Abstract
Heterotrimeric G‐proteins are cellular signal transducers. They mainly relay signals from G‐protein‐coupled receptors (GPCRs). GPCRs function as guanine nucleotide‐exchange factors to active these G‐proteins. Based on the sequence and functional similarities, these G‐proteins are grouped into four subfamilies: Gs, Gi, Gq, and G12/13. The G12/13 subfamily consists of two members: G12 and G13. G12/13‐mediated signaling pathways play pivotal roles in a variety of physiological processes, while aberrant regulation of this pathway has been identified in various human diseases. Here we summarize the signaling mechanisms and physiological functions of Gα13 in blood vessel formation and bone homeostasis. We further discuss the expanding roles of Gα13 in cancers, serving as oncogenes as well as tumor suppressors.
Keywords: G‐protein, G‐protein‐coupled receptors, blood vessel formation, bone homeostasis, endothelial cells, osteoclast resorption
Introduction
Heterotrimeric G‐proteins have three subunits: α, β, and γ. Gβ and Gγ subunits are associated together and function as one functional unit. G‐proteins are classified based on their Gα subunits. In the Gα12/13 subfamily, both Gα12 and Gα13 are expressed ubiquitously. As with other G‐proteins, Gα12/13 undergoes a GTPase cycle. In the inactive form, guanine diphosphate (GDP) bound Gα subunit binds to heterodimer Gβγ. Upon ligand binding to a G‐protein‐coupled receptor (GPCR), the receptor acts as a guanine nucleotide‐exchange factor (GEF), promoting the release of bound GDP from Gα. Nucleotide‐free Gα then binds to guanine triphosphate (GTP) and dissociates from Gβγ.1 Both Gα and Gβγ can signal to downstream effectors.
The most familiar action of the G12/13 subfamily is to activate the Ras‐superfamily small GTPase RhoA in response to a GPCR that influences a number of cellular responses including actin cytoskeletal reorganization, cell migration, phospholipase D, protein kinase D, Na+–H+ exchange, JNK activation and serum response factor (SRF)‐mediated gene transcription.2, 3, 4 The mechanism for Gα12/13 signaling to RhoA is through RhoGEFs which contain a RGS (regulator of G‐protein signaling)‐like domain (thus, called RGS‐RhoGEFs) that binds to G‐protein α subunits.5 Three different RGS‐RhoGEFs have been identified including p115‐RhoGEF, LARG, and PDZ‐RhoGEF.2 Gα12/13 also interacts with a variety of other interacting proteins including Btk/Tec‐family non‐receptor tyrosine kinases, cadherin, radixin, socius, endothelial nitric oxide synthase (eNOS), A‐kinase anchoring proteins, protein phosphatase 5, and Abl.2, 3, 4, 6, 7 Additionally, Gα12 and Gα13 appear to have their own separate interacting proteins. Gα12 interacts with Ras‐GAP1m (Ras‐specific GTPase‐activating protein), Axin, RGS1, AKAP‐Lbc (A‐kinase anchoring protein‐lymphoid blast crisis oncogenes), ZO‐1 (Zonula Occludens), PP2A (protein phosphatase 2A), p120‐catenin, αSNAP (soluble NSF attachment protein), and Hsp90 (heat‐shock protein 90).3, 8 Gα13 interacts with RGS16, Pyk2 (proline‐rich tyrosine kinase 2), Hax‐1 (HS1‐associating protein 1), JLP1 (JNK‐interacting leucine zipper protein), AKAP110 (A‐kinase anchoring protein 110), ASK‐1 (apoptosis signal‐regulating kinase 1), and GRK4γ (G‐protein‐coupled receptor kinase 4γ).3 Although the list of the interacting partners continues to increase, the physiological importance of these interactions is, in many cases, still unclear. Nevertheless, it becomes more and more apparent that Gα12/13‐mediated signaling operates in almost every tissue and manifests itself in a context specific manner. In this review, we will focus on our understandings of the signaling mechanisms and physiological/pathophysiological functions of Gα13 in specific cell types including endothelial cells (ECs), osteoclasts (OCs), and tumor cells.
Role of Gα13 signaling in endothelial cells and blood vessel formation
Formation of blood vessels during mammalian embryonic development is a complex and highly regulated process. Angioblasts proliferate, migrate, and differentiate to form primitive vascular structures composed of ECs. These structures remodel by sprouting, branching, growing and regressing, and mature by the recruitment and differentiation of pericytes and smooth muscle cells.9 The essential role of Gα13 signaling for proper blood vessel formation during mammalian embryonic development as well as adult angiogenesis has been recognized for 20 years now, however, the underlying molecular mechanisms are not completely understood. It was shown that Gα13 deficient (Gα13 −/−) mice died at embryonic Day 9.5.10 The yolk sac of Gα13 −/− mouse embryos (at E9.5) did not show any blood vessels. Importantly, the lack of Gα13 did not affect the differentiation of progenitor cells into ECs, which were present throughout the embryo. It was further confirmed that EC‐specific Gα13 knockout (KO) mouse embryos also died at E9.5 with the same phenotypes as conventional Gα13 KO embryos.11 Notably, Gα13 deficiency could be rescued by a transgene expressing Gα13 under the control of an EC‐specific promoter.11 These mouse genetic studies demonstrate that Gα13 is essential for proper blood vessel formation in vascular development. On the other hand, mice lacking Gα12 appeared normal.12 However, Gα12 and Gα13 double‐deficient mice died at embryonic day 8.5, and the Gα12‐deficient mice that carried only one intact Gα13 allele also died in utero.12 These observations suggest that Gα13 has an overlapping as well as distinct functions with Gα12 in early mouse development.
