Introduction
The catalysis by DNA polymerases has been thought for decades to go through a two‐metal‐ion mechanism [Fig. 1(a)], involving Metal A (MeA, catalytic metal) and Metal B (MeB, nucleotide binding metal). Since 2012, a third metal, Metal C (MeC), has been observed for Pol η,1, 2 Pol β,3, 4, 5, 6, 7 and Pol μ8 by time‐lapse X‐ray crystallography. For Pol β and Pol μ, reported by Wilson, Perera and coworkers, the role of MeC has been suggested to be in product stabilization.3, 4, 8, 9, 10 On the other hand, MeC has been suggested to play a key catalytic role in a new “three‐metal‐ion mechanism” [Fig. 1(b)] based on detailed crystallographic studies as well as kinetics and mutagenesis.2, 11 The results of Pol β on 8‐oxoG lesion by Suo's group also supported the catalytic role of the third metal.5, 6, 12 Since the two‐metal‐ion mechanism has been shown to be conserved among DNA polymerases as well as other related enzyme families, it is important to determine which of the two “competing mechanisms” is correct.
Figure 1.
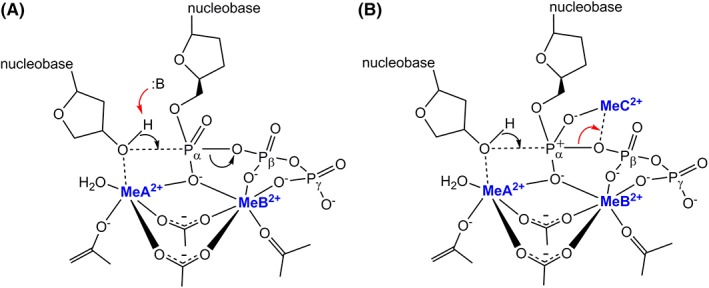
The proposed two‐metal‐ion mechanism (a) and three‐metal‐ion mechanism (b), reproduced from Ref. 16.
In this issue of Protein Science, Wang and Smithline13 report X‐ray crystallographic evidence for two‐metal‐ion catalysis in human Pol η, and conclude that the role of MeC in Pol η is to stabilize the product before its release. Their approach was to re‐analyze the metal occupancy (termed q MnA, q MnB, q MnC for Mn2+ ions A, B and C, respectively) in the structures published by Gao and Yang,2 using an improved method developed by Wang.14 The main conclusion is that “the formation of the product pyrophosphate precedes the binding of MnC.” In addition, the authors also suggested that “in the previous study,2 q MnC was substantially overestimated.”
The purpose of this Commentary is not to examine the validity of crystallographic analysis from Gao and Yang2 nor the re‐analysis by Wang and Smithline.13 Instead, I will address two issues: whether the crystal occupancy can be used to differentiate the two mechanisms and, if not, what other experimental evidence is available, particularly from the viewpoint of enzymology, for such distinction.
Clarification of the alternative mechanisms and related issues
First we need to clarify what are being compared. The two‐metal‐ion mechanism as first proposed by Steitz15 and the three‐metal‐ion mechanism as first proposed by Yang2 both refer to transition state (TS) structures. The TS structures are not directly observable by X‐ray crystallography. Over the years, the two‐metal‐ion mechanism has gained a great deal of support because both MeA and MeB have been observed in the crystal structures of the reaction intermediates of many DNA polymerases.16 However, reaction intermediates are not transition states, and it has never been shown directly that the TS involves only MeA and MeB.
The proposal of the three‐metal‐ion mechanism by Gao and Yang2 was based on the observation of an additional divalent ion, MeC, in the structures of reaction intermediates that could be closer to the TS, but still not the TS. At each time point in the time‐lapse experiment, the observed structure could contain substrates, products, or a mixture of both, but certainly not the TS. However, just as the observation of MeA and MeB led to the proposed two‐metal‐ion mechanism, observation of the third metal naturally led to the proposal of a three‐metal‐ion mechanism.
The validity of crystallographic occupancies
The rationale of Wang and Smithline13 is that if MeC is part of the TS, it should bind the ES complex before the TS is reached and the product formed, leading to higher crystal occupancy of MeC than the product PPi (q MnC ≥ q PPi). On the other hand, if MeC only binds to the product, then q MnC ≤ q PPi is expected. By re‐analyzing the electron densities of the structures of Gao and Yang,2 Wang and Smithline observed, using q MnA as a reference (1.0), that q MnB values are also ca. 1.0, while q MnC values fall between 0.30 and 0.52, and are smaller than the corresponding q PPi values by 10–30% for all five time points from 90 s to 600 s. Based on these data, they concluded that “MnC binds only to the already‐formed enzyme‐product (EP) complex after the catalysis.”
