Abstract
Background:
As the exact pathogenesis of inflammatory bowel disease (IBD) is not known, there is increasing evidence of clinical trials and animal models that indicate the beneficial effects of probiotics.
Methods:
Multiple databases were adopted to search for the relevant studies involving the comparison between probiotics and control groups. Review Manager 5.0 was used to assess the efficacy among included articles. Risk of bias for the articles included was also conducted.
Results:
Finally, 10 studies eventually met the inclusion criteria and 1049 patients were included. The meta-analyses showed that no significant differences of remission, relapse, and complication rate between Escherichia coli Nissle 1917 and mesalazine groups (RR = 0.94, 95%CI [0.86, 1.03], P = .21; RR = 1.04, 95%CI [0.82, 1.31], P = .77; RR = 1.12, 95%CI [0.86, 1.47], P = .39, respectively). Despite the fact that no significant differences of remission, relapse, and complication rate were observed in overall meta-analysis results between probiotics and placebo group, the subgroup analyses suggested that VSL#3 presented a higher remission rate and lower relapse rate (RR = 1.67, 95%CI [1.06, 2.63], P = .03; RR = 0.29, 95%CI [0.10, 0.83], P = .02, respectively).
Conclusion:
Some types of probiotics, such as E coli Nissle 1917 and VSL#3, could be used as alternative therapy for patients with IBD.
Keywords: inflammatory bowel disease, mesalazine, placebo, probiotics
1. Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn's disease (CD), is a type of chronic bowel inflammation diseases that relapse episodes with unknown aetiology.[1,2] It has been widely accepted that IBD is the consequence of overly activated response of mucosal immune system to the environmental, dietary, or infectious antigen in a genetically susceptible host.[3] Studies on the animal models have indicated that aggressive cell-mediated immune caused by commensal enteric bacteria plays a vital role in the development and maintenance of IBD.[4,5] Evidence from patients also showed innate immune system would be activated and aberrant immune response would be initiated through secreting inflammatory mediators caused by endogenous bacterial flora, which would result in IBD.[6]
Therapy of IBD often involves induction of remission and prevention of relapses.[7,8] Corticosteroids are initially used to induce remission, but the maintenance is often less successful, and patients treated with long time corticosteroids may suffer several complications including growth failure or osteopenia.[9] Guidelines[10] have recommend aminosalicylates as a maintenance treatment. Clinical treatment with aminosalicylates for patients with IBD is well established to maintain remission.[11] But also the effect is contentious and some potential complications are observed, such as infection, hepatitis, leucopenia, and pancreatitis.[12,13] Modification of the bacterial microenvironment in bowel is another therapy to induce or maintain remission in IBD.[14,15] Using antibiotics to remove the bacteria with potential inflammatory is a seemingly feasible solution, but the use of antibiotics is limited.[16,17] Another option is to use probiotics which could solve inflammation though improving its intestinal microbial balance.[18]
Probiotics are live microorganisms that intend to provide positive efficacy on the treatment of traveler's diarrhea, diarrhea caused by the human immunodeficiency virus, and difficile colitis relapses.[19,20] After ingested, probiotics could inhibit the overgrowth of potentially pathogenic bacteria to modify the composition in bowel, which have beneficial effects on human health.[21] Several animal models have proved the effectiveness of probiotic therapy for patients with IBD.[22] For patients, some studies have also conducted with Escherichia coli Nissle 1917 (EcN 1917), Saccharomyces boulardii and VSL#3, and these yeasts have been reported to have some beneficial effects in IBD.[23–25]
Despite the fact that several studies have studied the effect of different probiotics, inconsistent results about the therapeutic efficacy of probiotics have been reported. This study is aimed to evaluate the effect of probiotics to maintain remission and cause complication in IBD patients.
2. Materials and methods
This work was no request of patient consent and ethical approval because it is a meta-analysis.
