Abstract
Rational:
Intracranial hemorrhage (ICH) after carotid artery stenting (CAS) is a rare but fatal complication, and it primarily occurs in the corresponding vascular distribution area. Herein, a case of ICH after CAS in both anterior and posterior circulation has been described.
Patient concerns:
A 62-year-old female was referred to the hospital for left-side limb weakness and right nasal hemianopia for 1 month.
Diagnosis:
Cerebral MRI following hospitalization revealed cerebral infarction in the right posterior cortical watershed area. Computed tomography angiography (CTA) showed severe stenosis in the right internal carotid artery.
Interventions:
We performed CAS to treat the right internal carotid artery stenosis without any complications.
Outcomes:
After CAS, cerebral hemorrhage occurred under strict control of blood pressure in both anterior and posterior circulation. After 69 days of treatment, the patient was discharged from the hospital.
Lessons:
Hypertension is still one of the causes of ICH after CAS. The control of perioperative BP in patients with severe carotid stenosis is yet a major concern.
Keywords: carotid artery stenting, hyperperfusion syndrome, hypertension, intracranial hemorrhage
1. Introduction
Apart from medical treatment, there are 2 ways to treat carotid stenosis: carotid endarterectomy (CEA) and carotid artery stenting (CAS). Intracranial hemorrhage (ICH) after carotid revascularization with an incidence of 0.63% to 0.72%[1,2] has been associated with significant morbidity and mortality. In most cases, ICH primarily occurs in the corresponding vascular distribution area[3] and results from cerebral hyperperfusion syndrome (CHS). We present here a case of ICH which is not just limited in CAS performed vascular distribution area. The report may also indicate other reasons, besides CHS, for ICH after CAS.
2. Case report
A 62-year-old right-handed female was referred to the hospital following complaints of left-side limb weakness and right nasal hemianopia for 1 month. She had a history of hypertension for poor blood pressure control. After hospitalization, the cerebral MRI revealed cerebral infarction in the right posterior cortical watershed area, and the National Institutes of Health Stroke Scale (NIHSS) score was 6. Computed tomography angiography (CTA) showed severe stenosis in the right internal carotid artery (Fig. 1), and the CTA detected slender bilateral posterior cerebral artery (P1). A collateral blood flow was detected from the bilateral internal carotid artery through the bilateral posterior communicating artery. However, the platelet and neutrophil counts were in the normal range, and the coagulation function was normal.
Figure 1.
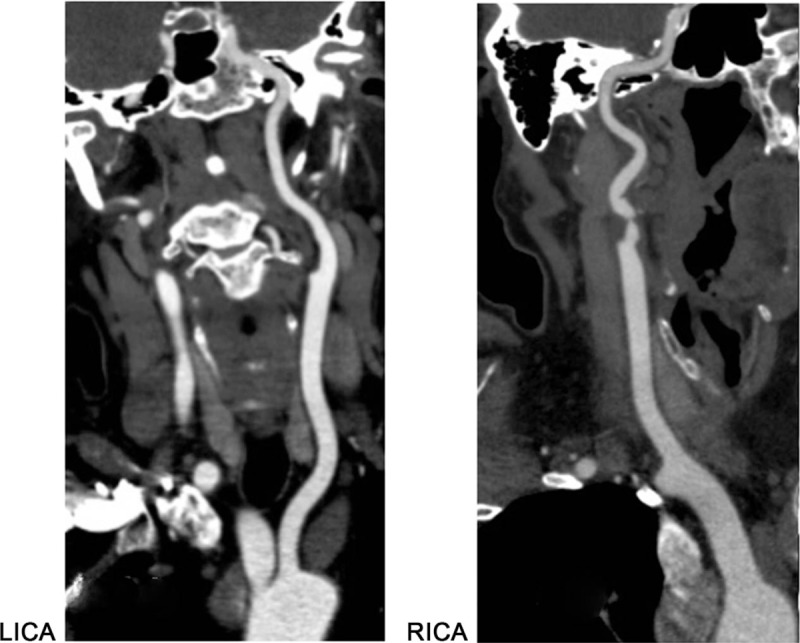
Preoperative CT angiography shows mild left stenosis of the left carotid artery and severe stenosis of the right carotid artery.
