Abstract
Background:
The World Health Organization recommends that antiretroviral therapy (ART) programs in resource-limited settings develop third-line ART policies. South Africa developed a national third-line ART program for patients who have failed both first-line non-nucleoside reverse transcriptase inhibitor–based ART and second-line protease inhibitor (PI)-based ART. We report on this program.
Methods:
Third-line ART in South Africa is accessed through a national committee that assesses eligibility and makes individual regimen recommendations. Criteria for third-line include the following: ≥1 year on PI-based ART with virologic failure, despite adherence optimization, and genotypic antiretroviral resistance test showing PI resistance. We describe baseline characteristics and resistance patterns of this cohort and present longitudinal data on virological suppression rates.
Results:
Between August 2013 and July 2014, 144 patients were approved for third-line ART. Median age was 41 years [interquartile range (IQR): 19–47]; 60% were women (N = 85). Median CD4+ count and viral load were 172 (IQR: 128–351) and 14,759 (IQR: 314–90,378), respectively. About 2.8% started PI-based ART before 2004; 11.1% from 2004 to 2007; 31.3% from 2008 to 2011; and 6.3% from 2012 to 2014 (48.6% unknown start date). Of the 144 patients, 97% and 98% had resistance to lopinavir and atazanavir, respectively; 57% had resistance to darunavir. All were initiated on a regimen containing darunavir, with raltegravir in 101, and etravirine in 33. Among those with at least 1 viral load at least 6 months after third-line approval (n = 118), a large proportion (83%, n = 98) suppressed to <1000 copies per milliliter, and 79% (n = 93) to <400 copies per milliliter.
Conclusion:
A high proportion of third-line patients with follow-up viral loads are virologically suppressed.
Key Words: virologic failure, HIV, antiretroviral therapy, third-line antiretroviral therapy
BACKGROUND
South Africa has the largest antiretroviral therapy (ART) program in the world, with more than 3.4 million HIV-infected individuals accessing antiretroviral (ARV) drugs, with about 145,000 (∼4%) accessing second-line ART.1 As the program continues to grow with the removal of CD4+ count thresholds as a criterion for ART initiation, it is anticipated that the numbers of patients failing current first-line non-nucleoside reverse transcriptase inhibitor (NNRTI)-based ART and needing second-line protease inhibitor (PI)-based ART will increase. Mathematical modeling suggests that by 2020, the percentage of people living with HIV globally may increase from 8.4% to 10.8%, and by 2030, up to 4.6 million will require second-line ART globally.2
While the absolute number of patients switching to second-line is increasing, the incidence of switching to second-line has typically been low,3 despite evidence that acquired resistance is increasing in resource-limited settings, and that PI-based second-line therapies would be effective in achieving viral load suppression in most cases if switching occurs timeously.4 With more patients moving to second-line regimens, which are more difficult to adhere to than currently recommended first-line regimens, as a result of tolerability issues and higher pill burden, the need for suppressive third-line ARV regimens is likely to increase.
The World Health Organization (WHO) has recommended that national ART programs in resource-limited settings develop policies for access to third-line ART, containing ritonavir-boosted darunavir, integrase inhibitors, and etravirine.5,6 These agents have been shown to be effective in highly treatment-experienced patients in trial settings.7–10 However, there are few countries in resource-limited settings that provide third-line ART in the public sector, and a paucity of data on second-line ART failure and the use of third-line ART.11–13 Several studies have shown that accumulated NRTI mutations after failure of first-line ART do not predispose to second-line failure.14–17 Other studies in resource-limited settings have shown that prolonged virologic failure, even on a PI-based regimen, results in resistance mutation accumulation.14,18–20 Given the high cost of second- and third-line regimens compared with first-line ART, it is essential to assess the effectiveness of these regimens to ensure optimal use of resources.21
In 2013, a third-line ART program was implemented in the South African public sector. Three ARV drugs were added to the national program to be used as third-line ART: ritonavir-boosted darunavir (DRV/r), raltegravir, and etravirine. DRV/r is a PI with a high barrier to resistance; raltegravir is an HIV-1 integrase strand–transfer inhibitor, the first one in the class to be introduced; etravirine is a second-generation NNRTI that has some activity against virus with resistance to first-generation NNRTIs.
We set out to describe the cohort of patients accessing third-line ART in South Africa during the first year of the third-line program, as well as virological outcomes where available.