2.1. Search strategy
A comprehensive search of the articles about the effect of probiotics for inflammatory bowel disease was performed adhering to the procedures of meta-analyses guidelines. The pertinent studies were published from inception to December 2017 among multiple databases including PubMed, Springer, Embase, OVID and Cochrane databases. There is no language restriction in our study. Patients of all age groups were evaluated. The following terms were used in the process of literature searching: inflammatory bowel disease OR IBD OR ulcerative proctocoliti OR ulcerative colitis OR UC OR Crohn's disease OR CD; probiotics OR Lactobacillus OR E coli Nissl OR S boulardii. To search out all the relevant studies, 2 team members searched the literature independently and the reference lists should also be examined to obtain additional studies that not identified before. The articles searched out were screened for further selection.
2.2. Citation selection
We screened the titles and abstracts of the articles identified above and downloaded full texts that met the inclusion criteria. Then the full texts were reviewed to extract data.
The inclusion criteria that studies included in this study must meet including:
-
(1)
A randomized control trial (RCT) or controlled clinical trial (CCT) study;
-
(2)
Comparison between probiotics and placebo;
-
(3)
Patients with inflammatory bowel disease;
-
(4)
Availability of full text.
The exclusion criteria including:
-
(1)
Nonrandomized studies;
-
(2)
Studies on other treatment measures;
-
(3)
Studies without comparable results;
The process of selection was conducted independently and attentively, and 2 members of our team determined the final target articles together. Then, these 2 researches met and reached a consensus. If any problems of poor agreement occurred or no consensus could be achieved, a third investigator involved to solve the controversy.
2.3. Data extraction
Two of the reviewers read the full text of the studies included independently and extracted the detail data from each study with a standard data extraction form. The data extracted included the first author's name, year of publication, year of onset, age range of patients, sample size (probiotics/placebo), sex distribution (male/female), and outcome parameters. In this study, outcome parameters included, which were collected to estimate the clinical effect.
2.4. Risk of bias
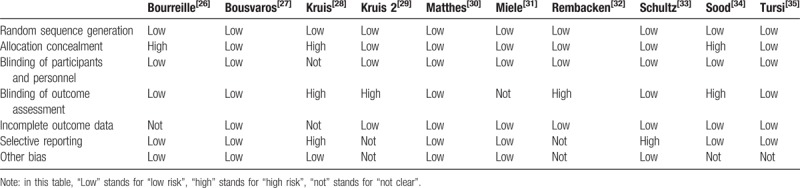
According to the Review Manager 5.3 Tutorial, risk of bias in this study was assessed (Table 1). The assessment including: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; other bias. The disagreements about the biases were resolved by discussion, and if it is necessary, a third investigator was the adjudicator.
Table 1.
Detailed characteristics of the included studies.

2.5. Statistical analysis
The meta-analyses in our study were performed with the Review Manager 5.0 (The Cochrane Collaboration, 2011) to estimate the different effect between probiotics and placebo group in patients with inflammatory bowel disease. Heterogeneities of the meta-analyses were investigated and reflected by the I2 statistic across studies. Random-effect models were adopted if I2 is >50%, which means significant heterogeneity was observed. Otherwise a fixed-effect model was chosen. For binary outcomes, related ratio (RR) with 95% confidence intervals (CIs) was calculated. In this study, P value < .05 was considered a statistically significant result.
3. Results
3.1. Search results
Totally 745 titles were initially searched out in databases after the primary selection, and finally 10 studies[26–35] eventually satisfied all the inclusion criteria mentioned. The other 735 articles were excluded for duplication, irrelevant studies, inappropriate outcomes, reviews, without primary outcomes, not RCT, or not a full-text. The process of the selection about our study has been shown in Figure 1. Among these 10 articles, 3 were involved in the comparison between probiotics and mesalazine, while the other 7 studies compared the effect between probiotics and placebo.
Figure 1.

Flow diagram of study identification and inclusion.
3.2. Characteristics of included studies
Detailed about the selected studies were performed in Table 2, which includes the first author's name, year of publication, year of onset, age range of patients, sex distribution (male/female), experiment group, control group, sample size (experiment/control), and outcome measurements. These articles were published from 1999 to 2013. The sample size ranges from 11 to 222. In total, 1049 patients were included in these studies, and experiment and control groups were 528 and 521, respectively.
