Abstract
Background/Objective: The objective is to present the development of a novel web-based patient registry for sarcoidosis. We describe recruitment efforts and assess efficacy of internet-based advertising on recruitment. Methods: “Worldwide Sarcoidosis Research Study (WISE)” started in 2011 under the domain www.sarcoidstudy.org. The registry includes thirteen patient-reported surveys about patient characteristics, diagnosis, and treatment. Effects of two internet-based advertising methods (geographically-broad versus geographically-targeted to high sarcoidosis search areas) on recruitment were analyzed with time series regression. Results: Since 2011, over 1500 participants have registered (82% whites, 9% African Americans, 5% mixed, 4% other), with 23% of participants providing saliva samples for DNA. Median age is 43 years (range 21-80). African Americans were more frequently recruited via support groups, while whites had a higher frequency of finding the registry via internet. Generalized internet-based advertising significantly improved recruitment in all demographic groups (p<0.001). However, a higher response rate to internet-based advertising was seen in whites compared to African Americans (p<0.001), females versus males (p=0.043), higher income categories (p=0.048), and increased education level (p<0.001). Targeting advertising campaigns to geographical areas with high internet-search patterns for sarcoidosis, with different demographics, was not effective in raising registry recruitment above baseline or increasing diversity. Conclusions: A web-based registry is an effective method for establishing a cohort of patients with sarcoidosis invested in clinical research with DNA specimens. Despite limitations, opportunities for research in patient-oriented outcomes and broad internet-based research methodology are possible. Our results demonstrate that web-based approaches to recruit study subjects need to be focused to match different target populations. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 26-34)
Keywords: sarcoidosis, registries, internet, patient recruitment, minority health, epidemiology
Background
Sarcoidosis is a rare systemic inflammatory disorder involving the presence of granulomatous inflammation of unknown cause in various organs of the body. The disease is thought to be both environmentally and genetically mediated, and morbidity and mortality may be increasing over the past decades (1-3). Unfortunately, clinical studies for sarcoidosis are difficult to perform because of two reasons: 1) It is a rare disease, and 2) There is vast variation in clinical manifestations and prognosis. This limits larger studies to multicenter efforts. Furthermore, although sarcoidosis is a disease that disproportionately affects women and African Americans in prevalence and severity, many multicenter clinical trials lack high involvement of minority populations (4,5).
Registries are tools in clinical and epidemiological research that enable analysis of different disease characteristics such as natural history, effectiveness and safety of treatments, and patient-centered outcomes. In rare diseases, patient-based registries are particularly important to be able to collect information rapidly on a large diverse cohort of patients in situations where clinical trials may be difficult, if not impossible, to perform. Depending on the function of the individual registry, the information collected may or may not involve patient interaction. Although the scope and purpose of registries differ vastly, patient-based registries are important to provide a broad real-world view of disease.
The purpose of this paper is to describe the development and characteristics of a novel web-based patient registry for sarcoidosis. We also describe patient recruitment and assess whether a specific internet-based directed advertising campaign can assist with recruitment.
Methods
Purpose and timeline
Planning for our national patient-based registry, “Worldwide Sarcoidosis Research Study” (WISE) was initiated in 2008 with the purpose of examining the entire community of patients with sarcoidosis, not just those who present to academic research centers. The registry was initiated by researchers within the Institute for Clinical and Translational Science (ICTS) at the University of Iowa. The focus of the registry was on detailed clinical phenotyping of the registrants and accompanying collection of genetic data. The methodology relied on patients themselves entering their own information into the database. The web-based registry study was released to the public in December 2011.
Survey development and technological infrastructure
The survey content was initially developed by two pulmonologists with expertise in sarcoidosis. Twelve questionnaires for the patient were developed: Demographics, Exposures, Family History, General Healthcare, Geography, Initial Diagnosis, Medications, Occupations, Organ System Involvement, Sarcoidosis Care, Women’s Health, and a Patient Quick Questionnaire. The Patient Quick Questionnaire was developed to include quality of life instruments and changes in medical care that would be completed every six months by the patient. A contact information survey was also incorporated for those willing to be contacted by mail or phone for further studies. The surveys’ content was reviewed and critiqued by five sarcoidosis experts (medical doctors) from other sarcoidosis centers and an epidemiologist from the primary center. After modifications, the content was then reviewed via telephone by four patients with sarcoidosis who were randomly selected from a group of sarcoidosis support group leaders. The purpose of this review was to identify questions that needed more explanation, were not relevant, or those that were sensitive.