METHODS
Public Sector HIV Treatment Regimens
South Africa follows a public health approach in its HIV program, similar to that described by WHO, using standardized treatment protocols and a decentralized model of care delivery.22 Although there have been a number of changes in the guidelines including when to start and changes in the first-line NRTIs, a policy decision from the outset was to use NNRTIs as first-line ART in South Africa. With the exception of the period when single-dose nevirapine was used for prevention of mother-to-child transmission, all HIV-infected individuals were given triple therapy from the start of the public sector program.
If virological failure occurs by the definition according to the national guidelines, then a second-line ritonavir-boosted protease inhibitor (PI/r) with appropriate NRTIs is chosen. The PI/r of choice in South Africa's public sector is ritonavir-boosted lopinavir (LPV/r), and more recently, ritonavir-boosted atazanavir (ATV/r) was introduced for those patients unable to tolerate LPV/r.
Based on the knowledge of patterns of resistance, a pragmatic choice of second-line NRTIs was chosen at a programmatic level, with sequencing of NRTIs in accordance with WHO recommendations. Thus, a genotypic antiretroviral resistance test (GART) does not form part of the strategy when changing from first- to second-line. Resistance development after first-line failure on an NNRTI-based regimen is predictable, and PI-based second-line ART should achieve virological suppression if adherence issues can be adequately addressed.23–25 Most patients who are not virologically suppressed on second-line ART in resource-limited settings do not have PI resistance mutations and are therefore failing as a result of poor adherence to ART.26–31
However, the development of PI resistance mutations on second-line ART is not as predictable as failure on an NNRTI-based first-line regimen. In addition, more PI resistance mutations are required to develop phenotypic resistance.32 GART is essential to determine whether PI resistance is present and to construct a third-line regimen. A simulation and cost-effectiveness analysis projected that genotype assays and third-line ART would increase survival and be cost-effective in resource-limited settings compared with a population-based approach (where all patients failing second-line would be switched to a potent third-line regimen comprising DRV/r, raltegravir, and etravirine, without a genotype test).33
In 2013, third-line ART was made available in South Africa through a national centralized third-line committee of HIV drug resistance expert clinicians appointed by the National Department of Health. Criteria to qualify for third-line treatment include a minimum of 1 year of PI-based ART with confirmed virologic failure, despite adherence optimization, and GART demonstrating PI resistance. PI resistance is defined as a score of 15 or more, using the Stanford scoring system. The National Department of Health informed clinicians of the availability of the third-line program, including the application process, and required paperwork, with the details of where applications should be submitted. It was also publicized by the Southern African HIV Clinicians Society.
The GART is ordered by the referring clinician, who then submits a motivation along with the GART result to the third-line coordinator, who is appointed and employed by the National Department of Health. The motivation includes demographic data; contact details of the referring clinician and facility; history of previous ART regimens and reasons for stopping or changing ART; weight and body mass index; adherence history; concomitant medications (such as tuberculosis treatment); basic laboratory results (creatinine and creatinine clearance; hemoglobin; and hepatitis B surface antigen) as well as recent CD4+ counts and viral load; and the genotype result. This information is collected and collated centrally by the third-line coordinator. The coordinator then circulates all the collated information through email with the resistance genotype and Stanford scores to the third-line committee.
The third-line committee is a virtual committee that operates by email consensus. The committee assesses eligibility for third-line ART and makes a regimen recommendation for each individual case based on the information received. Once the regimen is agreed upon, the medications are released on a named-patient basis to the facility attended by the patient. Third-line regimens include DRV/r, raltegravir, etravirine, and usually 2 NRTIs, based on the Stanford scores. Patients then continue to be followed up and monitored at their usual clinic, according to national HIV treatment guidelines, which include a viral load 6 and 12 months after initiating third-line, and at least twelve-monthly thereafter (or as clinically indicated).
Study Design and Population
To describe the population of patients on third-line therapy, we conducted a cross-sectional analysis on the national adult third-line ART cohort. We then conducted a longitudinal analysis to determine virological suppression among patients who initiated third-line therapy and for whom we were able to identify a follow-up viral load. We defined virological suppression according to the South African national treatment guidelines, which uses 1000 copies per milliliter as the cutoff for virological suppression, in accordance with WHO guidelines. We also looked at the suppression to below 400 copies per milliliter and below 50 copies per milliliter. We included all patients for whom third-line ART was applied from August 1, 2013, to July 31, 2014. We defined third-line therapy to be any treatment regimen that included one of darunavir, raltegravir, or etravirine after documented PI-based ART failure and resistance.
