Abstract
Both diabetic peripheral neuropathy and peripheral arterial disease (PAD) cause foot ulcers and often result in non-traumatic amputations in patients with type 2 diabetes. This study aimed to evaluate the association between clinical variables, PAD, and subclinical diabetic small fiber peripheral neuropathy detected by abnormal thermal thresholds of the lower extremities in patients with type 2 diabetes.
We investigated 725 consecutive patients with type 2 diabetes (male/female: 372/353; mean age, 67 ± 11 years) who did not have apparent cardiovascular disease (including coronary artery disease, arrhythmia, and stroke) and who underwent the quantitative sensory test for thermal (warm and cold) thresholds of the lower limbs and ankle-brachial index (ABI)/toe-brachial index (TBI) examinations in 2015. The analyses included glycated hemoglobin, estimated glomerular filtration rate, and other characteristics.
In total, 539 (74.3%) patients showed an abnormality of at least 1 thermal threshold in their feet. All patients with an abnormal ABI (<0.9) had concurrent impaired thermal thresholds, and 93% (87/94) of patients with an abnormal TBI experienced abnormal thermal thresholds in the lower limbs. Age- and sex-adjusted TBI and estimated glomerular filtration rate were significantly correlated to abnormal thermal thresholds. In the multivariate analysis, fasting plasma glucose, and glycated hemoglobin were independently associated with abnormal thermal thresholds in the lower extremities.
Subclinical thermal threshold abnormalities of the feet are significantly associated with PAD and nephropathy in patients who have type 2 diabetes without cardiovascular disease.
Keywords: ankle-brachial index, cardiovascular disease, peripheral arterial disease, toe-brachial index, type 2 diabetes
1. Introduction
Peripheral neuropathy is considered to be one of the earliest and most prevalent chronic complications of type 2 diabetes.[1,2] The early detection of diabetic peripheral neuropathy (DPN) is a challenge due to the heterogeneous presentation of its symptoms. Previous studies have reported that peripheral neuropathy affects approximately 50% of diabetes patients,[3,4] and up to 50% of DPN cases are asymptomatic.[1,2] Furthermore, it is extremely difficult to confirm the diagnosis or estimate the severity of DPN because peripheral neuropathy is a combination of subjective symptoms and signs, abnormal neurophysiological examination findings, and impaired activities of daily living.[1,2,4] Peripheral neuropathy may cause foot ulcers and subsequent non-traumatic amputations in patients with type 2 diabetes.[1,2,5] Once peripheral neuropathy occurs, the annual incidence of diabetic foot ulcer may increase from <1% to >7%.[6] These complications not only affect the quality of life but also result in a heavy economic burden on society.[6] DPN is a critical but often overlooked health issue that requires special attention.
It is widely recognized that both peripheral arterial disease (PAD) and neuropathy are responsible for diabetic foot diseases and amputations. Furthermore, diabetes patients with lower extremity amputation may have a reduced life expectancy because of an increased risk of cerebrovascular and coronary artery diseases.[1,3,6] However, the association between PAD and abnormal thermal thresholds has rarely been investigated. Although the ankle-brachial index (ABI) and toe-brachial index (TBI) are recommended as noninvasive tools for PAD screening,[7] data on the early diagnosis of DPN, especially small fiber dysfunction, remain unclear.
Our study was designed to evaluate the association between clinical variables, including the peripheral vascular functional markers of PAD, and subclinical DPN detected by abnormal thermal thresholds of the lower extremities in type 2 diabetes outpatients without apparent cardiovascular disease.
2. Methods
2.1. Study population
Our data were obtained from a retrospective cohort at the outpatient department of a hospital in central Taiwan. We collected data from patients with type 2 diabetes who regularly visited the outpatient departments of the division of metabolism and endocrinology for at least 1 year, and underwent quantitative sensory testing (QST) for thermal (warm/cold) thresholds in both lower limbs, and ABI/TBI examinations from January to December 2015. Patients with a history of apparent cardiovascular disease (coronary artery disease, arrhythmia, and stroke), amputations, and end-stage renal disease (estimated glomerular filtration rate [eGFR] <15 mL·min−1·per 1.73 m2), and those undergoing dialysis, or diagnosed with a malignancy or neuropathy of the lower limbs were excluded. Patients were also excluded if they had an ABI > 1.3, as this is indicative of calcification, which makes the assessment of possible obstructive lesions in the lower limbs by ABI/TBI examination difficult.[8] We collected clinical and demographic data, including sex, age, body mass index (BMI), and biochemical data, including fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), serum creatinine, lipid profiles, and the urine albumin-to-creatinine ratio (UACR). The study protocol was approved by the Institutional Review Board of the Tsaotun Psychiatric Center, Ministry of Health and Welfare, Taiwan (approval certificate number: 104057).