In addition to the essential role for Gα13 signaling in embryonic vascular function, Gα13 also plays critical roles in adult angiogenesis in various physiological and pathological processes. The ovary, for example, undergoes hormonally regulated changes manifested by growth of the ovarian follicles accompanied by the expansion of blood vessels.13 Gα13 signaling contributes to ovarian angiogenesis, which is necessary for proper fertility.14 In Gα13 +/− heterozygous mice, tumor angiogenesis is also impaired.14 The third example of postnatal angiogenesis for Gα13 signaling is in the retina which is avascular at birth and a single superficial layer of blood vessels grows progressively from the center toward the periphery from postnatal Day (P) 1 until P7.15 In this physiological condition, Gα13 controls angiogenesis through regulation of vascular endothelial growth factor‐2 (VEGFR‐2) expression.16 Gα13 –mediated VEGFR‐2 expression involves activation of the small GTPase RhoA and transcription factor NF‐κB. Importantly, EC‐specific Gα13 KO mice showed impaired retinal angiogenesis and tumor angiogenesis.16
In canonical G‐protein signaling pathways, Gα13 signaling is initiated by GPCRs (Fig. 1). It has been proposed that protease‐activated receptor 1 (PAR1) and sphingosine‐1‐phosphate receptors (such as S1PR1) have important roles in regulating EC functions during vascular development via signaling though Gα13.17, 18 Gα13 signaling can also be initiated through non‐canonical signaling pathways, which are less‐well defined. In ECs and fibroblasts, Gα13 is required for receptor tyrosine kinase (RTK)‐induced cell migration and actin cytoskeleton reorganization8, 19 (Fig. 1). Ric‐8A, a non‐GPCR GEF for some Gα proteins, is critical in relaying RTK signals to Gα13 suggesting that this non‐canonical Gα13 signaling pathway maybe GPCR‐independent.19, 20, 21 Cell migration and cell remodeling including cell shape changes are necessary for proper EC coordinated behavior and therefore functional blood vessel formation and angiogenesis,9 illustrating the importance of this non‐canonical Gα13 signaling pathway for vascular functions. Cell remodeling requires dynamic control of actin cytoskeletal reorganization. Gα13 has been shown to control actin cytoskeletal reorganization by regulating the disassembly of dorsal ruffles.8, 20, 21 Dorsal ruffles are induced in response to growth factors such as platelet‐derived growth factor (PDGF). They are intense bursts of ruffling of the dorsal surface plasma membranes as seen under the phase‐contrast microscope.22 It is thought dorsal ruffles are needed to reorganize the actin cytoskeleton to prepare a static cell for motility.23 Dorsal ruffles are also involved in other cell physiological functions such as micropinocytosis, invasion and receptor internalization.24 Although, Gα13 could act through Rho‐GEF to activate RhoA linking to actin cytoskeleton,5 RhoA activation seemed not to contribute to EC remodeling since, in Gα13‐deleted ECs, RhoA activation was observed to be normal.11 On the other hand, the duration of Rac activation is extended in Gα13‐deleted cells.8 Notably, re‐expression of Gα13 shortens the duration of Rac activity in cells.8 It is not clear how Gα13 is linked to Rac. However, Rac is activated by Abl tyrosine kinase in PDGF‐induced dorsal ruffle formation in mammalian cells.25, 26, 27 Abl is a multi‐functional kinase that regulates signaling pathways implicated in cytoskeleton reorganization that are important for cell migration, morphogenesis and adhesion, among others cellular functions (reviewed in Ref. 28). Abl could potentially provide such a link to connect Gα13 to Rac (Fig. 1). Gα13 directly interacts with Abl.7 Indeed, this direct interaction is critical for Gα13‐induced dorsal ruffle turnover, endothelial cell remodeling, and cell migration.28 Future investigation will be needed to explore the Gα13 –Abl axis signaling pathways in ECs and their contributions to blood vessel formation.
Figure 1.