Table 2.
The risk of bias table in this meta-analysis.
3.3. Meta-analysis about the remission rate between EcN 1917 and mesalazine group
The 3 included articles in our study were involved in the comparison of the remission between EcN 1917 and mesalazine group. Figure 2 shows the forest plot of the remission in different groups. According to the forest plot, all these 3 studies showed no difference, and the meta-analysis suggested that these 2 groups have no significant difference in overall effect (RR = 0.94, 95%CI [0.86, 1.03], P = .21; P for heterogeneity = .84, I2 = 0%).
Figure 2.

A forest plot for the comparison of remission rate between EcN 1917 and mesalazine group.
3.4. Meta-analysis about the relapse rate between EcN 1917 and mesalazine groups
Forest plots for the relapse rate in EcN 1917 and mesalazine groups were shown in Figure 3. All the 3 articles included in the meta-analysis have a similar result, and the overall results suggested that in the articles included, probiotics group has a similar relapse risk compared with mesalazine group (RR = 1.04, 95%CI [0.82, 1.31], P = .77; P for heterogeneity = .57, I2 = 0%).
Figure 3.

A forest plot for the comparison of relapse rate between EcN 1917 and mesalazine group.
3.5. Meta-analysis about the complication rate between EcN 1917 and mesalazine groups
Only 2 of 10 included studies were involved in the complication of post-treatment. The forest plot for the rate of complication in EcN 1917 and mesalazine groups was shown in Figure 4. Both these 2 studies showed that no statistical difference of the rate of complication was observed, and the meta-analysis suggested similar rate of maternal complication (RR = 1.12, 95%CI [0.86, 1.47], P = .39; P for heterogeneity = .24, I2 = 27%).
Figure 4.

A forest plot for the comparison of complication rate between EcN 1917 and mesalazine group.
3.6. Meta-analysis about the remission rate between probiotics and placebo groups
The 4 articles selected in our study were involved in the comparison of the remission between probiotics and placebo groups. Figure 5 shows the forest plot of the remission in probiotics and placebo groups. Among these 4 studies, 3 studies showed no difference, while the other one showed that the remission rate in probiotics group was much higher than that of placebo group. The overall meta-analysis results suggested that these 2 groups have no significant difference in remission rate (RR = 1.46, 95%CI [0.94, 2.26], P = .09; P for heterogeneity = .06, I2 = 60%). Subgroup analyses were preformed basing on the types of probiotics. The forest plot of subgroup analyses was presented in Figure 6. Schultz conducted a comparison of lactobacillus GG, which showed no difference (RR = 0.96, 95%CI [0.55, 1.96], P = .89), while the combined results of other 3 studies involving in VSL#3 demonstrated that the rates of remission in probiotics group were much higher than that of placebo (RR = 1.67, 95%CI [1.06, 2.63], P = .03).
Figure 5.

A forest plot for the comparison of remission rate between probiotics and placebo groups.
Figure 6.

A forest plot for the subgroup comparison of remission rate between probiotics and placebo groups.
3.7. Meta-analysis about the relapse rate between probiotics and placebo groups
The 4 included articles were involved in the comparison of the relapse between probiotics and placebo groups. Figure 7 shows the forest plot of the relapse rate in these 2 groups. Among these 4 studies, 3 studies showed no difference, while the other one showed that the relapse rate in control group was much higher than that of probiotics group. Moreover, the overall meta-analysis results indicated no significant difference in relapse rate (RR = 0.84, 95%CI [0.46, 1.55], P = .59; P for heterogeneity = .07, I2 = 57%). Subgroup analyses were also conducted basing on the probiotics. The subgroup analyses forest plot was presented in Figure 8. Both the comparisons of S boulardii and lactobacillus GG have showed no significant difference (RR = 0.89, 95%CI [0.66, 1.22], P = .48; RR = 1.39, 95%CI [0.64, 2.99], P = .41, respectively), while the comparison of VSL#3 suggested that the relapse rate of control was much higher than that of probiotics (RR = 0.29, 95%CI [0.10, 0.83], P = .02).
Figure 7.