A website under the domain name www.sarcoidstudy.org was then created for the survey content. Sarcoidstudy.org is a custom application developed by University of Iowa Institute for Clinical and Translational Science Informatics Group. The application is a JAVA Web Application built using the Spring and Hibernate Frameworks. The system runs in a self-contained virtual machine which allows all user information to be securely encrypted once stored on the server. Users interact with the application through a SSL HTTP Session (HTTPS).
Pilot testing of the WISE website was performed by consultants from the Center for Social and Behavioral Research at the University of Northern Iowa (Cedar Falls, Iowa, USA). The consulting group completed thirty qualitative interviews via telephone with sarcoidosis patients in the United States (U.S.) and Canada to assess ease of administration, respondent comprehension, retrieval, interpretation, and response processes. These patients were recruited by waves of random sampling of a group of 71 support group leaders and the leader contacts. Technical issues were identified in website navigation. Content problems regarding variability in interpretation of items and recall of distant history were also noted. Based on this assessment, programming was revised and additional instructions regarding content interpretation were added. The website, survey content, and consent documents for retrieval of genetic samples and validation study were approved by the University of Iowa Institutional Review Board and the study was registered on ClinicalTrials.gov (NCT01610843). The website was released into public domain December 2011.
Recruitment
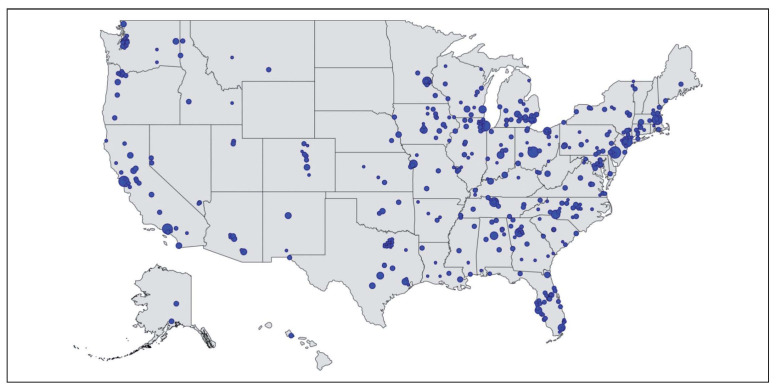
Patients were initially recruited with the help of 71 support group leaders who recruited subjects within local support communities. Internet-based recruitment included a Facebook page, Facebook advertising, Google AdWords, and links on the National Organization for Rare Diseases, and ClinicalTrials.gov. The research team also attended national patient meetings for study information dissemination. Flyers and traditional paper advertising were given to sarcoidosis physicians at multiple institutions to disseminate to patients. Spikes in registry recruitment were most associated with episodes of direct patient contact (telephone contact with support groups and patient meetings). Geographical distribution of website traffic in the U.S. is shown in Figure 1 (modified from Google analytics).
Fig. 1.
Geographic scope of website traffic in the United States for the WISE sarcoidosis web-based registry.
Samples for DNA were collected after subjects agreed to be contacted through the website. After an informed consent document was returned by the subject through the mail, an Oragene Discover saliva collection kit (www.dnagenotek.com) was sent to the subject for collection of DNA.
