champagne in 1995 where his doctoral work focused on the regulation of glutathione homeostasis during chronic glutathione deficiencies and/or supplementation. He became an Assistant Professor and Director of the Biochemistry of Aging Laboratory in 1998 at the University of Florida. He is currently a Professor with the Department of Aging and Geriatric Research, College of Medicine and Institute on Aging at the University of Florida and is the Chief of the Division of Biology of Aging.
His major research focus is to understand the molecular mechanism of oxidative stress and apoptosis with age. His work on assessment of oxidative damage and apoptosis with age has been increasingly recognized and appreciated by gerontologists worldwide.

Demetra Christou, Ph.D. received her doctoral training at the University of Illinois at Urbana-Champaign in the area of Exercise Physiology/Body Composition. She then trained as a Research Associate for six years in the area of Human Cardiovascular Physiology at the University of Colorado at Boulder. Prior to coming to the University of Florida, Dr. Christou was an Assistant Professor in the Department of Health and Kinesiology and the Department of Internal Medicine, Division of Cardiology at Texas A&M University and Health Science Center.
For the past 4 years Dr. Christou has directed the Integrative Cardiovascular Physiology Laboratory. Her lab performs mechanistic biomedically-relevant research in humans from an integrative perspective using whole- body measures (e.g., flow mediated dilation via ultrasonography) complemented with cellular/molecular approaches (vascular endothelial protein expression, mRNA expression in peripheral blood mononuclear cells). The general research focus of her lab is the study of alterations in cardiovascular-autonomic function in aging and related risk factors for cardiovascular disease. In addition, her group is interested in the effect of lifestyle interventions such as physical activity/exercise training and diet on cardiovascular function. Current projects investigate the mechanisms responsible for vascular endothelial dysfunction and arterial stiffness in healthy aging and in older adults with metabolic syndrome.

Alvaro Gurovich, P.T., Ph.D. received his Physical Therapy degree from Pontificia Universidad Católica de Chile in 1990 and worked as a clinician for more than 15 years. Even though Dr. Gurovich had granted tenure in the School of Kinesiology and Physical Therapy at Pontificia Universidad Católica de Valparaíso, he moved to University of Florida where he received his doctoral degree in Health and Human Performance in 2010. Once graduated, he started his tenure as post-doctoral associate at University of Florida College of Medicine, in the Department of Physiology and Functional Genomics, under Dr. Judy M. Muller-Delp training, where he is learning some in vitro and in situ techniques that will strength his translational research background.

Judy Muller-Delp received her Ph.D. in Physiology from the University of Missouri in 1992, where her work focused on coronary microvascular adaptations to exercise training. She trained as a postdoctoral research associate at Texas A&M University and at the University of Missouri. She became an Assistant Professor of Kinesiology at Texas A&M University in 2000. She is currently an Associate Professor of Physiology and Functional Genomics at the University of Florida.
Research in Dr. Muller-Delp’s laboratory focuses on understanding microvascular adaptations to aging and interventional exercise training in cardiac and skeletal muscle, with a major emphasis on assessing the cellular mechanisms that underlie age- induced dysfunction of the endothelium and vascular smooth muscle in resistance arteries.