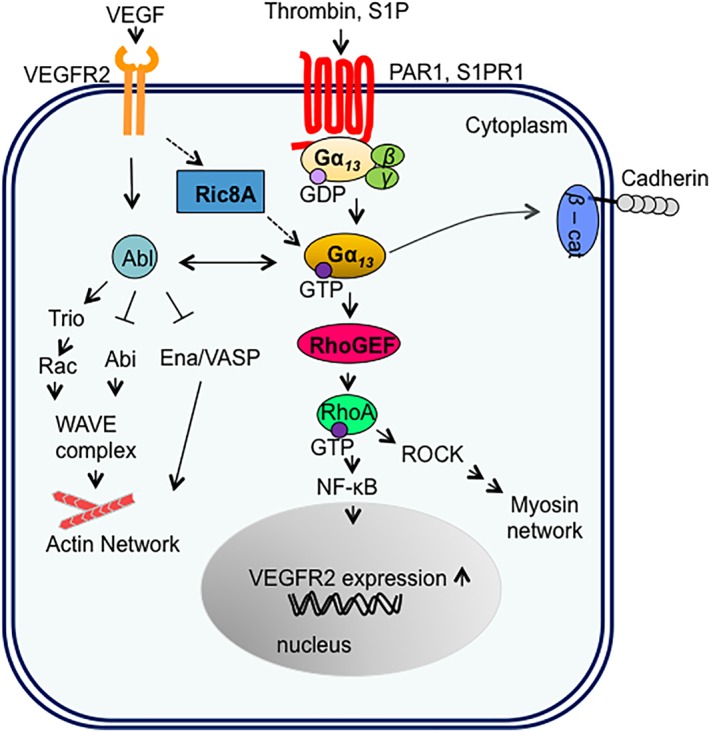
Signaling pathways regulated by Gα13 in endothelial cells (ECs). Both GPCRs, including protease‐activated receptor 1 (PAR1) or sphingosine‐1‐phosphate receptor (S1PR), and RTKs, including VEGFR2, can signal to Gα13 in ECs. β‐cat, β‐catenin; Abl, Abl tyrosine kinase; Abi, Abl interactor; GDP, guanosine diphosphate; GTP, guanosine triphosphate; NF‐kB, nuclear factor kappa‐light‐chain‐enhancer of activated B; RhoGEF, Rho‐specific guanine nucleotide exchange factor; RhoA, Ras homolog gene family, member A; ROCK, Rho‐associated protein kinase; Ric8A, resistance to inhibitors of cholinesterase‐8A; RTK, receptor tyrosine kinase; VEGF, vascular endothelial growth factor; VEGFR, Vascular endothelial growth factor receptor; VASP, vasodilator‐stimulated phosphoprotein; WAVE, Wiskott–Aldrich syndrome protein family member.
Role of Gα13 signaling in osteoclast cells and bone homeostasis
Osteoclasts (OCs) are multi‐nucleated bone resorptive cells derived from fusion of bone marrow cells of the monocyte/macrophage lineage.29 In response to extracellular stimuli, OCs can switch from resorptive to migratory states (Fig. 2). OC activation is initiated with matrix recognition, adhesion to the bone surface followed by actin cytoskeletal reorganization. The actin cytoskeleton in OCs is a unique structure that polarizes the resorptive machinery to the bone‐cell interface where it creates an isolated resorptive microenvironment consisting of an actin ring surrounding a ruffled border.30 The actin ring is also known as sealing zone (SZ) that is a prerequisite for the function of OCs. Inhibition of the actin ring leads to the inhibition of bone reabsorption.31 The SZ is composed of tightly packed dot‐like actin‐ and integrin‐rich attachment structures called podosomes. Podosomes are similar to dorsal ruffles, and they differ in their cellular locations (basal versus dorsal cell surfaces).32 Podosomes in OCs first appear as small actin dots/patches which are then reorganized into small rings or rosettes with a diameter of 0.5‐1 μm.33 Those small rings transform into sealing zones by fusing and positioning the podosomes at the basal periphery of the OCs. A particular feature of the OC podosome ring is that the SZ contains organized cortical actin cytoskeleton, which consists of a dense circumferential band of actin filaments.32 Once done with degrading a specific spot of bone, the OC then disassembles SZ and adopts a migratory state characterized by the formation of a “front‐to‐back” migratory polarity.33 Thus, organization of the OC actin cytoskeleton is an essential component of its capacity to resorb bone (Fig. 2).
Figure 2.
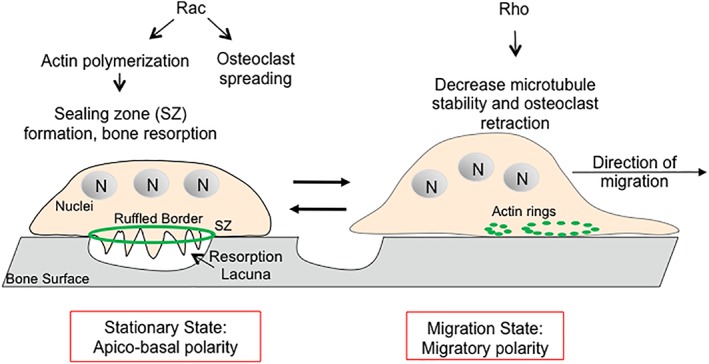
Regulation of the actin cytoskeleton in osteoclast resorption and migration states. In the stationary state, OCs are apical‐basally polarized with sealing zone (SZ) that contains actin and integrins, and area of membrane tightly juxtaposed to the bone surface that defines the resorption lacuna. SZ delineates the ruffled border where bone degradation takes places. Once the resorption stops, OCs enter into the migration state where they disassemble the SZ, flatten, and start the migration by becoming polarized with a leading and a trailing edge. Rac and Rho contribute to different states of OC biology. N, nuclei.
