Abstract
Hypoxia-inducible factor 1-alpha (HIF-1a) has been shown to contribute to resistance to chemotherapy in breast cancer. The purpose of this study was to investigate whether HIF-1a is predictive for pathological response and the prognostic value of HIF-1a in local advanced breast undergoing neoadjuvant chemotherapy.
Two hundred twenty patients with none-metastatic locally advanced invasive breast cancer (stages II–III) that subsequently received neoadjuvant chemotherapy were included in an observational study to assess the HIF-1a protein expression by immunohistochemistry. Associations between HIF-1a expression and pathological complete response (pCR) were analyzed using univariate and multivariate analysis. Independent prognostic factors for RFS were identified by multivariate Cox's proportional hazard analysis. A P value < .05 was considered to be statistically significant.
The median age was 46 years, Luminal A, Luminal B, HER2-positive, and triple-negative accounted for 3.6%, 57.7%, 7.0% and 16.0%, respectively. A total of 41 patients (18.6%) achieved a pCR after neoadjuvant chemotherapy in the present study. HIF-1α negative patients had a significantly higher pCR rate than HIF-1α positive patients (P = .027). Multivariate analysis demonstrated that HIF-1α negative expression is an independent favorable predictor of pCR. Multivariate Cox regression analysis demonstrated that the HIF-1a expression before NCT showed an independent prognostic value for RFS (HR = 4.168, 95% CI: 1.012–17.170, P = .048).
HIF-1a expression correlates with pCR in breast cancer undergoing neoadjuvant chemotherapy. Absent expression of HIF-1a was associated with a better pathological response and could indicate a favorable prognosis in non-pCR breast cancer patients.
Keywords: breast cancer, HIF-1a, neoadjuvant chemotherapy, pathological complete response
1. Introduction
About 20% of newly diagnosed breast cancers are locally advanced, with associated more invasive behavior and higher risk of relapse. The standard treatment option for locally advanced breast cancer (LABC) is neoadjuvant therapy, which represents a unique opportunity to learn how to tailor the treatment to patients with high-risk breast cancers.[1] Neoadjuvant chemotherapy for locally advanced breast cancer helps to screen new biomarkers and test new drugs based on these biomarkers.[2,3] For neoadjuvant chemotherapy, pathological complete response (pCR) can be used as a surrogate marker for clinical outcomes after neoadjuvant therapy in breast cancer patients.
Among the established clinicopathological markers, only Ki67, ER, and HER2, have clearly defined clinical applicability, but deficiencies in the prediction of neoadjuvant therapy response for breast cancer.[4,5] Molecular subtyping based on ER, HER2, and Ki67 of breast cancer plays an important role in predicting the complete response rate of neoadjuvant chemotherapy, triple-negative breast cancer has a higher rate of complete pathological response and Luminal subtype is lower. Overexpression of HIF-1a protein has been found in many tumor types with high levels profoundly influencing the growth rate and metastatic potential of these cancers.[6,7] In breast cancers, the frequency of HIF-1a-positive cells increases in parallel with increasing pathological stage and associated with a poor prognosis in lymph node–negative patients.[8,9] Furthermore, upregulation of the hypoxia pathway by HIF-1a has also been shown to contribute to resistance to radiation therapy and chemotherapy in cancers.[10]
Few previous studies have investigated the association between HIF-1 expression and response to neoadjuvant chemotherapy in breast cancer. Therefore, in the current study, we investigated whether absent expression of HIF-1 is more likely to respond to neoadjuvant chemotherapy in terms of pathological complete response (pCR) in a large cohort of 220 breast cancer patients who received neoadjuvant chemotherapy (NCT). This study was also designed to identify the prognostic value of HIF-1 expression among patients with breast cancer.