2.2. QST for the thermal (warm/cold) thresholds in the lower limbs
The QST values for the thermal (warm/cold) thresholds in both lower limbs were measured using the sensory threshold evaluation system (Q-Sense Thermal Sensory Analyzer, Medoc Advanced Medical Systems, Ramat Yishai, Israel) following established protocols.[9,10] Normative QST data was obtained for the age-specific thermal thresholds of the software based on a published collection of normal controls, and the repeatability was established.[11] Two operators were responsible for the examinations in daily clinical practice; they underwent standard training to ensure there was no significant operator-derived variability. The thermode test sites for insertion were present on the dorsal surface of patients’ foot, with an active area of 9 cm2 (3 cm × 3 cm). The thermode had a baseline adaptation temperature of 32 °C, and the rate of temperature change was maintained at 1 °C/s with a return rate of 1 °C/s. Tests were performed to record the warm threshold; the cold threshold was detected by patients. All patients received trials to experience warm and cold sensations without training. The temperature was increased for the detection of the warm threshold and decreased for the cold threshold. At any time during the course of the examination, patients could immediately push a button on the handheld response unit and the machine would stop delivering the stimulus. The next trial started from the baseline value with a 15 s interval. If the patient failed to push the button before the temperature reached the predefined limits, the system automatically terminated the trial and returned the temperature to the baseline setting. The trials were conducted four times, and the average of the four trials was defined as the thermal threshold.[9,10] If the standard deviation (STD) of these four QST values was >1, the data of this examination was not acceptable, and this examination was identified as failed. A previous study demonstrated the high reproducibility of QST, and it is considered a reliable tool for the indirect evaluation of human small fiber nerve function.[12] An abnormal thermal threshold was defined as a QST abnormality in at least 1 cold or warm thermal threshold in the foot.
2.3. Measurement of PAD indices (ABI and TBI)
The ABI and TBI were measured using Vascular Profiler 1000 (Colin Co. Ltd., Komaki, Aichi, Japan) according to the standard method reported in our previous studies.[13,14] Patients were examined after resting for at least 5 min in the supine position in an air-conditioned room (approximately 25 °C). Electrocardiograph electrodes were attached to both wrists and a microphone for phonocardiography was placed at the third intercostal space on the left margin of the sternum. Pneumatic pressure cuffs were placed around the arms and ankles. Blood pressure and electrocardiogram data were recorded simultaneously. The highest brachial systolic blood pressure was recorded and automatically used to calculate the ABI and TBI. Toe blood pressure was measured using 25 mm wide digital cuffs placed around the bases of both halluces. The ABI was determined as the ratio of the ankle systolic blood pressure (SBP) and highest brachial SBP, and the TBI was determined as the ratio of the toe SBP and highest brachial SBP. These methods have been validated in previous studies.[15,16] All patients underwent bilateral ABI and TBI examinations, and data from the leg with the lower ABI values were analyzed. An abnormal ABI was defined as a value <0.9 and abnormal TBI as a value <0.6.[17]
2.4. Clinical and laboratory examinations
Clinical data, including age, sex, height, and weight, were recorded. The device measured these data simultaneously: SBP refers to the highest brachial SBP of the patient and diastolic blood pressure (DBP) is the brachial DBP measured on the same side as the SBP. Patients’ medical records were reviewed to obtain data on histories and medications. Overnight fasting blood samples were collected to measure plasma glucose, HbA1c, and serum lipid profile levels. FPG levels were measured using the glucose oxidase-peroxidase method. HbA1c levels were measured using boronate affinity high-performance liquid chromatography (Bio-Rad variant II HbA1c REORDER PACK 1000TEST/PKG, Alfred Nobel Drive Hercules, California, USA). Serum lipid profiles were assayed using the enzymatic method and triglyceride levels were assayed using the enzymatic GPO-Trinder. The homogeneous method was used to measure high-density lipoprotein cholesterol and low-density lipoprotein cholesterol concentrations. The UniCel DxC 800 Chemistry Analyzer (Beckman Coulter, Inc., USA) was used to determine serum creatinine concentrations. Urinary albumin levels were measured using the turbidimetric method and urine creatinine levels were measured using the modified kinetic Jaffe method (Beckman Coulter, Inc., Fullerton, CA, USA). Urinary albumin excretion was evaluated using the UACR in a spot sample.
2.5. Measurement of renal function (UACR and eGFR)
The eGFR values were calculated using the 2009 CKD-EPI creatinine equation: GFR = 141 × min (Scr/κ,1)α × max (Scr/κ,1)−1.209 × 0.993Age × 1.018 {if female} × 1.159 {if black}, where Scr refers to the serum creatinine concentration (mg/dL), min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1, with κ = 0.7 for female patients and 0.9 for male patients, α = −0.329 for female patients and −0.411 for male patients.[18,19] Albuminuria was defined as normoalbuminuria and proteinuria, and proteinuria was defined as a UACR ≥30 mg/mmol.[20,21]
2.6. Statistical analyses
Data are presented as mean ± STD, percentage (%), or median, as appropriate. Independent t tests were performed to detect differences in the continuous variables between 2 groups, while chi-square tests were used to assess the differences in the categorical variables such as sex, albuminuria status, and medication use. The adjusted odds ratio (ORs) of the risk factors for abnormal thermal thresholds were determined by multivariate logistic regression analyses. The area under the receiver operating characteristic curve (AUROC) for predicting abnormal QST thermal thresholds in at least one lower limb according to the HbA1c and FPG levels was calculated. Statistical significance was defined as p < .05. All statistical analyses were performed using the SPSS version 19.0 for Windows (SPSS Inc., Chicago, Illinois, USA).