A forest plot for the comparison of relapse rate between probiotics and placebo groups.
Figure 8.

A forest plot for the subgroup comparison of relapse rate between probiotics and placebo groups.
3.8. Meta-analysis about the complication rate between probiotics and placebo groups
The 5 studies were involved in the comparison of the complication rate between probiotics and placebo groups. The forest plot of the complication rate in these 2 groups was performed in Figure 9. As all these 5 studies showed no difference, the overall meta-analysis results suggested no significant difference in complication rate between probiotics and placebo groups (RR = 1.06, 95%CI [0.84, 1.33], P = .64; P for heterogeneity = .67, I2 = 0%). According to the different types of probiotics, forest plot of subgroup analyses was showed in Figure 10. All types of probiotics including S boulardii, lactobacillus GG and VSL#3 have showed no significant difference (RR = 1.05, 95%CI [0.80, 1.37], P = .72; RR = 0.81, 95%CI [0.33, 2.00], P = .64; RR = 1.14, 95%CI [0.73, 1.79], P = .56, respectively).
Figure 9.

A forest plot for the comparison of complication rate between probiotics and placebo groups.
Figure 10.

A forest plot for the subgroup comparison of complication rate between probiotics and placebo groups.
3.9. Bias analysis
Relatively high heterogeneities in the meta-analysis of remission rate and relapse rate between probiotics and placebo groups were observed (I2 = 60% and 57%, respectively). Despite the fact that high heterogeneities were observed, we did not assess the publication bias in our study for the fact that few articles were included.[36]
4. Discussion
Until now, there have no standard therapy for IBD and the most common treatment option is to establish systemic or topical immunoregulation with mesalazine or sulfasalazine, which could reduce the associated risk of cancer in bowel.[37,38] Unfortunately, previous studies have reported several serious adverse effects about the use of mesalazine after long time follow-up[39,40]; thus an alternative therapy is required. It has been reported that almost 40% of adults and children who suffered with IBD have treated with alternative therapies such as probiotics, which may mediate the inhibition of nuclear factor kB.[41,42]
Organisms present in probiotic preparations include S boulardii, Lactobacillus GG, EcN 1917, and VSL#3. S boulardii has been showed the prevention of recurrences on Clostridium difficile infection, and animal models have reported the effects in IBD.[43,44] There were few data about the effect of S boulardii on patients with IBD, but the reduction of clinical features about inflammation and reinforcement of intestinal epithelial barrier were observed in previous studies.[45–47] Lactobacillus GG (LGG) has been used as the treatment of rotavirus, acute diarrhea, and atopic disease in at-risk infants.[48,49] It could modify bacterial flora in human bowel. EcN 1917 is one of the most common strains used as probiotics in IBD patients, and the specific characteristics like the unique structure of lipopolysaccharide and biofilms formation in different conditions make it survive in the gut.[50,51] VSL#3 consists of 8 different bacterial species, which has been shown to be effective in infection disease, such as chronic pouchitis.[52,53]
In our study, we preformed meta-analyses of the remission, relapse, and complication rate between EcN 1917 and mesalazine. Although safe and well tolerated, there was no significant difference either in the EcN1917 group or among patients treated with mesalazine. Generally, experiment-control studies are presented to test the difference. However, due to the fact that mesalazine has been regarded as the established gold standard therapy, the results in our study were aimed to demonstrate the equivalence. The meta-analyses suggested that EcN1917 provided similar efficacy in remission, relapse and complication rate compared with mesalazine.
We also conducted the comparison of the efficacy between IBD patients treated with probiotics and placebo. All the combined results about the efficacy in remission, relapse, and complication rate showed no significant difference between probiotics and placebo groups. As 3 types of probiotics including S boulardii, Lactobacillus GG and VSL#3 were involved in the meta-analyses, the subgroup analyses were conducted. Though no difference was observed in remission rate of Lactobacillus GG, VSL#3 showed higher remission rate than that of placebo group (RR = 1.67, 95%CI[1.06, 2.63]). Both S boulardii and Lactobacillus GG showed similar result about the relapse rate in subgroup mate-analysis, but the relapse of patients with placebo showed higher rate than VSL#3 (RR = 0.29, 95%CI[0.10, 0.83]). The frequency of complications was similar in all subgroups. The side-effects motioned in the studies were relatively minor including diarrhea, abdominal pain, arthralgia, bdominal bloating, and some discomfort.