Given the important roles of actin cytoskeleton to OC resorptive and migratory states, it is not surprising that Gα12 and Gα13 have been shown to play critical roles in the biology of OCs. Gα12 KO (Gα12 −/−) and Gα13 KO (Gα13 −/−) mice present defective bone phenotypes.34, 35 Whereas Gα12 −/− mice show osteopetrotic phenotype with increased trabecular bone volumes,35 Gα13 −/− mice have severe osteoporosis phenotype with weak bone density.34 It is unclear what led to such an opposite phenotype considering that Gα12 and Gα13 share an ~67% amino acid identity, and many of the same upstream GPCRs and downstream effector molecules.4 The differences might be due to that Gα12 −/− mice were with a conventional KO while Gα13 −/− mice were with osteoclast‐lineage‐specific conditional KO.34, 35
Gα13‐deficiency triggers an increase in both osteoclast number and activity. Mechanistically, it is proposed that Gα13 antagonizes osteoclast formation and activity by attenuating the Akt‐GSK3β‐NFATc1 signaling axis.34 In addition to the increase in bone resorption, Gα13‐deficient OCs exhibit an increase in the actin ring formation and those actin rings were observed to be three‐fold larger in Gα13 −/− cells than in wild‐type cells, similar to the observation in fibroblast cells.17 Moreover, Gα13‐deficient OCs secreted more of Cathepsin K, a protease that is necessary to degrade bone.36 This suggests a possibility that Gα13‐deficient osteoclasts display hyper‐activation due to a larger actin ring which may allow for more surface areas for those cells to secrete more bone‐degrading molecules and thus resorb more bones, leading to osteoporosis. Similar to Gα13 −/− fibroblasts, Gα13 −/− OCs have larger cell sizes. In the future, it is necessary to further investigate the actin ring formation and disassembly to understand the actin cytoskeletal dynamics in OCs, for example, by live‐cell microscopy.
It has been shown that both RhoA and Rac GTPases play important roles in OC biology37 (Fig. 2). The migratory state of OCs, where OCs acquire a thicker and less spread‐out morphology on bones, is correlated with high basal RhoA activity.38 Additionally, Rho GTPase activity is proposed to decrease as podosome pattern changes in OCs from dots/patches to small actin rings at the migratory state to stable actin ring at the SZ at the stationary state.33 In contrast, Rac contributes to resorptive state of OCs, where OCs are in a stationary and spread‐out morphology on bones.37 Injecting anti‐Rac antibodies into OCs to inhibit Rac1 or Rac2 disrupts actin ring formation, reduces OC resorption and causes retraction of OCs.39 Potentially, Gα13 may regulate the activation of both RhoA and Rac, serving as a decisive hub to regulate different states of OC functions (Fig. 2). In the future, it is important to demonstrate that RhoA and Rac activity is indeed perturbed in Gα13‐deficient OCs and to correlate this information to the states of OCs.
Role of Gα13 signaling in cancer cells
Gα13 as an oncogene
Gα12 and Gα13 were initially identified as oncogenes with the potential for neoplastic transformation of fibroblast cell lines.40, 41, 42, 43 They were further confirmed to stimulate mitogenic responses and cell growth in many different cell lines.43 Additionally, Gα12/13 subfamily has been shown to regulate cell migration, invasion and metastasis,2 which are all critical cellular processes for tumor progression. Hence, Gα12/13 proteins have been designated as “gep” oncogenes and are of great interested to cancer biologists.43 To date, the signaling pathways downstream of Gα12/13 that promote oncogenic transformation, tumor cells growth, cell migration, invasion and metastasis have been studied extensively.2, 44 Gα12/13 activation can promote tumorigenesis and cell growth in ovarian, small‐cell lung and hepatocellular cancer cells, but not in breast and prostate cancer cells.45, 46, 47, 48, 49, 50 Recently, it has also been shown that Gα12/13 signaling regulates proliferation of ovarian cancer cells via the Hippo pathway through activation of the transcriptional coactivator Yes‐associated protein (YAP).50 YAP promotes tissue growth and cell viability by regulating the activity of multiple transcription factors.50, 51 The precise mechanism is unknown, but it is clear that Gα12/13 regulation of YAP depends on both RhoA and F‐actin.50 Together, these suggest that a diverse repertoire of Gα12/13‐mediated pathways that preferentially activate downstream effectors in cell type specific /context‐dependent matters leading to differential effects in different types of cells.