Recruitment study
In 2014, a recruitment study was performed to assess if internet advertising could a) increase enrollment, and b) to increase diversity of enrollment based on geographically focused methods. We utilized Google AdWords in our internet-based advertising approach to see if there was benefit from targeting ads to high search areas for sarcoidosis which also had high census populations for African Americans (Figure 2). All advertising was ceased for a period of four months prior to the advertising intervention for baseline frequency of recruitment. Two separate internet advertising campaigns were initiated on February 20, 2014 for three month duration with a max of $30 U.S. dollars per day per campaign. The first campaign targeted to ten cities with the highest frequency of google searches for sarcoidosis in the U.S. (Indianapolis, Indiana; Boston, Massachusetts; Baltimore, Maryland; Detroit, Michigan; Kansas City, Missouri; Cleveland, Ohio; Columbus, Ohio; Philadelphia, Pennsylvania; Pittsburg, Pennsylvania; Nashville, Tennessee). These ten cities also had higher African-American populations than the average census estimates. The second advertising campaign was generalized to all locations in the U.S., excluding the ten metropolitan areas targeted by the first campaign. The generalized advertising campaign was turned off on June 20, 2014 and the targeted campaign was turned off on July 21, 2014. Changes in recruitment before, during, and after the advertising campaigns were analyzed.
Fig. 2.
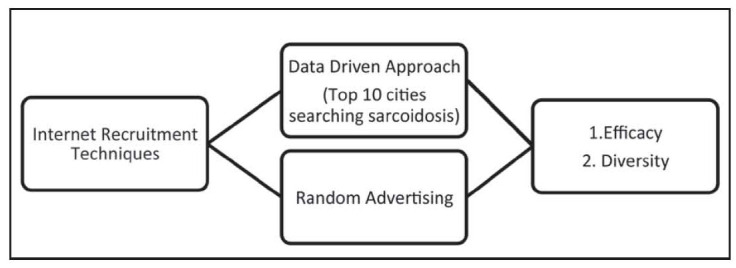
Conceptual diagram of recruitment study comparing a targeted ad campaign towards high sarcoidosis search areas to generalized national advertisment
Statistical analysis
We employed an interrupted time series regression model to assess the longitudinal mean level change of the time-delimited advertising interventions, specifically, to estimate the step-wise level change in the total registration (6). In addition to the total registration, we applied the same analyses to all the demographic variables, including racial background, gender, education, and household income, to assess the impact of web-based advertising on these individual groups. To investigate whether the mean level change differs across levels, the same modeling framework was applied on the differenced registration between the two levels within each demographics. Summary statistics were calculated for subject characteristics. All analyses were performed with SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Overall results