The patient was administered 100 mg aspirin and 75 mg clopidogrel daily for 5 days before the right CAS was performed under local anesthesia. The patient was administered heparin (3000 IU) to control the activated clotting time from 140 to 313 s. The right ICA lesion was approached through the left femoral artery using an 8F guiding catheter (Boston Scientific, Maple Grove, USA). The stenosis was crossed with a 190 cm EZ Filter Wire (Boston Scientific, Maple Grove, USA). The pre-dilatation was performed using a 4.0 to 30 mm Sterling balloon (Bosten Scientific, Natick MA, USA). A 6–8 × 40 mm XACT carotid stent (Abbott, Temecula, USA) was placed successfully. Angiography after stent placement demonstrated nearly total resolution of the stenosis (Fig. 2). The blood pressure (BP) of the patient was 182/84 mmHg before CAS. After stent deployment, with nimodipine and uradil, the BP of the patient was 124/61 mmHg.
Figure 2.
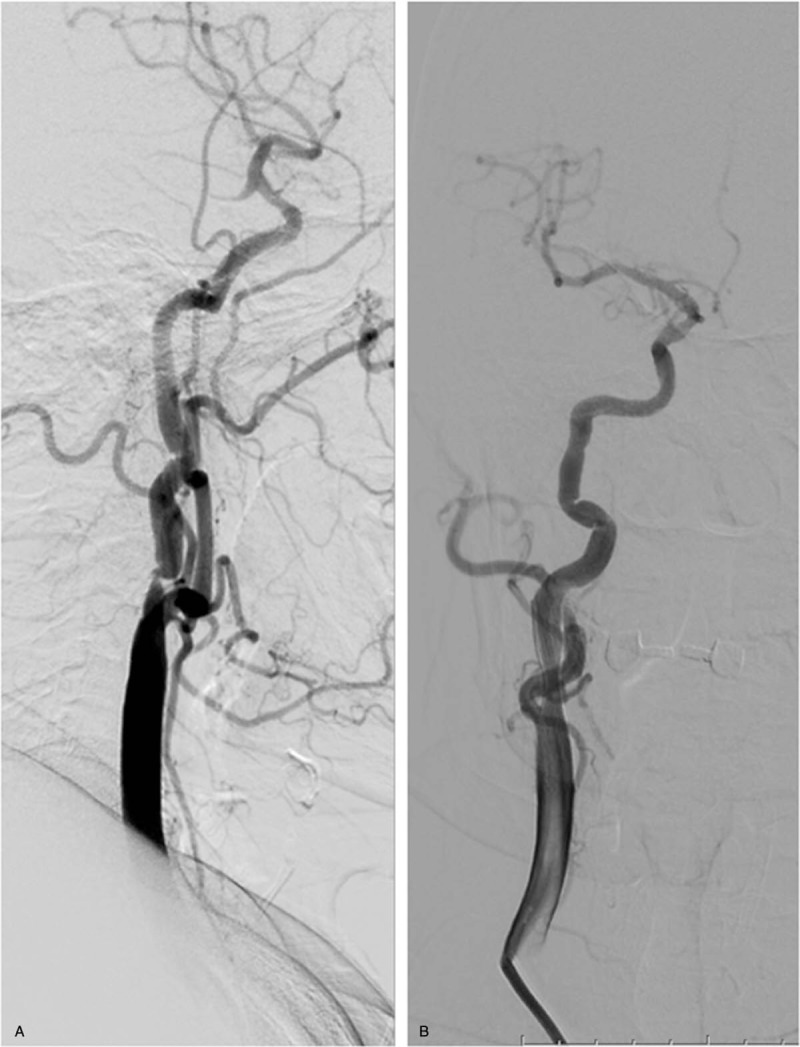
A. Angiography before stent placement demonstrates 95% stenosis before carotid artery stenting (CAS) placement. B. Angiography demonstrates nearly total resolution of the stenosis after CAS placement.