The levels of Gα12/13 mRNAs and/or proteins are elevated in patients with breast or prostate cancers.45, 46 Moreover, a systematic meta‐analysis of gene‐expression microarray datasets revealed that Gα12 and Gα13 are overexpressed in breast, oral, esophageal and colon cancers.52 In addition, the meta‐analysis of expression signatures from ∼18,000 human tumors with overall survival outcomes across 39 malignancies revealed associations between the expression of Gα12 and Gα13 and poor prognosis in patients with glioblastoma, oral, breast, lung, kidney, and ovarian cancers.53 Despite the transforming capacity of constitutive Gα12 and Gα13 mutants in experimental systems and numerous implications of these G‐proteins in cancers, activating mutants in the Gα12 and Gα13 genes in patient tumor samples have not been described.52
Gα13 as a tumor suppressor
Contrasting with the oncogenic roles of Gα12 and Gα13, loss‐of‐function mutations in Gα13 promote proliferation and dissemination of B‐cell‐derived lymphoma.54, 55 The presence of Gα13 gene loss‐of‐function mutations in Burkitt's lymphoma and diffuse large B‐cell lymphoma (DLBCL) were first identified in large‐scale genomic DNA sequencing studies.56, 57 Germinal center (GC) B cells, unlike most lymphocytes, are tightly confined in lymphoid organs and do not recirculate. Deficiency in Gα13 led to GC B‐cell dissemination into lymph and blood55 (Fig. 3). It has been proposed that the effector pathway for Gα13 in lymphocytes is the activation of ARH‐GEF1 which leads to activation of RhoA.54, 58 This Gα13–RhoA axis has suppressive effect on Akt phosphorylation which leads to the precise control of GC B cell proliferation and migration. Gα13 signaling helps to confine B cells to the GC and to limit B‐cell expansion. Loss of Gα13 would, therefore, allow cells to escape the GC to lymph nodes and blood stream55 (Fig. 3). Frequent mutations in Gα13 gene and RhoA gene are observed in B‐cell lymphomas.59 Interestingly, the expression of wild‐type Gα13 in B‐cell lymphoma cell line (with a mutant Gα13 gene) has limited impact on proliferation of these cells in vitro but results in a remarkable growth inhibition in vivo utilizing a tumor xenograft model.59
Figure 3.
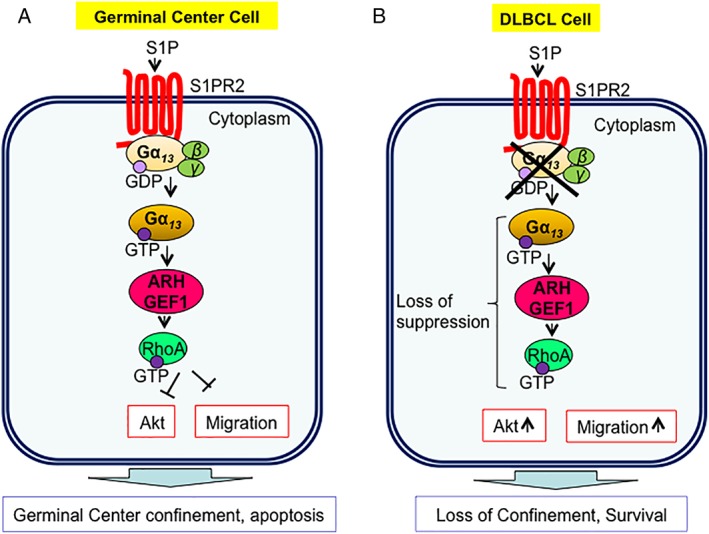
Gα13‐mediated signaling pathway in germinal center (GC) cells and diffuse large B‐cell lymphoma (DLBCL) cells. (A) In GC cells, S1PR2 initiates Gα13‐mediated signaling leading to the suppression of Atk phosphorylation, which leads the control of apoptosis and confinement of B‐cells to the GC. (B) In DLBCL cells, the loss of suppressive pathway via the absence of Gα13 leads to the increase in survival and migration of GC B‐cells.
Furthermore, it has been shown that Gα13‐mediated pathway in DLBCL could also be Atk‐independent.60 In these B‐cells, the signaling can be initiated through S1PR2 which also promotes apoptosis of GC B cells. S1PR2 gene expression is essential for the B‐cell confinement to the GC. Loss of Gα13 in DLBCL, results in a suppression of S1PR2 gene expression by the forkhead box protein (FOX1P) transcription factor leading to loss of B‐cell confinement to the GC and an increase of B‐cell survival.60
Although the detailed mechanisms by which Gα13 inhibits cell growth and migration of B‐cells requires further exploration, it is interesting to note that this signaling elicits the opposite effect in B‐cell lymphomas as compared to other cancer cell types. As discussed above, Gα13 is associated with cellular transformation and tumorigenesis in other cancer cells. The apparent‐opposing effects of a particular signaling molecule, based on cellular context, have been observed with other pathways such as the JNK and transforming growth factor beta (TGFβ) signaling pathways.61, 62 Further investigations will reveal the molecular mechanisms of Gα13 signaling for these different functions in different cancer cell types.
Conclusion
We have briefly reviewed the data on the signaling mechanisms and functions of Gα13 in endothelial cells, osteoclast cells, and cancer cells. In ECs and OCs, Gα13 seems to mainly control the actin cytoskeletal reorganization to contribute to blood vessel formation or bone resorption, respectively. In most types of cancer cells, Gα13 promotes cell proliferation; but in B‐cell lymphoma, Gα13 confines B‐cells in germinal centers and limits B‐cell expansion. Gα13 can be activated by the conical GPCRs, as well as by other signaling pathways, such as RTKs, possible through non‐GPCR GEFs such as Ric‐8A. The exact cellular and physiological or pathological outcomes of Gα13 signaling depend on cell‐types and cell functional states.