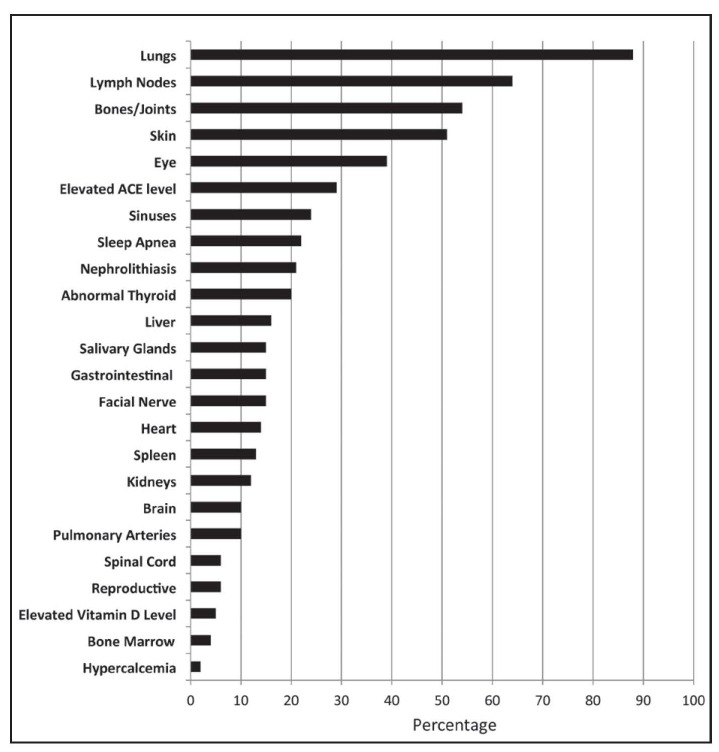
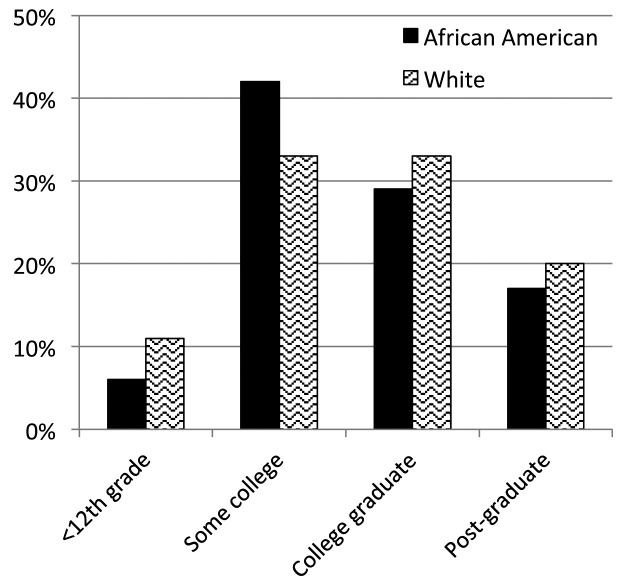
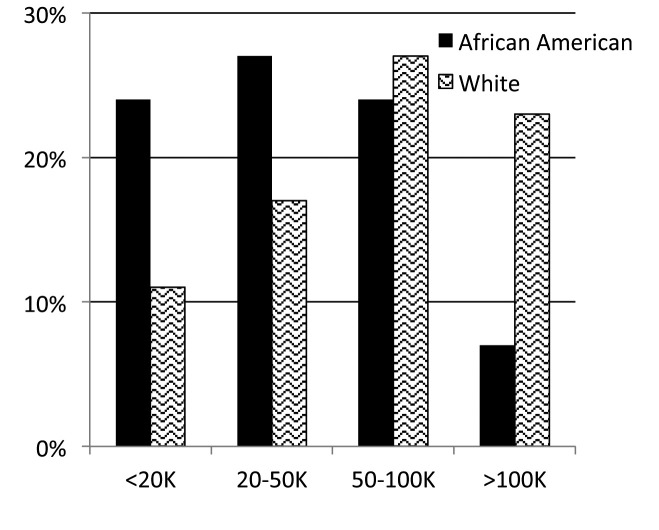
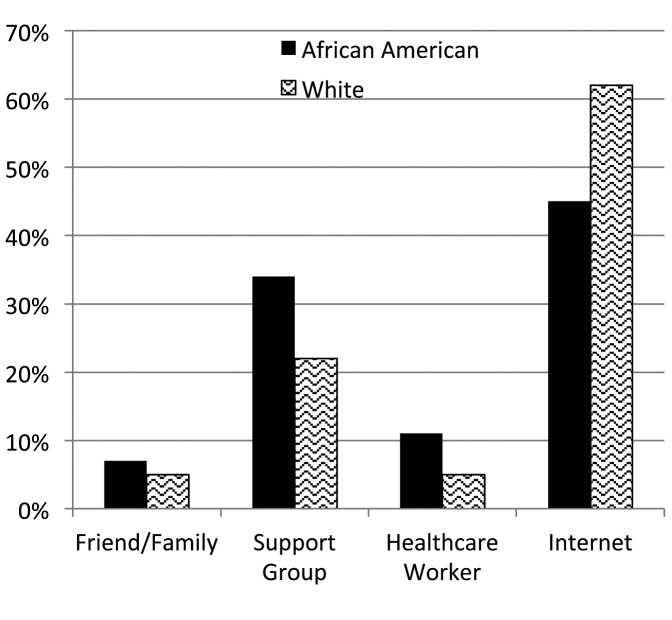
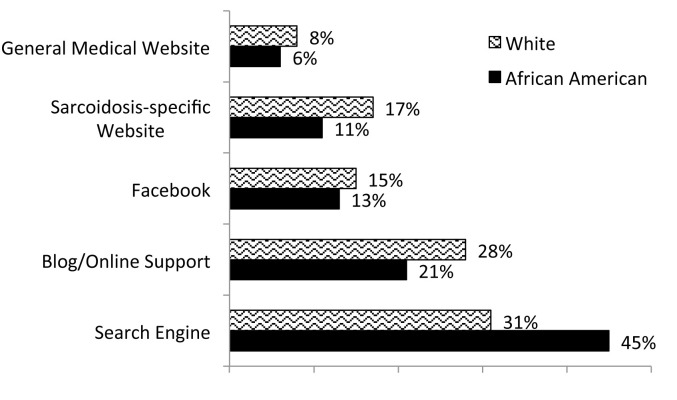
From December 2011 to September 2015, 1588 patients registered. Full contact information was entered by 67% (n=949) of the patients, with the other third completing the surveys anonymously. Patients were represented from forty-four states within the U.S. and seven countries. Demographic characteristics are shown in Table 1 and types of organ involvement in Figure 3. The median age of patients enrolled was 43 years (range 21-80). When analyzing study participants by race, African Americans and whites had similar educational backgrounds (Figure 4), but whites tended to be in higher income categories (Figure 5). African Americans reported higher frequency of being referred by a support group compared to whites, while whites had a higher frequency of finding the registry through the internet (Figure 6). Sources of internet recruitment are shown in Figure 7, with African Americans most likely to be recruited through searching the internet (search engine).
Table 1.
Patient characteristics in the Worldwide Sarcoidosis Research Study (WISE) registry

Fig. 3.
Frequency of patient-reported organ involvement of WISE sarcoidosis registry participants. Organ involvement is not mutually exclusive, as subjects can report multiple organs
Fig. 4.
Patient-reported education of the WISE registry cohort by race
Fig. 5.
Patient-reported household income of the cohort by race (United States dollars)
Fig. 6.
Patient-reported source of recruitment into the WISE cohort by race
Fig. 7.
Source of internet recruitment into the WISE cohort by race
Saliva samples for DNA storage were obtained from 353 (23%) of the total cohort. Demographics, education, and income distribution of the cohort that submitted DNA was similar to the overall cohort.
Recruitment by internet-based advertising
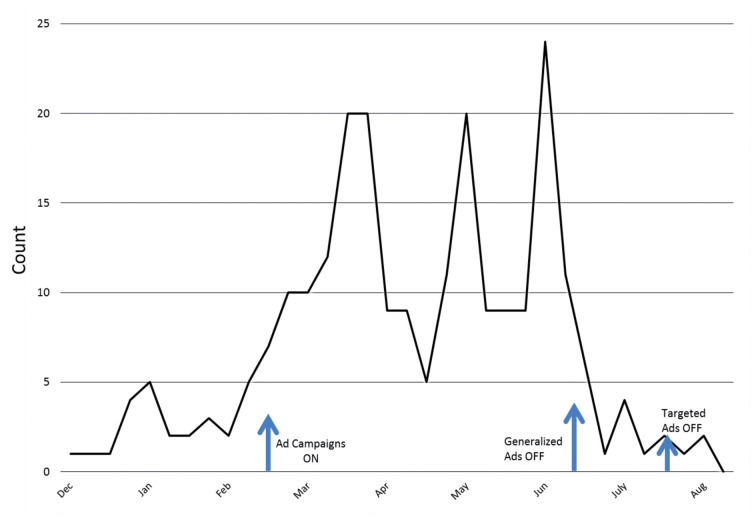
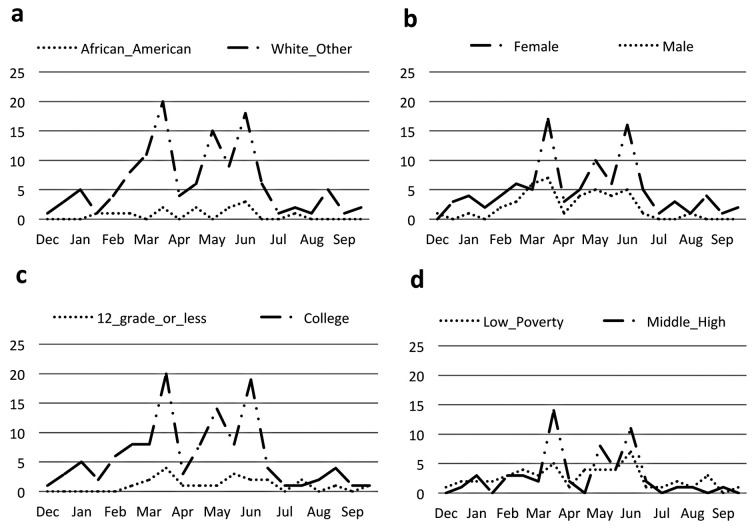