2. Materials and methods
2.1. Study population
From January 2013 to December 2014, HIF-1 in a total of 220 patients who were treated with neoadjuvant chemotherapy for stages II to III invasive breast cancer at The Affiliated Cancer Hospital of Zhengzhou University were investigated. Inclusion criteria were: female; invasive breast cancer; clinical IIb to III stage breast cancer; underwent neoadjuvant chemotherapy before mastectomy or breast-conserving surgery. Exclusion criteria were: stage IV breast cancer; carcinoma in situ; received any treatment before NCT (chemotherapy, radiotherapy, and endocrine therapy); bilateral breast cancer; male breast cancer and inflammatory breast cancer. Core needle biopsy was performed to confirm the diagnosis of invasive breast cancer before NCT, and fine needle aspiration of palpable or ultrasound-detected lymph nodes was performed at the time of diagnosis. Blood chemistry, bone scan, chest CT, and abdominal ultrasound examination were performed to evaluate organ function and exclude metastatic disease before the initiation of NCT.
2.2. Pathology and immunohistochemistry
Immunohistochemistry for HIF-1a of breast cancer patient tissues was performed according to the manufacturer's instructions, using a DAB Substrate Kit (Maxin,Jiehao Co., Ltd, Shanghai, China) on invasive component of core needle biopsy samples, and positive and negative controls were set. The results were scored by 2 examiners who were blinded to clinicopathologic data. Intensity of staining was scored as (0 = negative; 1 = low; 2 = medium; 3 = high). Extent of staining was scored as 0 = 0% stained; 1 = 1% to 25% stained; 2 = 26% to 50% stained; 3 = 51% to 100% stained. Five random fields were observed under a light microscope. The final score was determined by multiplying the scores of intensity with the extent of staining, ranging 0 to 9. Final scores of <1 were considered as negative staining (−), 1 to 2 as low staining (+), 3 to 4 as medium staining (2+), and 6 to 9 as high staining (3+), which were considered as positive.
TNM staging was based on the sixth American Joint Committee on Cancer criteria. Immunohistochemistry (IHC) analysis was performed on formalin-fixed, paraffin-embedded tissue sections using standard procedures for breast tumor specimens from CNB to evaluate the expression of ER, PR, and HER2. ER and PR positive were defined as tumors with >1% nuclear-stained cells. HER2-positivity was indicated by a 3+ or 2+ score from the immunohistochemical evaluation and was confirmed using a fluorescence in situ hybridization (FISH) test for HER2. A cut-off point of 14% was used for Ki-67 staining. The molecular subtypes were classified into 4 groups as follows: Luminal A (ER+ and/or PR+, HER2−, Ki-67<14%); luminal B subtype (luminal B, HER2 negative [ER+ and/or PR+, HER2− and Ki-67≥20%] and luminal B HER2 positive [ER+ and/or PR+ and HER2+]), HER2 positive (ER− and PR−, HER2+); and triple-negative (ER− and PR−, HER2−) according to the molecular subtype consensus of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011.[11]
2.3. Neoadjuvant chemotherapy regimens and response
Among the 220 patients who received neoadjuvant chemotherapy, most patients received 4 to 8 cycles. Treatments were categorized as follows: anthracycline-based regimen, NE (navelbine 25 mg/m2 on day 1 and 8 and epirubicin 60 mg/ m2 on day 1 every 3 weeks), CEF (cyclophosphamide 600 mg/m2 on day 1, epirubicin 90 mg/ m2 on day 1, and 5-fluorouracil 600 mg/ m2 on day 1 every 3 weeks). Anthracycline- and taxane-based regimen, EC-T (epirubicin 75 mg/ m2, cyclophosphamide 600 mg/m2 on day 1 followed by docetaxel 60 mg/ m2 on day 1 every 3 weeks), PE (paclitaxel 175 mg/m2 on day 1 and epirubicin 75 mg/ m2 on day 1 every 3 weeks). Taxane-based regimen, TC (paclitaxel 75 mg/m2 on day 1 and carboplatin AUC 6 on day 1 every 3 weeks), P (paclitaxel 75 mg/m2 once a week for 12 weeks).