There are however two problems in the interpretations of Wang and Smithline.13 First, whether MeC binds before or after the TS is reached, the difference in timing could be on the order of sub‐seconds, which could not be differentiated by the time lapse experiments that spread over many seconds. Second, the authors assumed that, in both scenarios mentioned above, MeC will always stay with the product PPi. This is not necessarily so, as some of the static structures of the product complexes have been shown to retain PPi with a metal at the position corresponding to MeB but not MeC.17, 18 In fact, their finding of q MnC < q PPi can be alternatively interpreted as supporting evidence for the catalytic role of MeC because a catalytic metal is likely to depart sometime after product formation.
Functional results supporting the three‐metal‐ion mechanism
While structural studies can lead to proposal of TS structures, verification of the TS structures can only come from functional studies, or structural studies of the complexes with TS analogs. The proposal of the three‐metal‐ion mechanism by Gao and Yang2 was not based on the crystallographic structures alone. An important piece of evidence, among others, is that MnC has a distinct affinity that is weaker (K1/2 = 3.2 mM) than MnA or MnB (K1/2 < 0.5 mM). Consistent with this, the early kinetic study of Dunlap and Tsai19 showed that the Kd,app value of MgdNTP was 30–50 μM while that of Mg2+ was 0.6–1.0 mM. This result supports the existence of an additional weak metal ion binding site during catalysis, though not so proposed at the time. This point was further validated by a stopped‐flow study, where it was shown that at low concentration of MgdATP (no excess Mg2+), the conformational change occurred but not the chemical reaction; the latter was triggered only after subsequent mixing with excess MgCl2. 20 Although this early study was performed with Pol β, it agrees with the results of Pol η.
Arguments and counter arguments of other evidence
Wang and Smithline13 cited literature in the DNA polymerase field and coordination studies to suggest that MgC and MnC can only bind stably to the product, not the ES complex. Here it is important to reiterate that the “three‐metal‐ion mechanism” proposes a role for MeC at the TS, not the ES complex as clearly stated by Gao and Yang2. In the relevant free energy diagrams in that work2 and in subsequent reviews,11, 16 it was shown that the main role of MeC occurs at the proposed TS. The available evidence suggests that MeC participates when ES is on the way to the TS.
Wang and Smithline13 cited the work of Liu and Tsai21 on the stereoselectivity of Pol β toward (Sp)‐dATPαS relative to the Rp isomer to support the two‐metal‐ion mechanism (the Sp/Rp ratio is 57.5 for Mg2+ and 7.6 for Mn2+). This point is well appreciated. However, as elaborated by Liu and Tsai,21 the use of “metal ion dependence of Rp/Sp stereoselectivity” to elucidate the metal ion binding site is very complicated due to many competing factors. For example, the same paper showed that a single mutation D276R abolished most of the stereoselectivity. Usually it requires three data sets from three different metal ions (e.g., Mg2+, Mn2+, and Cd2+) to make a clear case. In Liu and Tsai21, the data of Cd2+ fell out of order (Sp/Rp = 21), leading the authors to make only constrained interpretations in the paper.
In relation to the above approach, Wang and Smithline13 also pointed out that, since based on the three‐metal‐ion mechanism MeC should bind directly to the pro‐Sp oxygen of the α‐phosphate, (Sp)‐dATPαS should be highly disfavored (relative to dATP) by Mg2+ and favored by Mn2+, which was not supported by the kinetic data of Gao and Yang.2 Again there are many competing factors involved, but if the binding of MeC is singled out, it can be consistent with the prediction based on the three‐metal‐ion mechanism. As Gao and Yang2 reported, the Ka of Mg2+ and Mn2+ are relatively high (0.6 mM and 2.7 mM, respectively). When dATP was replaced by the Sp isomer of dATPαS, the K a values became 15 mM and 9 mM, respectively. The data suggest that the binding of MgC is weakened for (Sp)‐dATPαS (relative to dATP) more than that of MnC, consistent with binding of MeC to the pro‐Sp oxygen of the α‐phosphate of dNTP.
Reconciliation
It is important to restate that all studies mentioned agree that MeC exists and that it can stabilize the product PPi. The main point of controversy is whether it is part of the transition state, which is always a difficult question to address. Every method has its limitations in trying to decipher the TS structure, and many functional studies can be interpreted in alternative ways. However, considering that TS is the “transition” between the substrate and the product, it is reasonable that all parties from the substrates and the products convene together. MeA and MeB bind to the substrate and they can participate in the TS (which everyone agrees even though there is still no direct evidence). Likewise, MeC can participate in the TS and then remain with the product for some time before departing. In my view this scenario is preferable to one in which MeC comes from some ill‐defined location to bind the product, sometime after the product is formed. In another direction, the three‐metal‐ion mechanism has also been reported as the mechanism for Bacillus halodurans RNase H1.22 Certainly, more functional studies are highly warranted to provide further support for the structural interpretations.
Conflict of interest
The author declares no conflict of financial interest.