3. Results
A total of 725 (male/female: 372/353) patients with type 2 diabetes, with a mean age of 67 ± 11 years, were enrolled. Of the 725 patients, 539 (74%, male/female: 297/254) showed an abnormality in at least 1 thermal threshold, 46% had chronic kidney disease (eGFR < 60 mL·min−1·per 1.73 m2 or UACR > 30 mg/g), and 13% had abnormal TBI values. An abnormal thermal threshold in the lower limbs was the most prevalent chronic complication found in our study population (Fig. 1). Furthermore, all patients with abnormal ABI values had concomitant abnormal thermal threshold examination findings (n = 13), and 93% (87/94) of the patients with an abnormal TBI experienced an abnormality in at least one thermal threshold in the lower extremities. Among patients with an impaired thermal threshold in the lower limbs, 59% had normoalbuminuria.
Figure 1.
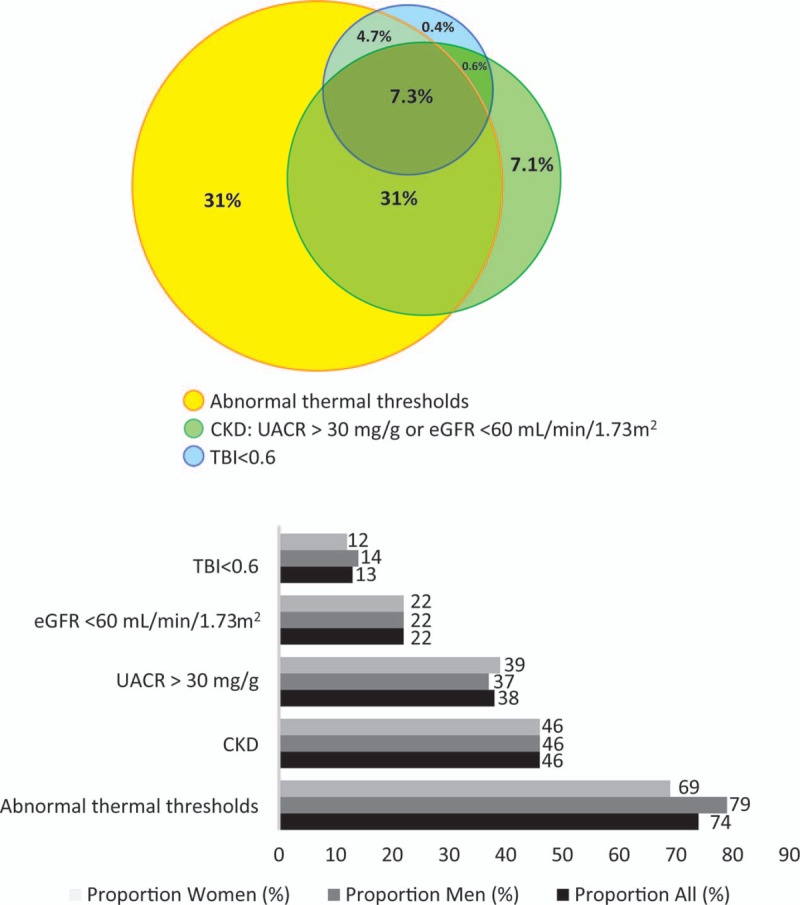
Proportions of chronic complications in patients with type 2 diabetes. Abnormal thermal thresholds = abnormal quantitative sensory testing for cool or warm thermal thresholds in at least 1 lower limb, CKD = chronic kidney disease, including UACR >30 mg/g or eGFR< 60 mL/min/1.73 m2, TBI = toe-brachial index, UACR = urine albumin-to-creatinine ratio; eGFR: estimated glomerular filtration rate.
Patients with abnormal QST values for cool or warm thermal thresholds in the lower limbs were likelier to be male, older in age, and have higher SBP, higher UACR, lower eGFR, and lower TBI values than patients possessing normal thresholds in both lower limbs (p < .05) (Table 1). The distribution of the antidiabetic medication profiles was similar between patients with and without abnormal thermal thresholds (Table 1). Low eGFR, low TBI, high FPG, and HbA1c levels remained significantly associated with impaired thermal thresholds after adjusting for age and sex (Table 2). The age- and sex-adjusted ORs of the abnormal thermal thresholds for eGFR, TBI, FPG, and HbA1c were 0.987 (95% confidence interval [CI], 0.978–0.997; p = .013), 0.096 (95% CI, 0.026–0.352; p < .001), 1.007 (95% CI, 1.002–1.011; p = .002), and 1.149 (95% CI, 1.026–1.288; p = .017), respectively.