Data on age, sex, duration of previous therapy, and 3 previous CD4+ counts and viral loads are collected and collated centrally by the National Department of Health. As this is a public sector cohort, all viral loads and most genotype tests were conducted by the National Health Laboratory Service (NHLS). Viral load outcome data, where available, were collected from the NHLS.
The Stanford HIV Drug Resistance Database was used to calculate resistance scores, and a cutoff of ≥15 was used to determine whether resistance was present for a particular drug. To access third-line ART, the Stanford score for the PI/r the patient was on, either LPV/r or ATV/r, of 15 at least was required. This cutoff was selected by the third-line committee.
For those with any resistance, we further grouped them into those with low-level (15–29), intermediate-level (30–59), and high-level (>59) resistance and assessed the proportion of patients with any resistance (>15), those with intermediate- or high-level resistance (>29) and those with high-level resistance (>59).
We described the population using proportions for categorical variables and medians and corresponding interquartile ranges (IQRs) for continuous variables. We estimated the proportion of third-line ART patients who suppressed their viral load in those in whom we were able to identify a follow-up viral load at least 6 months after third-line approval. Patients were followed from the date of third-line approval until their first suppressed viral load or June 14, 2017, when the data set was closed, whichever occurred earlier. We looked for predictors of suppression using Cox-proportional hazards regression. We defined suppressed as reaching a viral load below 1000 copies per milliliter, below 400 copies per milliliter, and below 50 copies per milliliter, but for the regression analysis, we used below 400 copies per milliliter.
Ethical approval was obtained from the Human Research Ethics Committee of the University of Witwatersrand (M140505) and the Boston University Institutional Review Board (H-33400).
RESULTS
Baseline Characteristics
Between August 2013 and July 2014, 174 applications were submitted to the national third-line committee, and 144 applications fulfilled the inclusion criteria for third-line ART. Thirty of the 174 applications submitted were rejected: 7 had no genotype test performed while the remaining 23 showed no PI resistance. The remaining 144 patients had PI resistance mutations conferring at least low-level resistance (Stanford score ≥15) to the PI they were taking (either LPV/r or ATV/r) and were eligible for third-line ART.
The baseline characteristics of the cohort are shown in Table 1. Median age at the time of third-line application was 41 years (IQR: 19–47), and 60% were women (N = 85). The median CD4+ count and viral load around submission were 172 cells/uL (IQR: 128–351) and 14,759 copies per milliliter (IQR: 314–90,378), respectively. A small proportion started second-line before large scale rollout of ART in South Africa (2.8%) but most started between 2004 and 2011 when the start date was known.
TABLE 1.
Description of the Third-Line Cohort With Genotypic PI Resistance

Those patients entering the third-line program with viral loads less than 1000 copies per milliliter were previously accessing third-line drugs through clinical trials, post-trial access, or through the private sector, who were no longer able to access third-line ART through these routes.