After completion of neoadjuvant chemotherapy, patients were treated with mastectomy or breast conserving surgery. Additional cycles of adjuvant chemotherapy were administered after the operation to complete a total of 6 to 8 cycles, and patients with axially positive lymph nodes and/or breast-conserving therapy received radiotherapy. Endocrine therapy was administered for patients with hormone receptor positive tumors. Trastuzumab was recommended for patients with human epidermal receptor-2 positive tumors in the neoadjuvant or adjuvant setting.
The clinical responses to NCT were evaluated based on MRI and ultrasound examinations in accordance with the response evaluation criteria in solid tumors (RECIST) version 1.1. A pathological complete response (pCR) after NCT was defined as the absence of invasive carcinoma in the breast tissue and lymph nodes of the resected specimen, noninvasive breast residuals were allowed (ypT0/is ypN0).
2.4. Ethics statement
This study was carried out in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of the Affiliated Cancer Hospital of Zhengzhou University. Written informed consent was obtained from every patient for the use of the pathological tissue samples and medical records for research purposes.
2.5. Statistical analysis
The associations between HIF-1α expression status, clinicopathologic characteristics, and pathological response to neoadjuvant chemotherapy were determined by Pearson's chi squared test or Fisher's exact test. A multivariate logistic hazard analysis was applied to determine whether a factor was an independent predictor of pCR in a multivariate analysis. Relapse-free survival (RFS) was calculated from the date of surgery to the date of disease relapse (local or distant relapse or death from any cause). Patients without events or death were censored at the last follow-up. Survival curves were estimated using the Kaplan-Meier method, and the log-rank test was used to test for differences between the groups. Independent prognostic factors for RFS were identified by multivariate Cox's proportional hazard analysis. All the statistical analyses were performed with SPSS 17.0 (SPSS, Chicago, IL) software. A P value < .05 was considered to be statistically significant.
3. Results
3.1. Patient and tumor characteristics
The study enrolled a total of 220 patients who were stages II to III invasive breast cancer. The clinicopathological characteristics of the patients are presented in Table 1. The median age of the study population was 46 years (range 23–66 years). Among all the cases, Luminal B (127, 57.7%) accounted for the largest proportion, 8 (3.6%) were classified as Luminal A, 50 (22.7%) as HER2-positive, and 35 (16.0%) as triple-negative.
Table 1.
Clinicopathological characteristic of subjects and correlation between HIF-1α expression and clinicopathological factors.
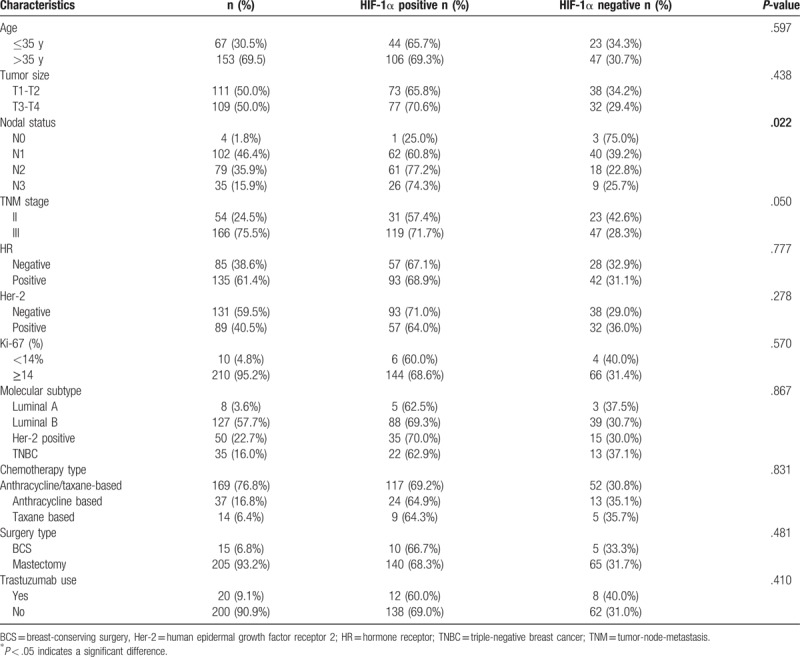
3.2. Relationship between HIF-1a and clinicopathological characteristics of patients
In this cohort of 220 patients, HIF-1a positive accounts for 68.2% of all breast carcinoma (150/220, 68.2%) (Fig. 1). No significant differences were found between HIF-1a positive and HIF-1a negative with regard to age, tumor size, ER or PR status, Her-2 status, Ki-67 expression, molecular subtype, chemotherapy regimens, surgery type, and trastuzumab use (Table 1). However, HIF-1α expression was significantly associated with lymph node status (P = .022). HIF-1α positive expression cases were more likely to be diagnosed in patients with positive lymph node status.