Acknowledgment
The author wishes to thank Dr Wen‐Jin Wu for discussion and help in preparing the manuscript. The work is supported by the Taiwan Protein Project (Grant no. AS‐KPQ‐105‐TPP) funded by the Ministry of Science and Technology, Taiwan.
References
- 1. Nakamura T, Zhao Y, Yamagata Y, Hua YJ, Yang W (2012) Watching DNA polymerase eta make a phosphodiester bond. Nature 487:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao Y, Yang W (2016) Capture of a third Mg(2)(+) is essential for catalyzing DNA synthesis. Science 352:1334–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freudenthal BD, Beard WA, Shock DD, Wilson SH (2013) Observing a DNA polymerase choose right from wrong. Cell 154:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freudenthal BD, Beard WA, Perera L, Shock DD, Kim T, Schlick T, Wilson SH (2015) Uncovering the polymerase‐induced cytotoxicity of an oxidized nucleotide. Nature 517:635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vyas R, Reed AJ, Tokarsky EJ, Suo Z (2015) Viewing human DNA polymerase β faithfully and unfaithfully bypass an oxidative lesion by time‐dependent crystallography. J Am Chem Soc 137:5225–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reed AJ, Suo Z (2017) Time‐dependent extension from an 8‐oxoguanine lesion by human DNA polymerase beta. J Am Chem Soc 139:9684–9690. [DOI] [PubMed] [Google Scholar]
- 7. Whitaker AM, Smith MR, Schaich MA, Freudenthal BD (2017) Capturing a mammalian DNA polymerase extending from an oxidized nucleotide. Nucleic Acids Res 45:6934–6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jamsen JA, Beard WA, Pedersen LC, Shock DD, Moon AF, Krahn JM, Bebenek K, Kunkel TA, Wilson SH (2017) Time‐lapse crystallography snapshots of a double‐strand break repair polymerase in action. Nat Commun 8:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perera L, Freudenthal BD, Beard WA, Shock DD, Pedersen LG, Wilson SH (2015) Requirement for transient metal ions revealed through computational analysis for DNA polymerase going in reverse. Proc Natl Acad Sci USA 112:E5228–E5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perera L, Freudenthal BD, Beard WA, Pedersen LG, Wilson SH (2017) Revealing the role of the product metal in DNA polymerase β catalysis. Nucleic Acids Res 45:2736–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang W, Weng PJ, Gao Y (2016) A new paradigm of DNA synthesis: three‐metal‐ion catalysis. Cell Biosci 6:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raper AT, Reed AJ, Suo Z (2018) Kinetic mechanism of DNA polymerases: contributions of conformational dynamics and a third divalent metal ion. Chem Rev 118:6000–6025. [DOI] [PubMed] [Google Scholar]
- 13. Wang J, Smithline Z (in press) Crystallographic evidence for two‐metal‐ion catalysis in human pol η. Protein Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang J (2018) Determination of chemical identity and occupancy from experimental density maps. Protein Sci 27:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steitz TA (1999) DNA polymerases: structural diversity and common mechanisms. J Biol Chem 274:17395–17398. [DOI] [PubMed] [Google Scholar]
- 16. Wu W‐J, Yang W, Tsai M‐D (2017) How DNA polymerases catalyse replication and repair with contrasting fidelity. Nat Rev Chem 1:0068. [Google Scholar]
- 17. Arndt JW, Gong W, Zhong X, Showalter AK, Liu J, Dunlap CA, Lin Z, Paxson C, Tsai M‐D, Chan MK (2001) Insight into the catalytic mechanism of DNA polymerase β: structures of intermediate complexes. Biochemistry 40:5368–5375. [DOI] [PubMed] [Google Scholar]
- 18. Reed AJ, Vyas R, Raper AT, Suo Z (2017) Structural insights into the post‐chemistry steps of nucleotide incorporation catalyzed by a DNA polymerase. J Am Chem Soc 139:465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dunlap CA, Tsai M‐D (2002) Use of 2‐aminopurine and tryptophan fluorescence as probes in kinetic analyses of DNA polymerase β. Biochemistry 41:11226–11235. [DOI] [PubMed] [Google Scholar]
- 20. Bakhtina M, Lee S, Wang Y, Dunlap C, Lamarche B, Tsai M‐D (2005) Use of viscogens, dNTPαS, and rhodium(III) as probes in stopped‐flow experiments to obtain new evidence for the mechanism of catalysis by DNA polymerase β. Biochemistry 44:5177–5187. [DOI] [PubMed] [Google Scholar]
- 21. Liu J, Tsai M‐D (2001) DNA polymerase β: pre‐steady‐state kinetic analyses of dATPαS stereoselectivity and alteration of the stereoselectivity by various metal ions and by site‐directed mutagenesis. Biochemistry 40:9014–9022. [DOI] [PubMed] [Google Scholar]
- 22. Samara NL, Yang W (2018) Cation trafficking propels RNA hydrolysis. Nat Struct Mol Biol 25:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]