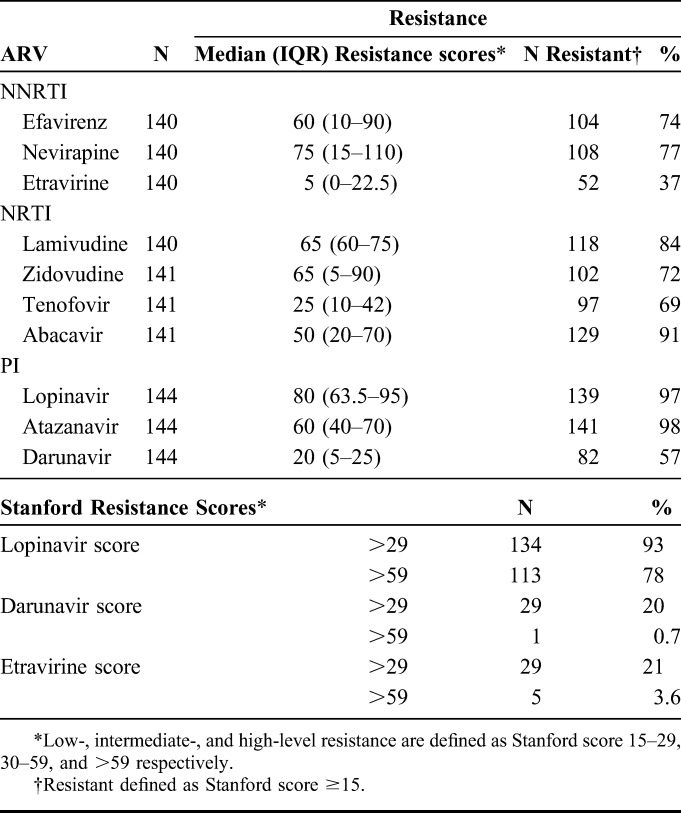
A high proportion of the cohort had baseline resistance to drugs available in the public sector in sub-Saharan Africa at second-line ART failure. The resistance profiles are shown in Table 2. Of the 144 patients with resistance test results, resistance was common for the NNRTIs commonly used in first-line therapy. Seventy-four percent (N = 104/140) and 77% (N = 108/140) had resistance (Stanford score ≥15) to efavirenz and nevirapine, respectively. Resistance to the NRTIs used in first-line ART was also common. Eighty-four percent (N = 118/140) had resistance to lamivudine; 72% (N = 102/141) to zidovudine; 69% (N = 97/141) to tenofovir; and 91% (N = 129/141) to abacavir. These patients also had high levels of resistance for PI/r that are commonly used in second-line therapy, as would be expected for a cohort failing second-line ART: 97% (N = 139/144) and 98% (N = 141/144) with resistance to lopinavir and atazanavir, respectively. Of those with lopinavir resistance, 93% (134/144) and 78% (113/144) had intermediate- and high-level resistance to lopinavir, respectively. In addition, 57% (N = 82/144) and 37% (N = 52/140) had resistance to darunavir and etravirine, respectively. Intermediate-level resistance and high-level resistance to darunavir were seen in 20% (29/144) and 0.7% (1/144), respectively. With regard to etravirine, 21% (29/140) and 3.6% (5/140) had intermediate and high levels of resistance to etravirine, respectively.
TABLE 2.
Resistance Profiles of Cohort of 144 Patients Failing PI-Based ART in South Africa
Third-Line Regimens Prescribed
Eighty-three percent (n = 144) of the 174 applications that were submitted to the committee in the first year was initiated on a third-line regimen containing some combination of the drugs mentioned previously. The following third-line drugs were prescribed: all the patients were given DRV/r (n = 144). In addition to the DRV/r, 101 (70%) were given raltegravir, and 33 (23%) patients were given etravirine in addition to DRV/r and raltegravir. NRTIs were added to 139 patients: lamivudine/emtricitabine (n = 139; 97%); tenofovir (n = 103; 72%); and zidovudine (n = 18; 13%).
Virological Outcomes
By 14 June 2017, we were able to identify 118 of 144 patients (82%) that had at least 1 viral load after third-line approval. A large proportion of these 118 patients were found to be virologically suppressed. Eight-three percent (98/118) had viral suppression to viral loads below 1000 copies per milliliter, with 93/118 (79%) suppressed to below 400 copies per milliliter, and 68/118 (58%) to below 50 copies per milliliter.
Using Cox-proportional hazards regression to identify predictors of suppression, including age, sex, and year of ART or PI/r initiation, we found few predictive factors (Table 3). Year of ART initiation and year of second-line were mildly predictive, with those initiating second-line later being more likely to suppress than those initiating earlier. However, our estimates were imprecise because of the relatively small numbers included in the analysis. These are shown in Table 3.
TABLE 3.
Predictors of Resuppression of Viral Load (<400 Copies/mL) in a Cohort of 144 Patients Failing Second-Line Therapy and Initiating Third-Line in South Africa

DISCUSSION
We present the findings of the first year of the South African public sector third-line ART cohort. This is the largest third-line cohort from a public sector program in a resource-limited setting. These findings are particularly important because few countries in resource-limited settings provide third-line ART in public sector programs, resulting in a paucity of data on third-line ART in such settings. We show that patients enrolled into the third-line ART program in its first year for which at least 1 viral load is available after the approval of third-line ART have high virological suppression rates, with 83% and 79% suppressed to below 1000 and 400 copies per milliliter, respectively. This is despite high levels of resistance to ARV drugs across all 3 classes of drug used in first- and second-line ART in the public sector ARV program in South Africa.