Generalized internet-based advertising did significantly improve overall recruitment in total registration (Figure 8, p<0.001). We also observed significant improvement in registration for each of the demographical variables (p<0.001). White populations were recruited at a higher rate than African Americans (Figure 9a, p<0.001) in response to advertising. Internet-based advertising also had a stronger effect on recruitment for females compared to males (Figure 9b, p=0.043), college education compared to high school or less (Figure 9c, p<0.001), and higher household income compared to lower household income (Figure 9d, p=0.048). When the generalized internet advertising campaign was turned off, the level of registrants fell to the baseline of no advertising. The targeted campaign was not effective in raising registry recruitment above baseline.
Fig. 8.
Frequency of registrants over time in response to internet advertising in two different methods: one which was generalized and the other was targeted to geographic locales with high “sarcoidosis” search traffic. The onset of the two advertising campaigns was February 20, 2014 and resulted in an increase in overall study recruitment by time series regression analysis (p<0.001). The generalized, geographically random advertising campaign was turned off on June 20, 2014 and the targeted campaign to high searching cities was turned off on July 20, 2014. The majority of recruitment effect was due to the geographically generalized advertising campaign compared to the campaign targeting high “sarcoidosis” searching areas
Fig. 9.
Frequency of registrants over time in response to internet advertising according to patient-reported race (a), gender (b), education (c), and income (d). By time series regression analysis, a higher rate of increase was seen in white populations compared to African Americans (p<0.001) and college or higher educated populations compared to high school education or less (p<0.001). A marginally higher rate of recruitment was seen in females compared to males (p=0.043) and higher income individuals (p=0.048)
Discussion
Our results demonstrate that the WISE registry study was successful in recruiting a large number of patients with clinical and genetic information. Successful recruitment for this patient-oriented, web-based study indicates that there is significant patient interest in participating in clinical research for this rare disease. Web-based registries hold promise for the future in investigating complex rare diseases.