In summary, EcN 1917 has a similar efficacy with the mesalazine, the commonly used drug for IBD patients. While both S boulardii and Lactobacillus GG showed no advantage compared with placebo, the mixed probiotics, VSL#3, presented better results.
There were some potential limitations in this study. Some high heterogeneity was observed in the meta-analyses. As subgroup analysis has been conducted, high heterogeneity was attributable to the different types to some extent. Few articles have been involved in our studies and few patients were enrolled in the trials, which could generate the possibility of bias. Besides, some other parameters of the patients could influence the result of the treatment and increases the risk of flare-up.[54] Future studies with high quality about the different probiotics used to IBD patients should be conducted.
5. Conclusion
In conclusion, according to its pathogenesis, the use of some types of probiotics could prevent the induction of inflammatory reactions in patients with IBD. EcN 1917 shows comparable efficacy and safety to mesalazine, and VSL#3 shows better effects than placebo. These probiotics could be considered as an alternative for patients with IBD.
Author contributions
Conceptualization: Kai Jia.
Data curation: Kai Jia, Xin Tong, Rong Wang, Xin Song.
Investigation: Xin Tong, Rong Wang, Xin Song.
Methodology: Kai Jia.
Supervision: Xin Tong, Rong Wang, Xin Song.
Writing – original draft: Kai Jia.
Writing – review & editing: Kai Jia.
Footnotes
Abbreviations: CCT = controlled clinical trial, CD = Crohn's disease, Cis = confidence intervals, IBD = inflammatory bowel disease, LGG = Lactobacillus GG, RCT = randomized control trial, RR = related ratio, UC = ulcerative colitis.
KJ and XT have equally contributed to this study.
Funding: This work was supported by special fund of special medical food of Beijing clinical nutrition quality control center.
The authors have no conflicts of interest to disclose.
References
- [1].Cosgrove M, Al-Atia RF, Jenkins HR. The epidemiology of paediatric inflammatory bowel disease. Arch Dis Child 1996;74:460–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andres P, Friedman L. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am 1999;28:255–81. [DOI] [PubMed] [Google Scholar]
- [3].Schreiber S, Raedler A, Stenson W, et al. The role of the mucosal immune system in the pathogenesis of inflammatory bowel disease. Gastroenterol Clin North Am 1992;21:451–502. [PubMed] [Google Scholar]
- [4].Duchmann R, Kaiser I, Hermann E, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 1995;102:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 1991;67:1033–6. [DOI] [PubMed] [Google Scholar]
- [6].Medzhitov R. Toll-like receptors and immunity. Nat Rev Immunol 2001;1:135–45. [DOI] [PubMed] [Google Scholar]
- [7].Freidman S. General principles of medical therapy for inflammatory bowel disease. Gastroenterol Clin North Am 2004;33:191–208. [DOI] [PubMed] [Google Scholar]
- [8].Katz J. Treatment of inflammatory bowel disease with corticosteroids. Gastroenterol Clin North Am 2004;33:171–89. [DOI] [PubMed] [Google Scholar]
- [9].Semeao E, Jawas A, Stouffer N, et al. Risk factors for low bone mineral density in children and young adults with Crohn's disease. J Pediatr 1999;135:593–600. [DOI] [PubMed] [Google Scholar]
- [10].Stange EF, Riemann J, von Herbay A, et al. Diagnosis and therapy of ulcerative colitis—results of an evidence-based consensus conference of the German Society of Digestive and Metabolic Diseases. Z Gastroenterol 2001;39:19–20. [DOI] [PubMed] [Google Scholar]
- [11].Allgayer H. Sulfasalazine and 5-ASA compounds. Gastroenterol Clin N Amer 1992;21:643–58. [PubMed] [Google Scholar]
- [12].Gisbert J, Gomollon F, Mate J, et al. Role of 5-aminosalicylic acid in treatment of inflammatory bowel disease: a systematic review. Dig Dis Sci 2002;47:471–88. [DOI] [PubMed] [Google Scholar]