The ratio of men to women is similar to most cohorts reported in sub-Saharan Africa. Two-thirds of the patients started ART before 2008, and 45% started second-line ART before 2012. There was a high proportion of patients with resistance to the drugs used in first- and second-line ART regimens. Before the inclusion of third-line into the national antiretroviral program, patients with detectable viral loads on PI/r-based ART were left on the regimen, to benefit from any residual activity of the drugs, preserving immune function and delaying clinical progression,34 in accordance with WHO guidelines. Mutations present could result in reduced replicative capacity and infectivity of the resistant virus. This, coupled with delayed switching to second-line ART on first-line failure, are the likely causes of the high levels of resistance noted in the cohort at baseline.
While high levels of resistance to drugs used in first-line and second-line therapy might be expected in a cohort of patients who have failed both first- and second-line regimens, resistance to third-line agents was also fairly common. Over half of the cohort (57%, 82/144) demonstrated some degree of resistance to darunavir at third-line initiation, mainly low- and intermediate-level resistance. The likely reason for this is that patients may experience prolonged virologic failure while on a second-line PI/r-based regimen before they are referred for assessment of eligibility for third-line ART. Furthermore, before the availability of third-line ART in South Africa, patients with virological failure on a PI/r-based regimen were maintained on that failing regimen in the absence of further options. Similarly, resistance to etravirine was noted in just over a third of patients (37%, 52/140), consisting mainly of low- and intermediate-level resistance. Current genotyping after second-line failure does not include integrase inhibitors; therefore, it is not possible to assess baseline resistance to raltegravir. As this class has not been used extensively within the public sector in South Africa before its introduction into third-line, it is unlikely that there would be integrase inhibitor resistance present at baseline.
In this public sector cohort of patients on third-line ART with at least 1 follow-up viral load, a high proportion achieved virological suppression. The rates of virological suppression to below 400 copies per milliliter are similar to those seen in third-line ART patients within the South African private sector HIV disease management programs, which incorporate both clinical and patient interventions, including ongoing adherence support.13 Such private programs follow guidelines that are similar to WHO guidelines, and patients enrolled usually access third-line/salvage ART through a clinical committee that reviews the treatment history and GART. However, there are significant differences between private sector and the public sector third-line ART program. For example, engagement with patients and the adherence support provided in private sector third-line programs, which may not be the case in patients accessing third-line ART through the public sector. This might account for higher number of patients with at least 1 viral load below 50 copies per milliliter seen in the private sector compared with the public sector cohort, at 74.5% versus 58%.13 Other observational cohorts of multiclass-resistant HIV patients conducted in other resource-limited settings have shown rates of suppression to less than 50 copies per milliliter in excess of 50%.35–37
The main limitations of our study are the relatively small sample size, short duration of follow-up, and missing data in those patients for whom no viral loads after third-line initiation are available. One of the challenges in South Africa's public sector health care services is the lack of implementation of a unique patient identifier. This makes it difficult to ascertain whether patients for whom no viral loads are available are lost to follow-up, have died or may simply have relocated, and are receiving care at other clinics (so called “silent transfers”). A further limitation is the lack of data regarding outcomes beyond viral loads, such as mortality and retention in care. Furthermore, the lack of accurate data with regard to numbers of patients on second-line ART and those with confirmed virological failure makes it difficult to contextualize the scope of PI resistance and need for third-line ART.
Programmatically, it is very likely that not all patients who are failing on PI/r-based regimens have genotype tests performed and therefore are not being referred for third-line ART. However, this is a real-world public sector third-line ART cohort, which demonstrates that good virological suppression rates on third-line regimens are achievable in resource-limited settings. These data are from only the first year of the third-line program, and although limited, they are reassuring that even in resource-limited settings, good outcomes are possible on third-line ART. We feel that other lower- and middle-income countries will find these data valuable and that the centralized model of a virtual committee is a useful approach in settings where there may be limited expertise to support a third-line program.