Electronic web-based registries offer certain advantages over traditional registries. First, these platforms allow recruitment of participants across a large geographic region, including rural or urban communities, as travel is not required for enrollment. Second, patient-provided information allows data input without need for extensive research support personnel. Third, patient-reported outcomes may also provide novel phenotypic information that is lacking in current phenotyping schema. For example, currently-used sarcoidosis phenotypes do not include patient-based outcomes such as fatigue, pain, or cognitive dysfunction (7,8). Fourth, web-based registries allow investigators to go back and ask extra questions that may have surfaced since creation of the registry. Finally, future potential includes integration with other electronic platforms and bioinformatic datasets that may incorporate genomic and phenomic characteristics.
Currently, there are few other national patient-based sarcoidosis registries against which to compare our results. In the future, it will be important to have multiple patient registries, such as one through the national Foundation for Sarcoidosis Research (9). This will allow further depth in registry development, allow patient-based questions to be validated in multiple cohorts, and to increase information about varying phenotypes. Comparing subgroups of patients with sarcoidosis from existing large epidemiologic cohorts (such as the Black Women’s Health Study or the Nurses Health Studies) will also be informative (10,11). Phenotypically, in our registry, we suspect that this particular group has chronic or more severe disease than may be generalized to the entire national prevalent sarcoidosis population, based on a high frequency of immunosuppressive use (particularly, combination therapy), organ involvement, disability applications, and hospitalizations for sarcoidosis. Inherently, patients who spontaneously resolve or have incidental findings of lymphadenopathy may not be as compelled to join a registry for longitudinal monitoring. Overall, because a number of phenotypes exist in patients with sarcoidosis, it is important to define our study population when interpreting and comparing survey data.