Table 1.
Clinical characteristics of patients with normal quantitative sensory test findings for cool or warm thermal thresholds in the lower limbs vs patients with abnormal thermal thresholds in at least one lower limb.

Table 2.
Age- and sex-adjusted odds ratios (95% confidence intervals) of abnormal thermal thresholds.

Table 3 shows the results of the multivariate logistic regression models derived by adding FPG and HbA1c separately after adjusting for sex, age, SBP, and lipid profiles. After adjusting for these potential confounders and demographic factors, FPG and HbA1c were found to be significant, independent modifiable risk factors associated with impaired QST for cool or warm thermal thresholds of the lower limbs in separate models. The ORs of impaired QST for cool or warm thermal thresholds of the lower limbs for FPG and HbA1c, after adjusting for demographic factors and clinical confounders, were 1.009 (95% CI, 1.003–1.015; p = .003) and 1.370 (95% CI, 1.146–1.639; p < .001), respectively.
Table 3.
Multiple logistic regression analysis for the assessment of the risk factors of abnormal thermal thresholds based on fasting plasma glucose and glycated hemoglobin levels after adjustment for potential confounders.

Receiver operating characteristic curves of the age- and sex-adjusted FPG and HbA1c values in predicting impaired QST values (Fig. 2) were constructed. For FPG and HbA1c, the age-adjusted AUROCs for predicting impaired QST from cool or warm thermal thresholds were 0.735 and 0.736, respectively. The AUROC values of FPG and HbA1c in predicting abnormal thermal thresholds in the lower limbs indicated acceptable discrimination (AUROC > 0.7, p < .001).
Figure 2.
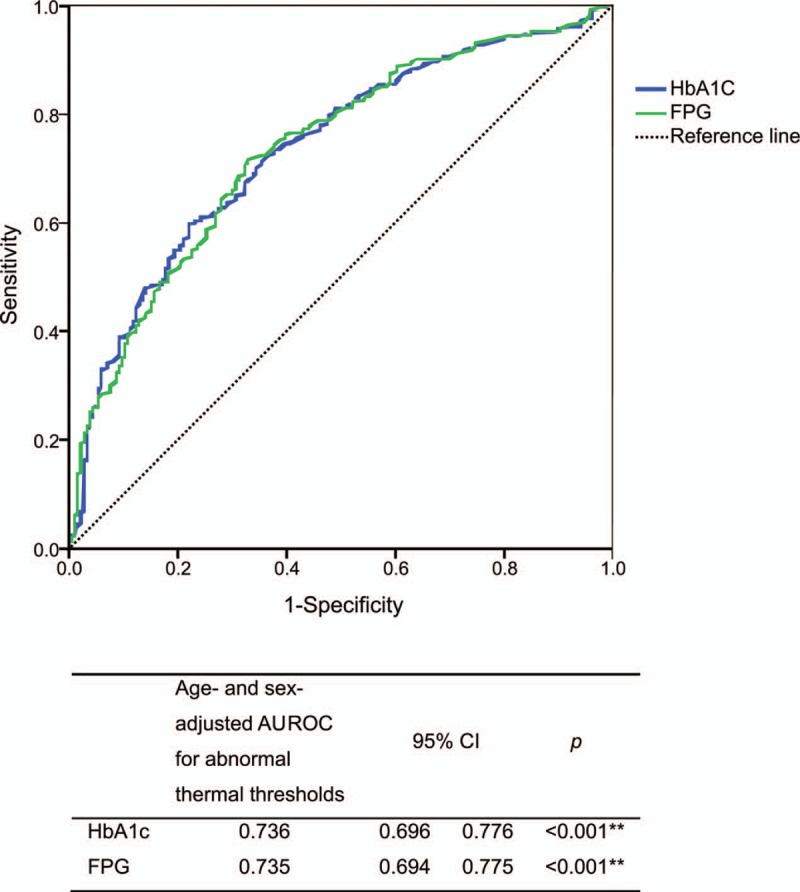
Age- and sex-adjusted area under the curve for the prediction of abnormal thermal thresholds in at least 1 lower limb according to HbA1c and FPG. AUROC = area under the receiver operating characteristic curve, CI = confidence interval, FPG = fasting plasma glucose, HbA1c = glycated hemoglobin.