CONCLUSIONS
Our analysis shows that patients failing second-line PI-based HIV treatment have a high level of resistance to drugs available in the public sector in South Africa. This article aims to document the third-line access program, and it cannot be seen as a surveillance of resistance present in the community. Despite the level of ARV resistance observed before third-line ART initiation, viral suppression rates were high in this programmatic rollout of third-line ART. These data are important, as they provide the first evidence of the effectiveness of third-line ART provided in a sub-Saharan African public sector programmatic setting, suggesting that patients failing second-line PI-based ART can achieve high rates of virological suppression on third-line ART regimens. These third-line regimens included ritonavir-based darunavir, raltegravir, etravirine, and NRTI drugs, selected according to genotype antiretroviral resistance test results.
Further analyses on the rates of suppression with third-line agents across the larger third-line cohort to include those patients enrolled into the third-line program after its first year in South Africa are necessary to determine the effectiveness of the program.
Footnotes
Presented at the meeting of the Conference of Retroviruses and Opportunistic Infections, Seattle, WA.F.C., M.P.F., M.M., G.M., W.D.F.V., M.-Y.M., and K.J. (February 2017). South Africa's national third-line ART cohort: descriptive analysis.
M.M. has received honoraria from pharmaceutical companies for speaking arrangements and attendance at advisory boards. W.D.F.V. has received honoraria from pharmaceutical, device manufacturer, and managed care organizations for speaking arrangements and attendance at advisory boards, all <$1000. M.P.F. was supported by Cooperative Agreement AID 674-A-12-00029 from the United States Agency for International Development (USAID), in partnership with PEPFAR. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government. The remaining authors have no conflicts interest to disclose.
REFERENCES
- 1.Venter WDF, Kaiser B, Pillay Y, et al. Cutting the cost of South African antiretroviral therapy using newer, safer drugs. S Afr Med J. 2017;107:28–30. [DOI] [PubMed] [Google Scholar]
- 2.Estill J, Ford N, Salazar-Vizcaya L, et al. The need for second-line antiretroviral therapy in adults in sub-Saharan Africa up to 2030: a mathematical modelling study. Lancet HIV. 2016;3:e132–e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madec Y, Leroy S, Rey-Cuille MA, et al. Persistent difficulties in switching to second-line ART in sub-Saharan Africa—a systematic review and meta-analysis. PLoS One. 2013;8:e82724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO HIV Drug Resistance Report 2012. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 5.WHO Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 6.WHO Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva, Switzerland: WHO; 2016. [PubMed] [Google Scholar]
- 7.Arasteh K, Yeni P, Pozniak A, et al. Efficacy and safety of darunavir/ritonavir in treatment-experienced HIV type-1 patients in the POWER 1, 2 and 3 trials at week 96. Antivir Ther. 2009;14:859–864. [DOI] [PubMed] [Google Scholar]
- 8.Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382:700–708. [DOI] [PubMed] [Google Scholar]
- 9.Steigbigel RT, Cooper DA, Teppler H, et al. Long-term efficacy and safety of Raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 Phase III trials. Clin Infect Dis. 2010;50:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazdanpanah Y, Fagard C, Descamps D, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009;49:1441–1449. [DOI] [PubMed] [Google Scholar]
- 11.Cesar C, Shepherd BE, Jenkins CA, et al. Use of third line antiretroviral therapy in Latin America. PLoS One. 2014;9:e106887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan S, Das M, Andries A, et al. Second-line failure and first experience with third-line antiretroviral therapy in Mumbai, India. Glob Health Action. 2014;7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meintjes G, Dunn L, Coetsee M, et al. Third-line antiretroviral therapy in Africa: effectiveness in a Southern African retrospective cohort study. AIDS Res Ther. 2015;12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boender TS, Hamers RL, Ondoa P, et al. Protease inhibitor resistance in the first 3 Years of second-line antiretroviral therapy for HIV-1 in sub-Saharan Africa. J Infect Dis. 2016;214:873–883. [DOI] [PubMed] [Google Scholar]
- 15.Paton NI, Kityo C, Hoppe A, et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med. 2014;371:234–247. [DOI] [PubMed] [Google Scholar]
- 16.La Rosa AM, Harrison LJ, Taiwo B, et al. Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): a randomised, phase 3, non-inferiority study. Lancet HIV. 2016;3:e247–e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd MA, Kumarasamy N, Moore CL, et al. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet. 2013;381:2091–2099. [DOI] [PubMed] [Google Scholar]