Immediately after revascularization, the patient complained of left limb hemiplegia that aggravated without headache and seizure. Thus, the CT scanning was performed immediately, and cerebral hemorrhage was found in the right basal ganglia that received the blood supply from the right middle cerebral artery (Fig. 3). Consequently, the administration of aspirin and clopidogrel was ceased immediately. To mitigate hemorrhage, we reduced and maintained the patient's systolic BP below 110 mmHg. At 7 h after revascularization, no increase was detected in intracranial hemorrhage (ICH), while 10 h after revascularization, the patient slipped into coma. The diameter of the pupil was not equal bilaterally, and the pupil reflex was not detected. Thus, we repeated the CT scanning, which revealed cerebral hemorrhage in the right basal ganglia and right thalamus, and the high-density sign in the lateral ventricle (Fig. 4).
Figure 3.
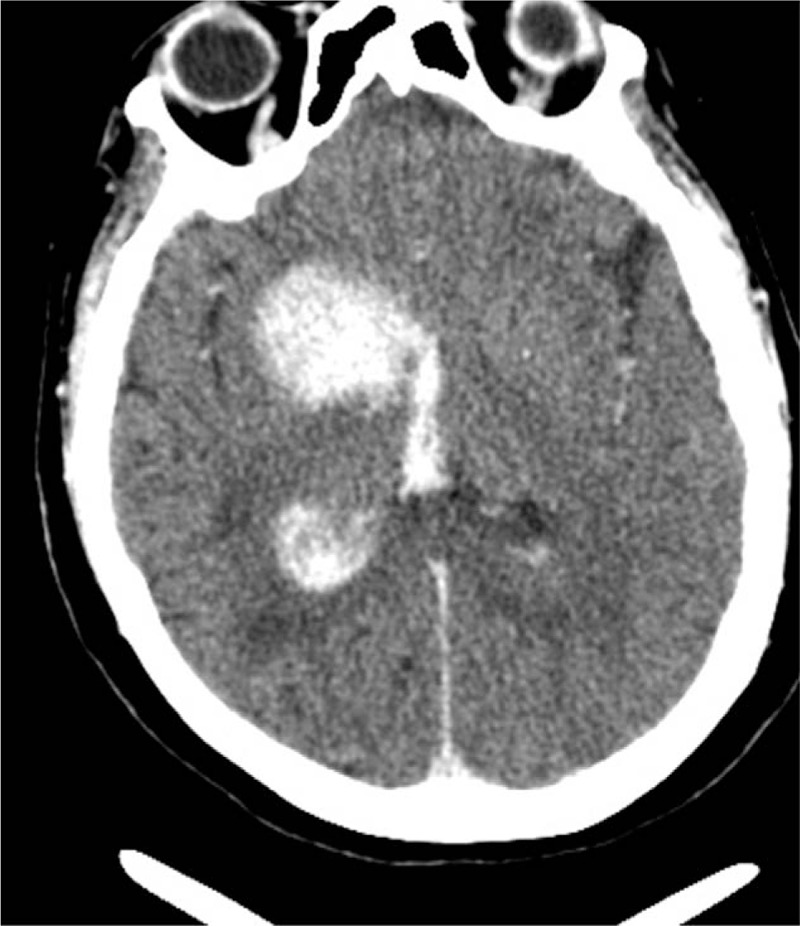
Cerebral hemorrhage seen in the right basal ganglia immediately after revascularization.
Figure 4.