4. Discussion
One major finding of our study was that the prevalence of subclinical abnormal peripheral small fiber dysfunction—identified as impaired QST values of the cold or warm thermal thresholds in the lower extremities—was extremely high among patients with type 2 diabetes. We found that 74% of the patients presented abnormalities in at least one thermal threshold. According to previous studies, DPN prevalence varies considerably depending on the form of examination used for diagnosis. A multicenter cross-sectional study conducted in 118 hospitals and diabetes clinics in the UK reported that the prevalence of DPN (detected by neuropathy disability score, including examinations of the ankle reflex, vibration, pin-prick and temperature sensation at the great toe, and neuropathy symptom score) among patients with type 2 diabetes was 32.1%, with the proportions increasing with age.[22] Another study found that 67% of 156 type 2 diabetes patients had at least 1 DPN variable, through the use of questionnaires and detailed neurological examinations, including monofilaments and tuning forks.[23] A multiregional cross-sectional study of 2644 patients reported the prevalence of DPN as 24.1% (22.4–25.9%) using a standard scoring system for symptoms and signs of polyneuropathy.[24] Using a questionnaire as a tool, the presence of DPN was reported in 50.8% of type 2 diabetes patients with or without neuropathic pain.[25] Using monofilament examination, the prevalence of DPN was found to be 77.4% in a sample of Indian patients with type 2 diabetes.[26] Using the Michigan Neuropathy Screening Instrument (MNSI),[27] and defined by an MNSI exam score >2.5, DPN was reported in 89% of 79 Pima Indian type 2 diabetes patients.[28] Our findings are in accordance with these trends; however, most of the above-mentioned studies evaluated DPN using clinical symptoms and examinations of large fiber function. Subclinical small fiber abnormalities have rarely been investigated.
In general, it is difficult to diagnose small nerve fiber dysfunction because it cannot be investigated using routine electrophysiological tests. In a previous study, QST for assessing cold and warm sensation thresholds and skin biopsy (for evaluating the quantification of somatic intraepidermal nerve fibers) were performed to detect small nerve fibers damages.[29] A small clinical trial was also conducted on 30 age-matched controls and 58 patients with impaired glucose tolerance. Abnormal QST findings for thermal threshold were identified in 27.6% of patients; the authors suggested that QST was one of the most sensitive techniques for the detection of neuropathy.[30] Another study investigated 35 patients with type 1 diabetes (8–16 years old), and found that 43% of patients showed abnormality of at least 1 sensory threshold in the arm or foot.[31] Yet another study included 57 type 2 diabetes patients and found that 39% had abnormal QST findings.[32] It should be noted that the numbers of participants in previous studies were relatively small, and information on the prevalence of abnormal thermal thresholds or small fiber dysfunction among type 2 diabetes patients is lacking. Abnormal thermal sensation is usually an asymptomatic subclinical condition that may escape detection. Our study revealed a high prevalence of asymptomatic small fiber DPN among patients with type 2 diabetes.
We also found that asymptomatic impaired cold or warm thermal thresholds were significantly associated with chronic kidney disease (defined by eGFR and UACR) in type 2 diabetes patients without apparent cardiovascular disease. It is known that DPN is frequently associated with comorbidities, such as depression, cognitive dysfunction, PAD, nephropathy, retinopathy, medial arterial calcification, and cardiovascular disease.[33] The association between DPN and diabetic nephropathy is bi-directional. A study conducted in Singapore involving 1861 type 2 diabetes patients revealed that patients with a high risk of chronic kidney disease (defined and classified using the 2012 Kidney Disease: Improving Global Outcomes Guidelines and Classification) had a higher prevalence of DPN (23%, detected by a monofilament test for light touch) than the low-risk group (3.6%).[34] A study that enrolled 3000 type 2 diabetes patients across India found that chronic kidney disease and DPN presented more frequently in patients with nephropathy.[35] As a result, patients with diabetic nephropathy may exhibit more pronounced DPN, and have an increased risk of severe foot disease. The frequency of foot complications is more than 2-fold higher in diabetes patients with end-stage renal disease, while the amputation rate is 6.5–10 times higher than in the general diabetes population.[33,36]
DPN was independently associated with PAD among type 2 diabetes patients. A study investigating 156 type 2 diabetes patients showed that PAD was three times likelier to occur (52%) in patients with DPN than in those without DPN (16%), and DPN was independently associated with PAD.[33,37] Another study including 126 patients with type 2 diabetes showed that ABI was significantly lower in the diabetic patients with DPN than in their non-diabetic counterparts, and an ABI <0.9 exhibited 47% sensitivity and 90.7% specificity for the detection of DPN.[33,38] Both DPN and PAD were responsible for diabetic foot disease and non-traumatic amputation. Based on many current guidelines, routine PAD screening is recommended using ABI examinations. In our study, the ABI values were lower among patients with abnormal thermal thresholds than those who had normal thresholds; however, this difference was not statistically significant. ABI sensitivity <0.9 for the diagnoses of PAD was limited (the ABI sensitivity was only 29.9% in patients undergoing hemodialysis) and the TBI may provide better accuracy along with the possibility for early PAD detection.