- 18.Magambo B, Nazziwa J, Bbosa N, et al. The arrival of untreatable multidrug-resistant HIV-1 in sub-Saharan Africa. AIDS. 2014;28:1373–1374. [DOI] [PubMed] [Google Scholar]
- 19.Rawizza HE, Chaplin B, Meloni ST, et al. Accumulation of protease mutations among patients failing second-line antiretroviral therapy and response to salvage therapy in Nigeria. PLoS One. 2013;8:e73582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saravanan S, Vidya M, Balakrishnan P, et al. Viremia and HIV-1 drug resistance mutations among patients receiving second-line highly active antiretroviral therapy in Chennai, Southern India. Clin Infect Dis. 2012;54:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Access to Antiretroviral Drugs in Low- and Middle-income Countries. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 22.Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. [DOI] [PubMed] [Google Scholar]
- 23.Garone DB, Conradie K, Patten G, et al. High rate of virological re-suppression among patients failing second-line antiretroviral therapy following enhanced adherence support: a model of care in Khayelitsha, South Africa. S Afr J HIV Med. 2013;14:166–169. [Google Scholar]
- 24.Collier D, Iwuji C, Derache A, et al. Virological outcomes of second-line protease inhibitor-based treatment for human immunodeficiency virus type 1 in a high-prevalence rural South African setting: a competing-risks prospective cohort analysis. Clin Infect Dis. 2017;64:1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox MP, Berhanu R, Steegen K, et al. Intensive adherence counselling for HIV-infected individuals failing second-line antiretroviral therapy in Johannesburg, South Africa. Trop Med Int Health. 2016;21:1131–1137. [DOI] [PubMed] [Google Scholar]
- 26.Van Zyl GU, Liu TF, Claassen M, et al. Trends in genotypic HIV-1 antiretroviral resistance between 2006 and 2012 in South African patients receiving first- and second-line antiretroviral treatment regimens. PLoS One. 2013;8:e67188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy RA, Sunpath H, Castilla C, et al. Second-line antiretroviral therapy- long-term outcomes in South Africa. J Acquir Immune Defic Syndr. 2012;61:158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ajose O, Mookerjee S, Mills EJ, et al. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. AIDS. 2012;26:929–938. [DOI] [PubMed] [Google Scholar]
- 29.Johnston V, Cohen K, Wiesner L, et al. Viral suppression following switch to second-line antiretroviral therapy: associations with nucleoside reverse transcriptase inhibitor resistance and subtherapeutic drug concentrations prior to switch. J Infect Dis. 2014;209:711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallis CL, Mellors JW, Venter WD, et al. Protease inhibitor resistance is uncommon in HIV-1 subtype C infected patients on failing second-line lopinavir/r-containing antiretroviral therapy in South Africa. AIDS Res Treat. 2011;2011:769627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steegen K, Bronze M, Papathanasopoulos MA, et al. Prevalence of antiretroviral drug resistance in patients who are not responding to protease inhibitor-based treatment: results from the first national survey in South Africa. J Infect Dis. 2016;214:1826–1830. [DOI] [PubMed] [Google Scholar]
- 32.Rosenbloom DI, Hill AL, Rabi SA, et al. Antiretroviral dynamics determines HIV evolution and predicts therapy outcome. Nat Med. 2012;18:1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenzana SB, Hughes MD, Grinsztejn B, et al. Genotype assays and third-line ART in resource-limited settings: a simulation and cost-effectiveness analysis of a planned clinical trial. AIDS. 2012;26:1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deeks SG, Lewin SR, Ross AL, et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med. 2016;22:839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidal JE, Song AT, Matos ML, et al. High rate of virologic suppression with darunavir/ritonavir plus optimized background therapy among highly antiretroviral-experienced HIV-infected patients: results of a prospective cohort study in Sao Paulo, Brazil. Braz J Infect Dis. 2013;17:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biscione FM, Westin MR, Ribeiro KM, et al. Virologic and immunologic effectiveness at 48 weeks of darunavir-ritonavir-based regimens in treatment-experienced persons living with HIV-1 infection in clinical practice: a multicenter Brazilian cohort. J Int Assoc Provid AIDS Care. 2014;13:63–68. [DOI] [PubMed] [Google Scholar]
- 37.Mata-Marin JA, Huerta-Garcia G, Dominguez-Hermosillo JC, et al. Effectiveness and risk factors for virological outcome of darunavir-based therapy for treatment-experienced HIV-infected patients. AIDS Res Ther. 2015;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]