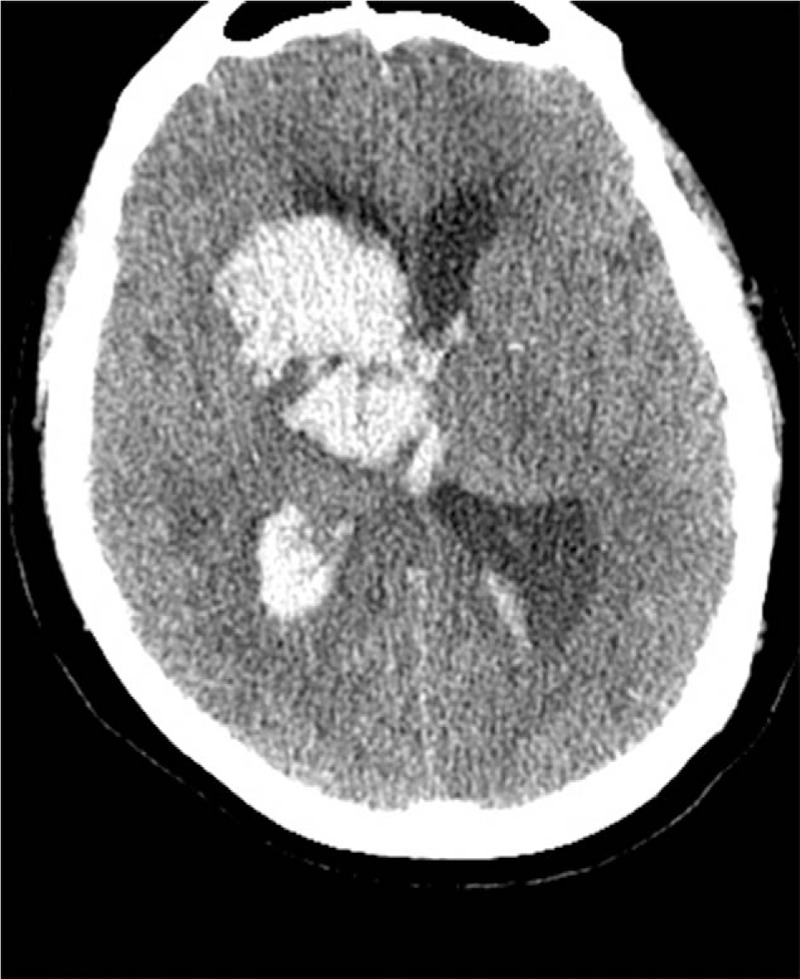
Ten hours after revascularization, cerebral hemorrhage observed in the right basal ganglia and right thalamus, and high-density sign seen in the lateral ventricle.
Subsequently, we conducted trepanation and drainage to treat the ICH. The patient also suffered a tracheotomy. After 69 days of treatment, the patient was discharged from the hospital with NIHSS score 12 and modified Rankin scale (mRS) score 4 (Fig. 5). At the 3-month of follow-up, the NIHSS score was 8, and the mRS score was 2. The patient has provided informed consent for publication of the case.
Figure 5.
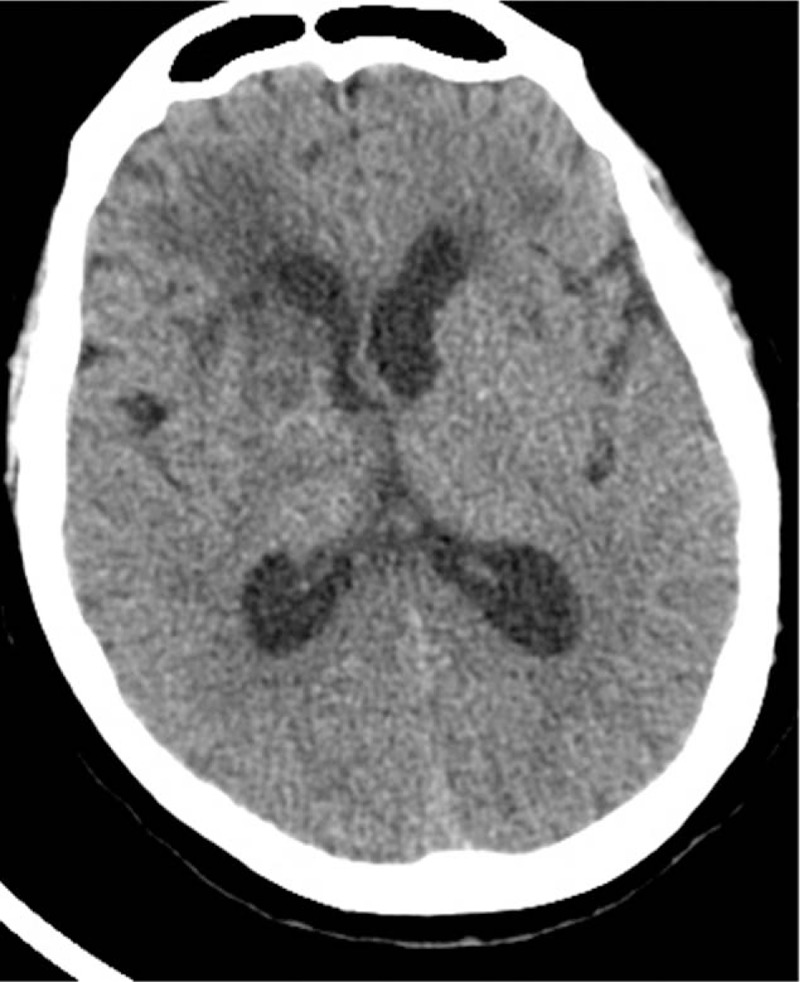
Head CT taken before discharge shows that the cerebral hemorrhage has been fully absorbed.