[39] It was suggested that the TBI is important for evaluation of hemodynamic disorders, and can accurately identify patients with arterial calcification.[13] Because medial arterial calcification is less frequent in the toe than in the ankle, TBI is recommended for early detection of atherosclerotic cardiovascular disease.[13,40] In the present study, we used the TBI to evaluate PAD in the study population, and found a significant independent association between the TBI and small fiber DPN among patients with type 2 diabetes without apparent cardiovascular disease, but the relationship between ABI and small fiber DPN was not significant. The results of our study show that peripheral nerve pathology associated with diabetes may commonly occur with atherosclerotic changes in small arteries rather than damage of large arteries. Patients with type 2 diabetes with subclinical sensory DPN should be advised about the greater risk of PAD. Risk factors for DPN may be classified as non-modifiable or modifiable. Patients’ age and duration of diabetes are well-known non-modifiable risk factors of DPN, including abnormal QST values for thermal thresholds.[22,33,34,41] While some reports have suggested a higher risk among men,[41,42] others have reported a similar prevalence between men and women[22,32]; no significant difference has been observed between ethnic groups.[22] The modifiable risk factors for DPN (large-fiber abnormalities) include poor glycemic control (evaluated by FPG, HbA1c, and glycemic variability), hypertension, hyperlipidemia (triglyceride[33] and low-density lipoprotein[26]), obesity (BMI), alcoholism, and smoking.[3,4,26,33,41,42] However, the risk factors for abnormal thermal thresholds or small fiber DPN have rarely been investigated. In addition to the age of patients and duration of diabetes, HbA1c/hyperglycemia, serum cholesterol level, and alcoholism were independent risk factors for abnormal small fiber neuropathy.[32,43] Our study found that higher fasting glucose and HbA1c levels were additional independent modifiable variables among patients of older age and male sex. Patients with abnormal QST values for the cool or warm thermal thresholds in the lower limbs were likelier to have higher SBP and higher triglyceride levels, but statistical significance was not obtained in the multivariate logistic regression models for these variables. This could be because the sample size was not sufficiently large. In addition, patients with symptomatic cardiovascular disease were excluded, and it is possible that our study population had relatively early-stage small fiber DPN.
The main limitation of our study was its retrospective nature. Our study was a hospital-based, cross-sectional observation and its external validity may be limited. However, the demographic characteristics of the patients in our study were similar to the national population-based distribution of diabetes.[44] Moreover, although records of health behaviors (such as smoking and exercise) were not available, and the definite duration of diabetes was not recorded, we reviewed the past history of comorbidities and complications of the study population and recorded blood glucose levels and HbA1c data. Glycemic control and disease severity were also investigated. In addition, we excluded patients with an ABI > 1.3, due to difficulties with assessing possible obstructive lesions, and the association between asymptomatic small fiber DPN and calcification of the lower limbs among patients with type 2 diabetes was not evaluated. Finally, thermal QST may have a multifactorial basis; for example, patients’ reaction time and mental concentration. Thus, we performed the trial 4 times in each patient, and the average of the 4 examinations was defined as the thermal threshold. If the STD of the four QST values was >1, this examination was identified as failed. To avoid operator-derived variability, the 2 operators responsible for the examinations received training. Normative data, including age and region-specific data, for the thermal threshold have been reported.[45] Previous studies have evaluated the reproducibility of QST, performed with the method-of-limits at different test intervals, by assessing the inter- and intra-individual variation of thermal thresholds, and showed that it is a reliable tool for the indirect evaluation of human small nerve fiber function.[12,46]
In conclusion, our findings demonstrate that asymptomatic small fiber DPN—defined by abnormal thermal cold and warm perception thresholds—occurs quite commonly in patients with type 2 diabetes but without apparent cardiovascular diseases. Subclinical abnormal thermal thresholds are independently associated with PAD and chronic kidney disease among such type 2 diabetes patients. Peripheral vascular disease should be examined in type 2 diabetes patients with abnormal thermal thresholds to avoid subsequent diabetic foot disease. Glucose control, including serum glucose and HbA1c levels, are independent modifiable risk factors for abnormal thermal thresholds in the lower extremities in patients with type 2 diabetes. Further large-scale, multicenter, prospective randomized controlled trials are required to investigate if targeting optimal blood glucose levels can reduce the impairment of small sensory fibers in patients with type 2 diabetes.
Acknowledgments
We thank our colleagues from Taichung Hospital, Ministry of Health and Welfare, who provided insight and expertise that greatly assisted the research.
Author contributions
Conceptualization: Yi-Jing Sheen, Tsai-Chung Li, Jiann-Liang Lin, Wen-Chen Tsai, Chuen-Der Kao, and Wayne H-H Sheu.
Data curation: Yi-Jing Sheen, Tsai-Chung Li, Jiann-Liang Lin, and Cho-Tsan Bau.
Formal analysis: Yi-Jing Sheen, Tsai-Chung Li, and Wen-Chen Tsai.
Investigation: Jiann-Liang Lin, Cho-Tsan Bau.