REFERENCES
- 1.Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vase Surg 42: 574–581, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 231: 237–251, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol 275: H1283–H1289, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Belik J, Jerkic M, McIntyre BA, Pan J, Leen J, Yu LX, Henkelman RM, Toporsian M, Letarte M. Age-dependent endothelial nitric oxide synthase uncoupling in pulmonary arteries of endoglin heterozygous mice. Am J Physiol Lung Cell Mol Physiol 297: L1170–L1178, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol 252: H721–H732, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Burke-Wolin T, Abate CJ, Wolin MS, Gurtner GH. Hydrogen peroxide-induced pulmonary vasodilation: role of guanosine 3’,5’-cydic monophosphate. Am J Physiol 261: L393–L398, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Cai H Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res 68: 26–36, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Cai H, Davis ME, Drummond GR, Harrison DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca(2+)/calmodulin-dependent protein kinase ll/janus kinase 2-dependent pathway. Arterioscler Thromb Vase Biol 21: 1571–1576, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Cai H, Li Z, Davis ME, Kanner W, Harrison DG, Dudley SC Jr. Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol Pharmacol 63: 325–331, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol 588: 373–385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey DP, Walker BG, Curry TB, Joyner MJ. Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol 589: 1477–1488, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conklin DJ, Cowley HR, Wiechmann RJ, Johnson GH, Trent MB, Boor PJ. Vasoactive effects of methylamine in isolated human blood vessels: role of semicarbaz- ide-sensitive amine oxidase, formaldehyde, and hydrogen peroxide. Am J Physiol Heart Circ Physiol 286: H667–H676, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Csiszar A, Labinskyy N, Orosz Z, Xiang-min Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Heart Circ Physiol 293: H919–H927, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 15.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Devan AE, Umpierre D, Harrison ML, Lin HF, Tarumi T, Renzi CP, Dhindsa M, Hunter SD, Tanaka H. Endothelial ischemia-reperfusion injury in humans: association with age and habitual exercise. Am J Physiol Heart Circ Physiol 300: H813–H819, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin ll-stimulated superoxide and hydrogen peroxide production. Free Radie Biol Med 45: 1340–1351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobrosielski DA, Arce AA, Allen JD, Wood RH, Welsch MA. Biphasic responses of the brachial artery diameter following forearm occlusion: a blunted response in the elderly. Dyn Med 5: 4, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res 86: 347–354, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Egashira K, Inou T, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Kuga T, Urabe Y, Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation 88: 77–81, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Elvebak RL, Eisenach JH, Joyner MJ, Nicholson WT. The function of vascular smooth muscle phosphodiesterase III is preserved in healthy human aging. Clin Transi Sei 3: 239–242, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Frisard MI, Broussard A, Davies SS, Roberts LJ, Rood J, Jonge L, Fang X, Jazwin-ski SM, Deutsch WA, Ravussin E. Aging, resting metabolic rate, and oxidative damage: results from the Louisiana Healthy Aging Study. J Gerontol Ser A Biol Sei Med Sei 62: 752, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimoto S, Asano T, Sakai M, Sakurai K, Takagi D, Yoshimoto N, Itoh T. Mechanisms of hydrogen peroxide-induced relaxation in rabbit mesenteric small artery. Eur J Pharmacol 412: 291–300, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao YJ, Lee RM. Hydrogen peroxide induces a greater contraction in mesenteric arteries of spontaneously hypertensive rats through thromboxane A(2) production. Br J Pharmacol 134: 1639–1646, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerassimou C, Kotanidou A, Zhou Z, Simoes DC, Roussos C, Papapetropoulos A. Regulation of the expression of soluble guanylyl cyclase by reactive oxygen species. Br J Pharmacol 150: 1084–1091, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O’Driscoll G, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol 562: 617–628, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halliwell B Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radie Biol Med 7: 645–651, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton CA, McPhaden AR, Berg G, Pathi V, Dominiczak AF. Is hydrogen peroxide an EDHF in human radial arteries? Am J Physiol Heart Circ Physiol 280: H2451–H2455, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem 276: 16587–16591, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Hatoum OA, Binion DG, Miura H, Telford G, Otterson MF, Gutterman DD. Role of hydrogen peroxide in ACh-induced dilation of human submucosal intestinal microvessels. Am J Physiol Heart Circ Physiol 288: H48–H54, 2005. [DOI] [PubMed] [Google Scholar]