References
- 1. Bourne HR, Sanders DA, McCormick F (1990) The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348:125–132. [DOI] [PubMed] [Google Scholar]
- 2. Kelly P, Casey PJ, Meigs TE (2007) Biologic functions of the G12 subfamily of heterotrimeric g proteins: growth, migration, and metastasis. Biochemistry 46:6677–6687. [DOI] [PubMed] [Google Scholar]
- 3. Worzfeld T, Wettschureck N, Offermanns S (2008) G(12)/G(13)‐mediated signalling in mammalian physiology and disease. Trends Pharmacol Sci 29:582–589. [DOI] [PubMed] [Google Scholar]
- 4. Syrovatkina V, Alegre KO, Dey R, Huang XY (2016) Regulation, signaling, and physiological functions of G‐proteins. J Mol Biol 428:3850–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siehler S (2009) Regulation of RhoGEF proteins by G12/13‐coupled receptors. Br J Pharmacol 158:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hewavitharana T, Wedegaertner PB (2012) Non‐canonical signaling and localizations of heterotrimeric G proteins. Cell Signal 24:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang L, Wang D, Xing B, Tan YC, Huang J, Liu B, Syrovatkina V, Espenel C, Kreitzer G, Guo L, Zhang JJ, Huang XY (2017) G‐protein Galpha13 functions with Abl kinase to regulate actin cytoskeletal reorganization. J Mol Biol 429:3836–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang D, Tan YC, Kreitzer GE, Nakai Y, Shan D, Zheng Y, Huang XY (2006) G proteins G12 and G13 control the dynamic turnover of growth factor‐induced dorsal ruffles. J Biol Chem 281:32660–32667. [DOI] [PubMed] [Google Scholar]
- 9. Herbert SP, Stainier DY (2011) Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 12:551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Offermanns S, Mancino V, Revel JP, Simon MI (1997) Vascular system defects and impaired cell chemokinesis as a result of Galpha13 deficiency. Science 275:533–536. [DOI] [PubMed] [Google Scholar]
- 11. Ruppel KM, Willison D, Kataoka H, Wang A, Zheng YW, Cornelissen I, Yin L, Xu SM, Coughlin SR (2005) Essential role for Galpha13 in endothelial cells during embryonic development. Proc Natl Acad Sci USA 102:8281–8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gu JL, Muller S, Mancino V, Offermanns S, Simon MI (2002) Interaction of G alpha(12) with G alpha(13) and G alpha(q) signaling pathways. Proc Natl Acad Sci USA 99:9352–9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bochner F, Fellus‐Alyagor L, Kalchenko V, Shinar S, Neeman M (2015) A novel intravital imaging window for longitudinal microscopy of the mouse ovary. Sci Rep 5:12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen L, Zhang JJ, Rafii S, Huang XY (2009) Suppression of tumor angiogenesis by Galpha(13) haploinsufficiency. J Biol Chem 284:27409–27415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gariano RF, Gardner TW (2005) Retinal angiogenesis in development and disease. Nature 438:960–966. [DOI] [PubMed] [Google Scholar]
- 16. Sivaraj KK, Takefuji M, Schmidt I, Adams RH, Offermanns S, Wettschureck N (2013) G13 controls angiogenesis through regulation of VEGFR‐2 expression. Dev Cell 25:427–434. [DOI] [PubMed] [Google Scholar]
- 17. Griffin CT, Srinivasan Y, Zheng YW, Huang W, Coughlin SR (2001) A role for thrombin receptor signaling in endothelial cells during embryonic development. Science 293:1666–1670. [DOI] [PubMed] [Google Scholar]
- 18. Allende ML, Yamashita T, Proia RL (2003) G‐protein‐coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102:3665–3667. [DOI] [PubMed] [Google Scholar]
- 19. Shan D, Chen L, Wang D, Tan YC, Gu JL, Huang XY (2006) The G protein G alpha(13) is required for growth factor‐induced cell migration. Dev Cell 10:707–718. [DOI] [PubMed] [Google Scholar]
- 20. Wang L, Guo D, Xing B, Zhang JJ, Shu HB, Guo L, Huang XY (2011) Resistance to inhibitors of cholinesterase‐8A (Ric‐8A) is critical for growth factor receptor‐induced actin cytoskeletal reorganization. J Biol Chem 286:31055–31061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xing B, Wang L, Guo D, Huang J, Espenel C, Kreitzer G, Zhang JJ, Guo L, Huang XY (2013) Atypical protein kinase Clambda is critical for growth factor receptor‐induced dorsal ruffle turnover and cell migration. J Biol Chem 288:32827–32836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mellstrom K, Heldin CH, Westermark B (1988) Induction of circular membrane ruffling on human fibroblasts by platelet‐derived growth factor. Exp Cell Res 177:347–359. [DOI] [PubMed] [Google Scholar]