- [13].Markowitz J, Grancher K, Kohn N, et al. the Pediatric 6MP collaborative group A multicenter controlled trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn's disease. Gastroenterology 2000;119:895–902. [DOI] [PubMed] [Google Scholar]
- [14].Zachos M, Tondeur M, Griffiths A. Enteral nutritional therapy for inducing remission of Crohn's disease. Cochrane Database Syst Rev 2001;3:CD000542. [DOI] [PubMed] [Google Scholar]
- [15].Isaacs K, Sartor R. Treatment of inflammatory bowel disease with antibiotics. Gastroenterol Clin North Am 2004;33:335–46. [DOI] [PubMed] [Google Scholar]
- [16].Mantzaris GJ, Hatzis A, Kontogiannis P, et al. Intravenous tobramycin and metronidazole as an adjunct to corticosteroids in acute, severe ulcerative colitis. Am J Gastroenterol 1994;89:43–6. [PubMed] [Google Scholar]
- [17].Cummings JH, Macfarlane GT. Jewell DP, Warren BF, Mortensen NJ. Is there a role for microorganisms? Challenges in Inflammatory Bowel Disease. Oxford: Blackwell Science; 2001. 47–8. [Google Scholar]
- [18].Macfarlane GT, Cummings JH. Probiotics, infection and immunity. Curr Opin Infect Dis 2002;15:501–6. [DOI] [PubMed] [Google Scholar]
- [19].McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994;271:1913–8. [PubMed] [Google Scholar]
- [20].Kirchhelle A, Frühwein N, Tobüren D. Treatment of persistent diarrhea with S. boulardii in returning travelers: results of prospective study. Fortschr Med 1996;114:136–40. [PubMed] [Google Scholar]
- [21].Shanahan F. Probiotics in inflammatory bowel disease—therapeutic rationale and role. Adv Drug Deliv Rev 2004;56:809–18. [DOI] [PubMed] [Google Scholar]
- [22].Mao Y, Nobaek S, Kasravi B, et al. The effects of Lactobacillus strains and oat fiber on methotrexate-induced enterocolitis in rats. Gastroenterology 1996;111:334–44. [DOI] [PubMed] [Google Scholar]
- [23].Guslandi M, Mezzi G, Sorghi M, et al. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Dig Dis Sci 2000;45:1462–4. [DOI] [PubMed] [Google Scholar]
- [24].Rembacken BJ, Snelling AM, Hawkey PM, et al. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomized trial. Lancet 1999;354:635–9. [DOI] [PubMed] [Google Scholar]
- [25].Kruis W, Schutz E, Fric P, et al. Double blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther 1997;11:853–8. [DOI] [PubMed] [Google Scholar]
- [26].Bourreille A, Cadiot G, Dreau GL, et al. Saccharomyces boulardii does not prevent relapse of Crohn's disease. Clin Gastroenterol Hepatol 2013;11:982–7. [DOI] [PubMed] [Google Scholar]
- [27].Bousvaros A, Guandalini S, Baldassano RN, et al. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn's disease. Inflamm Bowel Dis 2005;11:833–9. [DOI] [PubMed] [Google Scholar]
- [28].Kruis W, Schutz E, Fric P, et al. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther 1997;11:853–8. [DOI] [PubMed] [Google Scholar]
- [29].Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004;53:1617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Harald Matthes, Thomas Krummenerl, Manfred Giensch, et al. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN). BMC Complement Altern Med 2010;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miele E, Pascarella F, Giannetti E, et al. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol 2009;104:437–43. [DOI] [PubMed] [Google Scholar]
- [32].Rembacken BJ, Snelling AM, Hawkey PM, et al. Non pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomized trial. Lancet 1999;354:635–9. [DOI] [PubMed] [Google Scholar]
- [33].Schultz M, Timmer A, Herfarth HH, et al. Lactobacillus GG in inducing and maintaining remission of Crohn's disease. BMC Gastroenterol 2004;4:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sood A, Midha V, Makharia GK, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol 2009;7:1202–9. [DOI] [PubMed] [Google Scholar]