- 33.He M, Siow RC, Sugden D, Gao L, Cheng X, Mann GE. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr Metab Cardiovasc Dis 21: 277–285, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 284: H1662–H1667, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Holowatz LA, Thompson CS, Kenney WL. L-Arginine supplementation or argi- nase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol 574: 573–581, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol 105: 370–372, 2008. [DOI] [PubMed] [Google Scholar]
- 37.lida Y, Katusic ZS. Mechanisms of cerebral arterial relaxations to hydrogen peroxide. Stroke 31: 2224–2230, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Jin N, Rhoades RA. Activation of tyrosine kinases in H2O2-induced contraction in pulmonary artery. Am J Physiol 272: H2686–H2692, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Kang LS, Chen B, Reyes RA, Leblanc AJ, Teng B, Mustafa SJ, Muller-Delp JM. Aging and estrogen alter endothelial reactivity to reactive oxygen species in coronary arterioles. Am J Physiol Heart Cire Physiol 300: H2105–H2115, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H(2)0(2). Am J Physiol Heart Circ Physiol 297: H1087–H1095, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leblanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, Muller-Delp JM. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am J Physiol Ftegul Integr Comp Physiol 297: R1713–R1723, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol 295: H2280–H2288, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Li W, Altura BT, Altura BM. Perox-ynitrite-induced relaxation in isolated rat aortic rings and mechanisms of action. Toxicol Appl Pharmacol 209: 269–276, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003. [DOI] [PubMed] [Google Scholar]
- 45.van der Loo B, Bachschmid M, Skepper JN, Labugger R, Schildknecht S, Hahn R, Mussig E, Gygi D, Luscher TF. Age-associated cellular relocation of Sod 1 as a self-defense is a futile mechanism to prevent vascular aging. Biochem Biophys Res Commun 344: 972–980, 2006. [DOI] [PubMed] [Google Scholar]
- 46.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens 23: 571–579, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Machii H, Saitoh S, Kaneshiro T, Takeishi Y. Aging impairs myocardium-induced dilation in coronary arterioles: role of hydrogen peroxide and angiotensin. Mech Ageing Dev 131: 710717, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Majeed F, Kelemen MD. Acute coronary syndromes in the elderly. Clin Geriatr Med 23: 425–440, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Martin-Garrido A, Gonzalez-Ramos M, Griera M, Guijarro B, Cannata-Andia J, Rodriguez-Puyol D, Rodriguez-Puyol M, Saura M. H2O2 regulation of vascular function through sGC mRNA stabilization by HuR. Arterioscler Thromb Vase Biol 31: 567–573, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Matoba T, Shimokawa H. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Pharmacol Sei 92: 1–6, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun 290: 909–913, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Matoba T, Shimokawa H, Morikawa K, Kubota H, Kunihiro I, Urakami-Harasawa L, Mukai Y, Hirakawa Y, Akaike T, Takeshita A. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler Thromb Vasc Biol 23: 1224–1230, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Mayhan WG, Arrick DM, Sharpe GM, Sun H. Age-related alterations in reactivity of cerebral arterioles: role of oxidative stress. Microcirculation 15: 225–236, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Mayhan WG, Faraci FM, Baumbach GL, Heistad DD. Effects of aging on responses of cerebral arterioles. Am J Physiol 258: H1138–H1143, 1990. [DOI] [PubMed] [Google Scholar]
- 57.Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J Appl Physiol 111: 20–26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: e31–e40, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Morikawa K, Fujiki T, Matoba T, Kubota H, Hatanaka M, Takahashi S, Shimokawa H. Important role of superoxide dismutase in EDHF-mediated responses of human mesenteric arteries. J Cardiovasc Pharmacol 44: 552–556, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res 97: 618–628, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Nacitarhan C, Bayram Z, Eksert B, Usta C, Golbasi I, Ozdem S. The effect of hydrogen peroxide in human internal thoracic arteries: role of potassium channels, nitric oxide and cydooxygenase products. Cardiovasc Drugs Ther 21: 257–262, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Nossaman BD, Bivalacqua TJ, Champion HC, Baber SR, Kadowitz PJ. Analysis of vasodilator responses to peroxynitrite in the hindlimb vascular bed of the cat. J Cardiovasc Pharmacol 50: 358–366, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Nossaman BD, Kadowitz PJ. Potential benefits of peroxynitrite. Open Pharmacol J 2: 31–53, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohashi M, Faraci F, Heistad D. Peroxynitrite hyperpolarizes smooth muscle and relaxes internal carotid artery in rabbit via ATP-sensitive K+ channels. Am J Physiol Heart Circ Physiol 289: H2244–H2250, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Padilla J, Simmons GH, Fadel PJ, Laughlin MH, Joyner MJ, Casey DP. Impact of aging on conduit artery retrograde and oscillatory shear at rest and during exercise: role of nitric oxide. Hypertension 57: 484–489, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pattwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radie Biol Med 37: 1064–1072, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Pelaez NJ, Osterhaus SL, Mak AS, Zhao Y, Davis HW, Packer CS. MAPK and PKC activity are not required for H(2)0(2)-induced arterial muscle contraction. Am J Physiol Heart Circ Physiol 279: H1194–H1200, 2000. [DOI] [PubMed] [Google Scholar]