Demographically, this registry has a much higher percentage of Caucasians compared to what is thought to be in a general population of patients with sarcoidosis in the U.S. The exact national prevalence of sarcoidosis by demographics is not currently known; however, based on the previously published high prevalence of African American women with sarcoidosis, a higher percentage of African Americans would have been expected (10). However, prior registry studies in other diseases similarly report lower-than-expected minority participation, indicating that even web-based registries have challenges with diversity in recruitment.
The objective of our recruitment study was to investigate whether internet advertising could be targeted in order to expand the diversity. Because a majority of our African-American participants reported entry to the registry website by a search engine, we hypothesized that targeted recruitment advertising campaigns in high search areas in cities with higher than average census populations for African Americans would increase enrollment, and help build diversity in our cohort. However, although overall recruitment was increased significantly, the proportion of African Americans did not increase, indicating that internet-based advertising, although effective for boosting the number of registrants, does not necessarily increase diversity in healthcare-related internet survey research.
Published themes behind racial differences in recruitment into traditional clinical trials varies, but may be due to mistrust or skepticism of clinical trials, barriers to trial opportunity, and lack of facilitated encouragement (12,13). Access to internet is not likely a direct contributor as overall internet use is not significantly different in the U.S. by race, but rather, varies more by education, income, and age (http://www.pewinternet.org/). The diminished potential cost and time commitment of clinical trials by an internet interface may not be sufficient to overcome other inherent racial disparities in clinical trial recruitment. Our results are somewhat conflicting with a prior review finding that social marketing was successful in more recruitment studies compared to community outreach. However, this review did not address internet-based methods, and focused more on traditional mass media (14). There are very few studies that evaluate the effects of recruitment techniques in a rigorous, standardized fashion (14). It is most likely that multiple methods of recruitment are necessary to increase diversity (12,15). These could include further community-based interventions and active researcher involvement in community minority organizations (16,17). Differences in recruitment for clinical studies may also be related to whether patients see subspecialists or are seen in academic institutions.
In general, population-based registry studies do have inherent limitations including selection bias, misclassification of race and ethnicity, validity of patient-reported data, and residual confounding (18,19). In our registry, selection bias is likely, as the patients are self-selecting and may be favoring certain phenotypes of sarcoidosis (e.g. more severe and chronic). This limits the generalizability to every patient with this disease, particularly those with a shorter, or more benign course. However, our cohort population likely reflects those with the greatest healthcare needs. Second, the disparity of minority participation can impact the generalizability of results and limit culturally competent clinical management. However, as the registry grows, the absolute number of African Americans will provide an increasing amount of data about this demographic, even if the ratio is lower than expected. Third, validation of patient input is a limitation, and validation processes for our cohort are ongoing. Last, in our experience, it is likely that our surveys are too long and may benefit from more focused questionnaires, with the risk of excluding unknown confounding variables.
In conclusion, we have shown a novel internet based registry survey is a feasible way to establish a cohort of patients with sarcoidosis invested in clinical research with DNA specimens. Although challenges continue to exist regarding recruitment and retention of participants, electronic registries offer opportunity to further study both the disease and web-based registry methods. Future studies will focus on longitudinal participation, outcomes, and recruitment methods.
Research Funding Support:
This work was supported by the National Institutes of Health, Grant 1K23HL114640-01A1 (AKG) and the Institute for Clinical and Translational Science, University of Iowa, grants UL1RR0024979-03S2 and U54TR001356. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies.
Author Contributions:
AKGwas responsible for design, analysis, interpretation, writing and revising all drafts. FT was responsible for data analyses, and contributed to writing and revising the manuscript. YCC was responsible for data analysis/interpretation and critically revising the manuscript. MTL contributed to the design of the study, data analyses, and to the revising of the manuscript. JS contributed to the design of the study and to the critical revisions of the manuscript. EP contributed to the design of the study, data analyses, and to the writing and revising of the manuscript. PMP contributed to the design of the study, data analyses, and to the writing and revising of the manuscript. All authors approved the final version.