Methodology: Yi-Jing Sheen, Tsai-Chung Li, Chuen-Der Kao, and Wayne H-H Sheu.
Resources: Yi-Jing Sheen, Jiann-Liang Lin, and Cho-Tsan Bau.
Software: Tsai-Chung Li, Wen-Chen Tsai.
Supervision: Tsai-Chung Li, Wen-Chen Tsai, Chuen-Der Kao, and Wayne H-H Sheu.
Validation: Tsai-Chung Li, Jiann-Liang Lin, Wen-Chen Tsai, Cho-Tsan Bau, and Wayne H-H Sheu.
Writing – original draft: Yi-Jing Sheen.
Writing – review & editing: Tsai-Chung Li, Wen-Chen Tsai, and Wayne H-H Sheu.
Footnotes
Abbreviations: ABI = ankle-brachial index, AGI = Alpha-glucosidase inhibitors, BMI = body mass index, CI = confidence interval, DBP = diastolic blood pressure, DPN = diabetic peripheral neuropathy, DPP4I = dipeptidyl peptidase 4 inhibitors (DPP4 inhibitors), eGFR = estimated glomerular filtration rate, FPG = fasting plasma glucose, HbA1c = glycated hemoglobin, OR = odds ratio, PAD = peripheral arterial disease, QST = quantitative sensory testing, SBP = systolic blood pressure, SU = sulfonylurea, TBI = toe-brachial index, TZD = thiazolidinedione, UACR = urine albumin-to-creatinine ratio.
The authors declare no conflicts of interest.
References
- [1].Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:136–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev 2012;28:8–14. [DOI] [PubMed] [Google Scholar]
- [4].Tesfaye S. Recent advances in the management of diabetic distal symmetrical polyneuropathy. J Diabetes Investig 2011;2:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Won JC, Park TS. Recent advances in diagnostic strategies for diabetic peripheral neuropathy. Endocrinol Metab (Seoul) 2016;31:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Murphy-Lavoie HM, Bhimji SS. Diabetic, Foot Infections, https://www.ncbi.nlm.nih.gov/books/NBK441914/; 2017. Accessed July 01, 2018. [Google Scholar]
- [7].Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e726–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fleck CA. Measuring ankle brachial pressure index. Adv Skin Wound Care 2007;20:645–6. 648–649. [DOI] [PubMed] [Google Scholar]
- [9].Huang HW, Wang WC, Lin CC. Influence of age on thermal thresholds, thermal pain thresholds, and reaction time. J Clin Neurosci 2010;17:722–6. [DOI] [PubMed] [Google Scholar]
- [10].Chao CC, Hsieh SC, Yang WS, et al. Glycemic control is related to the severity of impaired thermal sensations in type 2 diabetes. Diabetes Metab Res Rev 2007;23:612–20. [DOI] [PubMed] [Google Scholar]
- [11].Yarnitsky D, Sprecher E. Thermal testing: normative data and repeatability for various test algorithms. J Neurol Sci 1994;125:39–45. [DOI] [PubMed] [Google Scholar]
- [12].Heldestad V, Linder J, Sellersjo L, et al. Reproducibility and influence of test modality order on thermal perception and thermal pain thresholds in quantitative sensory testing. Clin Neurophysiol 2010;121:1878–85. [DOI] [PubMed] [Google Scholar]
- [13].Sheen YJ, Lin JL, Lee IT, et al. Low estimated glomerular filtration rate is a major determinant of low ankle-brachial index and toe-brachial index in type 2 diabetes. Angiology 2012;63:55–61. [DOI] [PubMed] [Google Scholar]
- [14].Sheen YJ, Lin JL, Li TC, et al. Peripheral arterial stiffness is independently associated with a rapid decline in estimated glomerular filtration rate in patients with type 2 diabetes. Biomed Res Int 2013;2013: 309294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Motobe K, Tomiyama H, Koji Y, et al. Cut-off value of the ankle-brachial pressure index at which the accuracy of brachial-ankle pulse wave velocity measurement is diminished. Circ J 2005;69:55–60. [DOI] [PubMed] [Google Scholar]
- [16].Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 2002;25:359–64. [DOI] [PubMed] [Google Scholar]
- [17].Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med 2001;344:1608–21. [DOI] [PubMed] [Google Scholar]
- [18].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713–35. [DOI] [PubMed] [Google Scholar]
- [20].Andrassy KM. Comments on ’KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int 2013;84:622–3. [DOI] [PubMed] [Google Scholar]
- [21].Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care 2004;27Suppl 1:S79–83. [DOI] [PubMed] [Google Scholar]
- [22].Young MJ, Boulton AJ, MacLeod AF, et al. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36:150–4. [DOI] [PubMed] [Google Scholar]
- [23].Karvestedt L, Mårtensson E, Grill V, et al. The prevalence of peripheral neuropathy in a population-based study of patients with type 2 diabetes in Sweden. J Diabetes Complicat 2011;25:97–106. [DOI] [PubMed] [Google Scholar]