- [35].Tursi A, Brandimarte G, Papa A, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL # 3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol 2010;105:2218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Song F, Eustwood AJ, Gilbody S, et al. Publication and related biases. Health Technol Assess 2000;4:1–15. [PubMed] [Google Scholar]
- [37].van Bodegraven AA, Mulder CJ. Indications for 5-aminosalicylate in inflammatory bowel disease: is the body of evidence complete? World J Gastroenterol 2006;12:6115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Stange EF. Review article: the effect of aminosalicylates and immunomodulation on cancer risk in inflammatory bowel disease. Aliment Pharmacol Ther 2006;24suppl 3:64–7. [DOI] [PubMed] [Google Scholar]
- [39].Frandsen NE, Saugmann S, Marcussen N. Acute interstitial nephritis associated with the use of mesalazine in inflammatory bowel disease. Nephron 2002;92:200–2. [DOI] [PubMed] [Google Scholar]
- [40].Uslu N, Demir H, Saltik-Temizel IN, et al. Acute tubular injury associated with mesalazine therapy in an adolescent girl with inflammatory bowel disease. Dig Dis Sci 2007;52:2926–9. [DOI] [PubMed] [Google Scholar]
- [41].Neish AS, Gewirtz AT, Zeng H, et al. Prokaryotic regulation of epithelial esponses by inhibition of IkappaB-alpha ubiquitination. Science 2000;289:1560–3. [DOI] [PubMed] [Google Scholar]
- [42].Heuschkel R, Afzal N, Wuerth A, et al. Complementary medicine use in children and young adults with inflammatory bowel disease. Am J Gastroenterol 2002;97:382–8. [DOI] [PubMed] [Google Scholar]
- [43].Guslandi M, Giollo P, Testoni PA. A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur J Gastroenterol Hepatol 2003;15:697–8. [DOI] [PubMed] [Google Scholar]
- [44].Kalliomaki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001;357:1076–9. [DOI] [PubMed] [Google Scholar]
- [45].Garcia Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo Da Gama Torres H, et al. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn's disease in remission. Scand J Gastroenterol 2008;43:842–8. [DOI] [PubMed] [Google Scholar]
- [46].Dalmasso G, Cottrez F, Imbert V, et al. Saccharomyces boulardii inhibits inflammatory bowel disease by trapping T cells in mesenteric lymph nodes. Gastroenterology 2006;131:1812–25. [DOI] [PubMed] [Google Scholar]
- [47].Lee SK, Kim YW, Chi SG, et al. The effect of Saccharomyces boulardii on human colon cells and inflammation in rats with trinitrobenzene sulfonic acid-induced colitis. Dig Dis Sci 2009;54:255–63. [DOI] [PubMed] [Google Scholar]
- [48].Vanderhoof JA, Whitney DB, Antonson DL, et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr 1999;135:564–8. [DOI] [PubMed] [Google Scholar]
- [49].Van Niel CW, Feudtner C, Garrison MM, et al. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics 2002;109:678–84. [DOI] [PubMed] [Google Scholar]
- [50].Grozdanov L, Raasch C, Schulze J, et al. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle. J Bacteriol 2004;186:5432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Blum-Oehler G, Oswald S, Eiteljorge K, et al. Development of strainspecific PCR reactions for the detection of the probiotic Escherichia coli strain Nissle 1917 in fecal samples. Res Microbiol 2003;154:59–66. [DOI] [PubMed] [Google Scholar]
- [52].Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL #3) for maintaining remission in recurrent or refractory pouchitis. Gut 2004;53:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 2000;119:305–9. [DOI] [PubMed] [Google Scholar]
- [54].Cosnes J, Carbonnel F, Carrat F, et al. Effects of current and former cigarette smoking on the clinical course of Crohn's disease. Aliment Pharmacol Ther 1999;13:1403–11. [DOI] [PubMed] [Google Scholar]