3. Discussion
Both CEA and CAS are routine methods for the prevention of primary and secondary stroke in patients with significant carotid artery disease[4] and may be complicated by ICH, which is rare but could be fatal.[5–10] In a recent study, the incidence of ICH is less than 1% in asymptomatic patients, irrespective of CEA or CAS, while that in CEA and CAS was 0.8% and 4.4%, respectively in symptomatic patients.[11] Intracranial hemorrhage after CAS is attributed to CHS, hemorrhage caused by anticoagulation and antiplatelet therapy after stent implantation, hypertensive cerebral hemorrhage (mainly located in basal ganglia), and post-infarction hemorrhagic transformation combined with cranial internal bleeding disorders.[12,13] A previous study demonstrated that a majority of the ICH incidents were attributed to CHS.[14]
As a rare complication, CHS is likely to occur in elderly patients or in those with poor BP control, severe bilateral carotid stenosis, and collateral circulation.[13,15] In this case, the patient had only unilateral carotid artery stenosis, and the collateral circulation was satisfactory. The only risk factor for CHS was the poor BP control before hospitalization. Also, before ICH, the patient did not complain about any typical clinical symptoms for CHS, such as a unilateral headache, vomiting, facial and eye pain, and seizures.[5] We hypothesized that the CHS was not the only reason for her ICH. The patient had basal ganglia hemorrhage and thalamic hemorrhage. Although both have different blood supplies, hypertension is the leading cause of these hemorrhages.[16] Abou-Chebl et al[13] reported that the incidence of CHS and ICH can be reduced with stringent BP control; thus, in our case, the ICH may be attributed to hypertension.
Herein, we reduced and maintained the systolic blood pressure of the patients below 130 mmHg immediately after revascularization. In order to mitigate the hemorrhage, we reduced and maintained the SBP below 110 mmHg at 10 h after revascularization. Despite strict BP control, the patient still developed thalamic hemorrhage. Ogasawara et al[17] speculated that after long-term cerebral ischemia, low resistance is observed in the cerebral circulatory system, the brain tissue may lack effective protection by cerebrovascular self-regulation, and the normal BP may cause severe damage to the cerebral blood vessels. Thus, although the BP is regulated stringently, cerebral hemorrhage in this patient was increased. In some patients, an extremely low BP during the perioperative period may cause ischemic symptoms due to the poor compensatory ability of the carotid stenosis to cerebral ischemia. However, the “normal” BP may be a risk factor for ICH. Therefore, controlling the BP at an appropriate level in these patients is the challenge in the treatment of CAS. Theoretically, BP should be strictly controlled until the recovery of cerebrovascular self-regulation. However, the time required for restoration of cerebrovascular self-regulation after CAS varies from person to person.[17] Therefore, objective indicators are essential for determining the recovery time of cerebrovascular self-regulation ability.
4. Conclusion
ICH after CAS is a rare but catastrophic complication. Most ICHs are associated with hyperperfusion syndrome. However, hypertension is still one of the causes of ICH after CAS, and the control of perioperative BP in patients with severe carotid stenosis is crucial.
Author contributions
Conceptualization: Pian Wang.
Data curation: Pian Wang.
Formal analysis: Pian Wang.
Investigation: Yan Wang.
Methodology: Yan Wang.
Project administration: Yan Wang.
Writing – original draft: Pian Wang.
Writing – review & editing: Qingbin Zhang.
Footnotes
Abbreviations: BP = blood pressure, CAS = carotid artery stenting, CEA = carotid endarterectomy, CHS = hyperperfusion syndrome, CTA = computed tomography angiography, ICH = intracranial hemorrhage, NIHSS = National Institutes of health stroke scale.
Pian Wang and Yan Wang contributed equally to this work.