- [24].Cabezas-Cerrato J. The prevalence of clinical diabetic polyneuropathy in Spain: a study in primary care and hospital clinic groups. Neuropathy Spanish study group of the Spanish Diabetes Society (SDS). Diabetologia 1998;41:1263–9. [DOI] [PubMed] [Google Scholar]
- [25].Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab 2009;35:206–13. [DOI] [PubMed] [Google Scholar]
- [26].Kulshrestha M, Seth S, Tripathi A, et al. Prevalence of complications and clinical audit of management of type 2 diabetes mellitus: a prospective hospital based study. J Clin Diagn Res 2015;9:OC25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Feldman EL, Stevens MJ, Thomas PK, et al. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–9. [DOI] [PubMed] [Google Scholar]
- [28].Jaiswal M, Fufaa GD, Martin CL, et al. Burden of diabetic peripheral neuropathy in Pima Indians with type 2 diabetes. Diabetes Care 2016;39:e63–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008;131:1912–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kannan MA, Sarva S, Kandadai RM, et al. Prevalence of neuropathy in patients with impaired glucose tolerance using various electrophysiological tests. Neurol India 2014;62:656–61. [DOI] [PubMed] [Google Scholar]
- [31].Abad F, Diaz-Gomez NM, Rodriguez I, et al. Subclinical pain and thermal sensory dysfunction in children and adolescents with type 1 diabetes mellitus. Diabet Med 2002;19:827–31. [DOI] [PubMed] [Google Scholar]
- [32].Loseth S, Mellgren SI, Jorde R, et al. Polyneuropathy in type 1 and type 2 diabetes: comparison of nerve conduction studies, thermal perception thresholds and intraepidermal nerve fibre densities. Diabetes Metab Res Rev 2010;26:100–6. [DOI] [PubMed] [Google Scholar]
- [33].Papanas N, Ziegler D. Risk factors and comorbidities in diabetic neuropathy: an update 2015. Rev Diabet Stud 2015;12:48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Low SK, Sum CF, Yeoh LY, et al. Prevalence of chronic kidney disease in adults with type 2 diabetes mellitus. Ann Acad Med Singapore 2015;44:164–71. [PubMed] [Google Scholar]
- [35].Prasannakumar M, Rajput R, Seshadri K, et al. An observational, cross-sectional study to assess the prevalence of chronic kidney disease in type 2 diabetes patients in India (START -India). Indian J Endocrinol Metab 2015;19:520–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Papanas N, Liakopoulos V, Maltezos E, et al. The diabetic foot in end stage renal disease. Ren Fail 2007;29:519–28. [DOI] [PubMed] [Google Scholar]
- [37].Kärvestedt L, Martensson E, Grill V, et al. Peripheral sensory neuropathy associates with micro- or macroangiopathy: results from a population-based study of type 2 diabetic patients in Sweden. Diabetes Care 2009;32:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Papanas N, Symeonidis G, Mavridis G, et al. Ankle-brachial index: a surrogate marker of microvascular complications in type 2 diabetes mellitus? Int Angiol 2007;26:253–7. [PubMed] [Google Scholar]
- [39].Okamoto K, Oka M, Maesato K, et al. Peripheral arterial occlusive disease is more prevalent in patients with hemodialysis: comparison with the findings of multidetector-row computed tomography. Am J Kidney Dis 2006;48:269–76. [DOI] [PubMed] [Google Scholar]
- [40].Muro Y, Sugiura K, Morita Y, et al. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009;27:26–31. [PubMed] [Google Scholar]
- [41].Jaiswal M, Divers J, Dabelea D, et al. Prevalence of and risk factors for diabetic peripheral neuropathy in youth with type 1 and type 2 diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care 2017;40:1226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Olamoyegun M, Ibraheem W, Iwuala S, et al. Burden and pattern of micro vascular complications in type 2 diabetes in a tertiary health institution in Nigeria. Afr Health Sci 2015;15:1136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bednarik J, Vlckova-Moravcova E, Bursova S, et al. Etiology of small-fiber neuropathy. J Peripher Nerv Syst 2009;14:177–83. [DOI] [PubMed] [Google Scholar]
- [44].Jiang YD, Chang CH, Tai TY, et al. Incidence and prevalence rates of diabetes mellitus in Taiwan: analysis of the 2000-2009 Nationwide Health Insurance database. J Formos Med Assoc 2012;111:599–604. [DOI] [PubMed] [Google Scholar]
- [45].Hafner J, Lee G, Joester J, et al. Thermal quantitative sensory testing: a study of 101 control subjects. J Clin Neurosci 2015;22:588–91. [DOI] [PubMed] [Google Scholar]
- [46].Ponirakis G, Odriozola MN, Odriozola S, et al. NerveCheck for the detection of sensory loss and neuropathic pain in diabetes. Diabetes Technol Ther 2016;18:800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