References
- 1.Swigris JJ, Olson AL, Huie TJ, et al. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183(11):1524–30. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20(5):472–8. doi: 10.1097/MCP.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerke AK, Yang M, Tang F, Cavanaugh JE, Polgreen PM. Increased hospitalizations among sarcoidosis patients from 1998 to 2008: a population-based cohort study. BMC Pulm Med. 2012;12:19. doi: 10.1186/1471-2466-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29(2):119–27. [PubMed] [Google Scholar]
- 5.Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145(3):234–41. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 6.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 7.Prasse A, Katic C, Germann M, Buchwald A, Zissel G, Muller-Quernheim J. Phenotyping sarcoidosis from a pulmonary perspective. Am J Respir Crit Care Med. 2008;177(3):330–6. doi: 10.1164/rccm.200705-742OC. [DOI] [PubMed] [Google Scholar]
- 8.Moller DR, Koth LL, Maier LA, et al. Rationale and Design of the Genomic Research in Alpha-1 Antitrypsin Deficiency and Sarcoidosis (GRADS) Study. Sarcoidosis Protocol. Ann Am Thorac Soc. 2015;12(10):1561–71. doi: 10.1513/AnnalsATS.201503-172OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarcoidosis Advanced Registry for Cures (SARC) Foundation for Sarcoidosis Research. 2015. [Accessed January 12, 2016]. https://fsr-sarc.patientcrossroads.org/
- 10.Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in black women in the United States: data from the Black Women’s Health Study. Chest. 2011;139(1):144–50. doi: 10.1378/chest.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumas O, Abramovitz L, Wiley AS, Cozier YC, Camargo CA., Jr Epidemiology of Sarcoidosis in a Prospective Cohort Study of U.S. Women. Ann Am Thorac Soc. 2016;13(1):67–71. doi: 10.1513/AnnalsATS.201508-568BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durant RW, Wenzel JA, Scarinci IC, et al. Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: enhancing minority participation in clinical trials (EMPaCT) Cancer. 2014;120(Suppl 7):1097–105. doi: 10.1002/cncr.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelick PB, Harris Y, Burnett B, Bonecutter FJ. The recruitment triangle: reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African-American Antiplatelet Stroke Prevention Study (AAASPS) J Natl Med Assoc. 1998;90(3):141–5. [PMC free article] [PubMed] [Google Scholar]
- 14.UyBico SJ, Pavel S, Gross CP. Recruiting vulnerable populations into research: a systematic review of recruitment interventions. J Gen Intern Med. 2007;22(6):852–63. doi: 10.1007/s11606-007-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green MA, Kim MM, Barber S, et al. Connecting communities to health research: development of the Project CONNECT minority research registry. Contemp Clin Trials. 2013;35(1):1–7. doi: 10.1016/j.cct.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abernethy AD, Magat MM, Houston TR, Arnold HL, Jr, Bjorck JP, Gorsuch RL. Recruiting African American men for cancer screening studies: applying a culturally based model. Health Educ Behav. 2005;32(4):441–51. doi: 10.1177/1090198104272253. [DOI] [PubMed] [Google Scholar]
- 17.Reed PS, Foley KL, Hatch J, Mutran EJ. Recruitment of older African Americans for survey research: a process evaluation of the community and church-based strategy in The Durham Elders Project. Gerontologist. 2003;43(1):52–61. doi: 10.1093/geront/43.1.52. [DOI] [PubMed] [Google Scholar]
- 18.Whitehouse SL, Bolland BJ, Howell JR, Crawford RW, Timperley AJ. Mortality following hip arthroplasty--inappropriate use of National Joint Registry (NJR) data. J Arthroplasty. 2014;29(9):1827–34. doi: 10.1016/j.arth.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Izquierdo JN, Schoenbach VJ. The potential and limitations of data from population-based state cancer registries. Am J Public Health. 2000;90(5):695–8. doi: 10.2105/ajph.90.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]