The authors declare that they have no competing interests.
References
- [1].Kang HS, Han MH, Kwon OK, et al. Intracranial hemorrhage after carotid angioplasty: a pooled analysis. J Endovasc Ther 2007;14:77–85. [DOI] [PubMed] [Google Scholar]
- [2].Ogasawara K, Sakai N, Kuroiwa T, et al. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg 2007;107:1130–6. [DOI] [PubMed] [Google Scholar]
- [3].Li S, Li BM, Zhou DB, et al. Cause and treatment for intracranial hemorrhage during the perioperative period of carotid artery stenting. Zhonghua Wai Ke Za Zhi 2010;48:582–4. [PubMed] [Google Scholar]
- [4].Hobson RW, II, Mackey WC, Ascher E, et al. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the society for vascular surgery. J Vasc Surg 2008;48:480–6. [DOI] [PubMed] [Google Scholar]
- [5].van Mook WN, Rennenberg RJ, Schurink GW, et al. Cerebral hyperperfusion syndrome. Lancet Neurol 2005;4:877–88. [DOI] [PubMed] [Google Scholar]
- [6].Coutts SB, Hill MD, Hu WY. Hyperperfusion syndrome: toward a stricter definition. Neurosurgery 2003;53:1053–8. discussion 1058–1060. [DOI] [PubMed] [Google Scholar]
- [7].Adhiyaman V, Alexander S. Cerebral hyperperfusion syndrome following carotid endarterectomy. QJM 2007;100:239–44. [DOI] [PubMed] [Google Scholar]
- [8].Abou-Chebl A, Reginelli J, Bajzer CT, et al. Intensive treatment of hypertension decreases the risk of hyperperfusion and intracerebral hemorrhage following carotid artery stenting. Catheter Cardiovasc Interv 2007;69:690–6. [DOI] [PubMed] [Google Scholar]
- [9].Kablak-Ziembicka A, Przewlocki T, Pieniazek P, et al. Assessment of flow changes in the circle of Willis after stenting for severe internal carotid artery stenosis. J Endovasc Ther 2006;13:205–13. [DOI] [PubMed] [Google Scholar]
- [10].Safian RD, Bresnahan JF, Jaff MR, et al. Protected carotid stenting in high-risk patients with severe carotid artery stenosis. J Am Coll Cardiol 2006;47:2384–9. [DOI] [PubMed] [Google Scholar]
- [11].Clark WM, Brott TG. Intracranial hemorrhage complicating carotid artery stenting and carotid endarterectomy. Stroke 2011;42:2720–1. [DOI] [PubMed] [Google Scholar]
- [12].Buhk JH, Cepek L, Knauth M. Hyperacute intracerebral hemorrhage complicating carotid stenting should be distinguished from hyperperfusion syndrome. AJNR Am J Neuroradiol 2006;27:1508–13. [PMC free article] [PubMed] [Google Scholar]
- [13].Abou-Chebl A, Yadav JS, Reginelli JP, et al. Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting: risk factors, prevention, and treatment. J Am Coll Cardiol 2004;43:1596–601. [DOI] [PubMed] [Google Scholar]
- [14].Galyfos G, Sianou A, Filis K. Cerebral hyperperfusion syndrome and intracranial hemorrhage after carotid endarterectomy or carotid stenting: a meta-analysis. J Neurol Sci 2017;381:74–82. [DOI] [PubMed] [Google Scholar]
- [15].Kaku Y, Yoshimura S, Kokuzawa J. Factors predictive of cerebral hyperperfusion after carotid angioplasty and stent placement. AJNR Am J Neuroradiol 2004;25:1403–8. [PMC free article] [PubMed] [Google Scholar]
- [16].Tokgoz S, Demirkaya S, Bek S, et al. Clinical properties of regional thalamic hemorrhages. J Stroke Cerebrovasc Dis 2013;22:1006–12. [DOI] [PubMed] [Google Scholar]
- [17].Ogasawara K, Mikami C, Inoue T, et al. Delayed cerebral hyperperfusion syndrome caused by prolonged impairment of cerebrovascular autoregulation after carotid endarterectomy: case report. Neurosurgery 2004;54:1258–61. discussion 1261–1252. [DOI] [PubMed] [Google Scholar]