- 23. Krueger EW, Orth JD, Cao H, McNiven MA (2003) A dynamin‐cortactin‐Arp2/3 complex mediates actin reorganization in growth factor‐stimulated cells. Mol Biol Cell 14:1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoon JL, Wong WK, Koh CG (2012) Functions and regulation of circular dorsal ruffles. Mol Cell Biol 32:4246–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM (1999) c‐Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev 13:2400–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hernandez SE, Krishnaswami M, Miller AL, Koleske AJ (2004) How do Abl family kinases regulate cell shape and movement? Trends Cell Biol 14:36–44. [DOI] [PubMed] [Google Scholar]
- 27. Sini P, Cannas A, Koleske AJ, Di Fiore PP, Scita G (2004) Abl‐dependent tyrosine phosphorylation of Sos‐1 mediates growth‐factor‐induced Rac activation. Nat Cell Biol 6:268–274. [DOI] [PubMed] [Google Scholar]
- 28. Bradley WD, Koleske AJ (2009) Regulation of cell migration and morphogenesis by Abl‐family kinases: emerging mechanisms and physiological contexts. J Cell Sci 122:3441–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asagiri M, Takayanagi H (2007) The molecular understanding of osteoclast differentiation. Bone 40:251–264. [DOI] [PubMed] [Google Scholar]
- 30. Soysa NS, Alles N (2016) Osteoclast function and bone‐resorbing activity: an overview. Biochem Biophys Res Commun 476:115–120. [DOI] [PubMed] [Google Scholar]
- 31. Li J, Zeng L, Xie J, Yue Z, Deng H, Ma X, Zheng C, Wu X, Luo J, Liu M (2015) Inhibition of osteoclastogenesis and bone resorption in vitro and in vivo by a prenylflavonoid xanthohumol from hops. Sci Rep 5:17605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buccione R, Orth JD, McNiven MA (2004) Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol 5:647–657. [DOI] [PubMed] [Google Scholar]
- 33. Georgess D, Machuca‐Gayet I, Blangy A, Jurdic P (2014) Podosome organization drives osteoclast‐mediated bone resorption. Cell Adh Migr 8:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu M, Chen W, Lu Y, Zhu G, Hao L, Li YP (2017) Galpha13 negatively controls osteoclastogenesis through inhibition of the Akt‐GSK3beta‐NFATc1 signalling pathway. Nat Commun 8:13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song MK, Park C, Lee YD, Kim H, Kim MK, Kwon JO, Koo JH, Joo MS, Kim SG, Kim HH (2018) Galpha12 regulates osteoclastogenesis by modulating NFATc1 expression. J Cell Mol Med 22:849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Troen BR (2004) The role of cathepsin K in normal bone resorption. Drug News Perspect 17:19–28. [DOI] [PubMed] [Google Scholar]
- 37. Weivoda MM, Oursler MJ (2014) The roles of small GTPases in osteoclast biology. Orthop Muscular Syst 3:1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saltel F, Destaing O, Bard F, Eichert D, Jurdic P (2004) Apatite‐mediated actin dynamics in resorbing osteoclasts. Mol Biol Cell 15:5231–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Razzouk S, Lieberherr M, Cournot G (1999) Rac‐GTPase, osteoclast cytoskeleton and bone resorption. Eur J Cell Biol 78:249–255. [DOI] [PubMed] [Google Scholar]
- 40. Chan AM, Fleming TP, McGovern ES, Chedid M, Miki T, Aaronson SA (1993) Expression cDNA cloning of a transforming gene encoding the wild‐type G alpha 12 gene product. Mol Cell Biol 13:762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu N, Bradley L, Ambdukar I, Gutkind JS (1993) A mutant alpha subunit of G12 potentiates the eicosanoid pathway and is highly oncogenic in NIH 3T3 cells. Proc Natl Acad Sci USA 90:6741–6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu N, Voyno‐Yasenetskaya T, Gutkind JS (1994) Potent transforming activity of the G13 alpha subunit defines a novel family of oncogenes. Biochem Biophys Res Commun 201:603–609. [DOI] [PubMed] [Google Scholar]
- 43. Goldsmith ZG, Dhanasekaran DN (2007) G protein regulation of MAPK networks. Oncogene 26:3122–3142. [DOI] [PubMed] [Google Scholar]
- 44. Juneja J, Casey PJ (2009) Role of G12 proteins in oncogenesis and metastasis. Br J Pharmacol 158:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kelly P, Moeller BJ, Juneja J, Booden MA, Der CJ, Daaka Y, Dewhirst MW, Fields TA, Casey PJ (2006) The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proc Natl Acad Sci USA 103:8173–8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kelly P, Stemmle LN, Madden JF, Fields TA, Daaka Y, Casey PJ (2006) A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. J Biol Chem 281:26483–26490. [DOI] [PubMed] [Google Scholar]
- 47. Grzelinski M, Pinkenburg O, Buch T, Gold M, Stohr S, Kalwa H, Gudermann T, Aigner A (2010) Critical role of G(alpha)12 and G(alpha)13 for human small cell lung cancer cell proliferation in vitro and tumor growth in vivo. Clin Cancer Res 16:1402–1415. [DOI] [PubMed] [Google Scholar]