- 69.Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol 292: H93–H100, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radi R, Cosgrove TP, Beckman JS, Freeman BA. Peroxynitrite-induced luminol chemiluminescence. Biochem J 290(Pt 1): 51–57, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vase Biol 31: 1368–1376, 2011. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez-Martinez MA, Garcia-Cohen EC, Baena AB, Gonzalez R, Salaices M, Marin J. Contractile responses elicited by hydrogen peroxide in aorta from normotensive and hypertensive rats. Endothelial modulation and mechanism involved. Br J Pharmacol 125: 1329–1335, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salmon AB, Richardson A, Pérez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Rad Biol Med 48: 642–655, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato A, Sakuma I, Gutterman DD. Mechanism of dilation to reactive oxygen species in human coronary arterioles. Am J Physiol Heart Circ Physiol 285: H2345–H2354, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Piastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. N0X4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shimokawa H, Morikawa K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Mol Cell Cardiol 39: 725–732, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sohal RS, Brunk UT. Mitochondrial production of pro-oxidants and cellular senescence. Mutat Res 275: 295–304, 1992. [DOI] [PubMed] [Google Scholar]
- 80.Sohal RS, Orr WC. Relationship between antioxidants, proxidants, and the aging process. Ann N Y Acad Sei 663: 74–84, 1992. [DOI] [PubMed] [Google Scholar]
- 81.Sohal RS, Sohal BH. Hydrogen peroxide release by mitochondria increases during aging. Mech Ageing Dev 57: 187–202, 1991. [DOI] [PubMed] [Google Scholar]
- 82.Somers MJ, Harrison DG. Reactive oxygen species and the control of vasomotor tone. Curr Hypertens Rep 1: 102–108, 1999. [DOI] [PubMed] [Google Scholar]
- 83.Sotnikova R Investigation of the mechanisms underlying H2O2-evoked contraction in the isolated rat aorta. Gen Pharmacol 31: 115–119, 1998. [DOI] [PubMed] [Google Scholar]
- 84.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol (Lond) 556: 947–958, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spier SA, Delp MD, Ramsey MW, MullerDelp J. Mechanisms of endothelium dependent flow-induced vasodilation in rat skeletal muscle arterioles. FASEB J 15: A51, 2001. [Google Scholar]
- 86.Stone PH, Thompson B, Anderson HV, Kronenberg MW, Gibson RS, Rogers WJ, Diver DJ, Theroux P, Warnica JW, Nasmith JB, Kells C, Kleiman N, McCabe CH, Schactman M, Knatterud GL, Braunwald E. Influence of race, sex, and age on management of unstable angina and non-Q-wave myocardial infarction: The TIMI III registry. JAMA 275: 1104–1112, 1996. [PubMed] [Google Scholar]
- 87.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol 286: H2249–H2256, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6: 662–680, 2007. [DOI] [PubMed] [Google Scholar]
- 89.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000. [DOI] [PubMed] [Google Scholar]
- 90.Thomas SR, Chen K, Keaney JF Jr. Hydrogen peroxide activates endothelial nitricoxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem 277: 6017–6024, 2002. [DOI] [PubMed] [Google Scholar]
- 91.Trott DW, Gunduz F, Laughlin MH, Woodman CR. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 106: 1925–1934, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trott DW, Seawright JW, Luttrell MJ, Woodman CR. NAD(P)H oxidase-derived reactive oxygen species contribute to age-related impairments of endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol 110: 1171–1180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta EG, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sei Med Sei 66: 866–875, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol 301: H363–H372, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wei EP, Kontos HA, Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol Heart Circ Physiol 271: H1262–H1266, 1996. [DOI] [PubMed] [Google Scholar]
- 96.Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 93: 1685–1690, 2002. [DOI] [PubMed] [Google Scholar]
- 97.Yada T, Shimokawa H, Hiramatsu O, Haruna Y, Morita Y, Kashihara N, Shinozaki Y, Mori H, Goto M, Ogasawara Y, Kajiya F. Cardioprotective role of endogenous hydrogen peroxide during ischemia-reperfusion injury in canine coronary microcirculation in vivo. Am J Physiol Heart Circ Physiol 291: H1138–H1146, 2006. [DOI] [PubMed] [Google Scholar]
- 98.Yang ZW, Zhang A, Altura BT, Altura BM. Endothelium-dependent relaxation to hydrogen peroxide in canine basilar artery: a potential new cerebral dilator mechanism. Brain Res Bull 47: 257–263, 1998. [DOI] [PubMed] [Google Scholar]
- 99.Yang Z, Zhang A, Altura BT, Altura BM. Hydrogen peroxide-induced endothelium-dependent relaxation of rat aorta involvement of Ca2+ and other cellular metabolites. Gen Pharmacol 33: 325–336, 1999. [DOI] [PubMed] [Google Scholar]
- 100.Yang ZW, Zheng T, Zhang A, Altura BT, Altura BM. Mechanisms of hydrogen peroxide-induced contraction of rat aorta. Eur J Pharmacol 344: 169–181, 1998. [DOI] [PubMed] [Google Scholar]