- 48. Yagi H, Tan W, Dillenburg‐Pilla P, Armando S, Amornphimoltham P, Simaan M, Weigert R, Molinolo AA, Bouvier M, Gutkind JS (2011) A synthetic biology approach reveals a CXCR4‐G13‐Rho signaling axis driving transendothelial migration of metastatic breast cancer cells. Sci Signal 4:ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang YM, Lee WH, Lee CG, An J, Kim ES, Kim SH, Lee SK, Lee CH, Dhanasekaran DN, Moon A, Hwang S, Lee SJ, Park JW, Kim KM, Kim SG (2015) Galpha12 gep oncogene deregulation of p53‐responsive microRNAs promotes epithelial‐mesenchymal transition of hepatocellular carcinoma. Oncogene 34:2910–2921. [DOI] [PubMed] [Google Scholar]
- 50. Yagi H, Asanoma K, Ohgami T, Ichinoe A, Sonoda K, Kato K (2016) GEP oncogene promotes cell proliferation through YAP activation in ovarian cancer. Oncogene 35:4471–4480. [DOI] [PubMed] [Google Scholar]
- 51. Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL (2012) Regulation of the Hippo‐YAP pathway by G‐protein‐coupled receptor signaling. Cell 150:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O'Hayre M, Vazquez‐Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, Gutkind JS (2013) The emerging mutational landscape of G proteins and G‐protein‐coupled receptors in cancer. Nat Rev Cancer 13:412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, West RB, Plevritis SK, Alizadeh AA (2015) The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 21:938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Green JA, Suzuki K, Cho B, Willison LD, Palmer D, Allen CD, Schmidt TH, Xu Y, Proia RL, Coughlin SR, Cyster JG (2011) The sphingosine 1‐phosphate receptor S1P(2) maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat Immunol 12:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Muppidi JR, Schmitz R, Green JA, Xiao W, Larsen AB, Braun SE, An J, Xu Y, Rosenwald A, Ott G, Gascoyne RD, Rimsza LM, Campo E, Jaffe ES, Delabie J, Smeland EB, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Vaidehi N, Staudt LM, Cyster JG (2014) Loss of signalling via Galpha13 in germinal centre B‐cell‐derived lymphoma. Nature 516:254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lohr JG, Stojanov P, Lawrence MS, Auclair D, Chapuy B, Sougnez C, Cruz‐Gordillo P, Knoechel B, Asmann YW, Slager SL, Novak AJ, Dogan A, Ansell SM, Link BK, Zou L, Gould J, Saksena G, Stransky N, Rangel‐Escareno C, Fernandez‐Lopez JC, Hidalgo‐Miranda A, Melendez‐Zajgla J, Hernandez‐Lemus E, Schwarz‐Cruz y Celis A, Imaz‐Rosshandler I, Ojesina AI, Jung J, Pedamallu CS, Lander ES, Habermann TM, Cerhan JR, Shipp MA, Getz G, Golub TR (2012) Discovery and prioritization of somatic mutations in diffuse large B‐cell lymphoma (DLBCL) by whole‐exome sequencing. Proc Natl Acad Sci USA 109:3879–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Love C, Sun Z, Jima D, Li G, Zhang J, Miles R, Richards KL, Dunphy CH, Choi WW, Srivastava G, Lugar PL, Rizzieri DA, Lagoo AS, Bernal‐Mizrachi L, Mann KP, Flowers CR, Naresh KN, Evens AM, Chadburn A, Gordon LI, Czader MB, Gill JI, Hsi ED, Greenough A, Moffitt AB, McKinney M, Banerjee A, Grubor V, Levy S, Dunson DB, Dave SS (2012) The genetic landscape of mutations in Burkitt lymphoma. Nat Genet 44:1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cattoretti G, Mandelbaum J, Lee N, Chaves AH, Mahler AM, Chadburn A, Dalla‐Favera R, Pasqualucci L, MacLennan AJ (2009) Targeted disruption of the S1P2 sphingosine 1‐phosphate receptor gene leads to diffuse large B‐cell lymphoma formation. Cancer Res 69:8686–8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O'Hayre M, Inoue A, Kufareva I, Wang Z, Mikelis CM, Drummond RA, Avino S, Finkel K, Kalim KW, DiPasquale G, Guo F, Aoki J, Zheng Y, Lionakis MS, Molinolo AA, Gutkind JS (2016) Inactivating mutations in GNA13 and RHOA in Burkitt's lymphoma and diffuse large B‐cell lymphoma: a tumor suppressor function for the Galpha13/RhoA axis in B cells. Oncogene 35:3771–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Flori M, Schmid CA, Sumrall ET, Tzankov A, Law CW, Robinson MD, Muller A (2016) The hematopoietic oncoprotein FOXP1 promotes tumor cell survival in diffuse large B‐cell lymphoma by repressing S1PR2 signaling. Blood 127:1438–1448. [DOI] [PubMed] [Google Scholar]
- 61. Bachman KE, Park BH (2005) Duel nature of TGF‐beta signaling: tumor suppressor vs. tumor promoter. Curr Opin Oncol 17:49–54. [DOI] [PubMed] [Google Scholar]
- 62. Liu J, Lin A (2005) Role of JNK activation in apoptosis: a double‐edged sword. Cell Res 15:36–42. [DOI] [PubMed] [Google Scholar]