Figure 1.
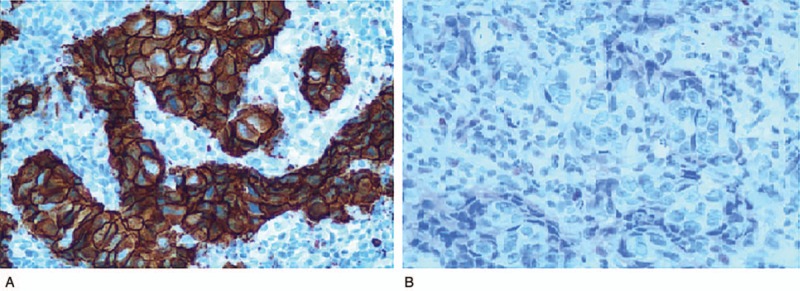
Immunohistochemistry for HIF-1a of breast cancer patient tissues (×200). (A) HIF-1α positive expression. (B) HIF-1α negative expression. HIF-1a = hypoxia inducible factor 1a.
3.3. Response to neoadjuvant chemotherapy in HIF-1α positive and negative patients
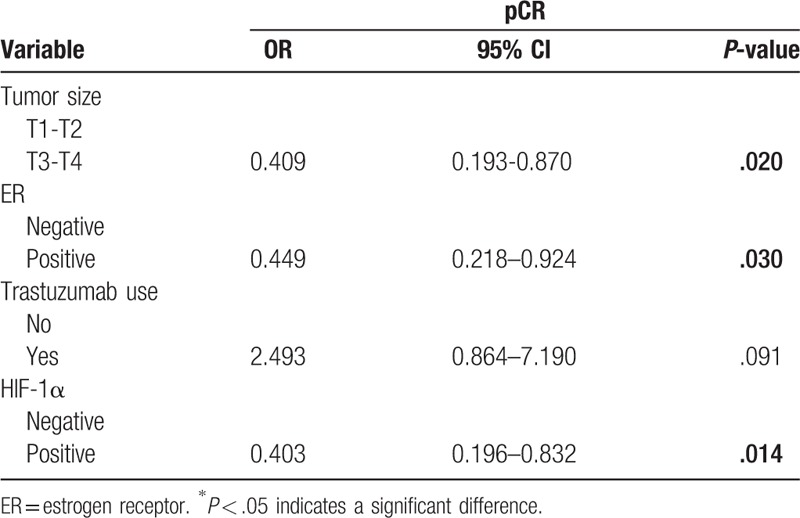
A total of 41 patients (18.6%) achieved a pCR after neoadjuvant chemotherapy in the present study. The pCR rate was 27.1% (19/70) for HIF-1α negative patients and 14.7% (22/150) for HIF-1α positive patients (Table 2), indicating that HIF-1α negative patients had a significantly higher pCR rate than HIF-1α positive patients (P = .027). In a univariate analysis, other factors including tumor size (P = .011), ER negative (P = .040) and trastuzumab therapy (P = .049) were significantly prognostic factors for pCR. The pCR rate was significantly higher in patients with larger tumor size, ER negative and who received trastuzumab in combination with neoadjuvant chemotherapy. Multivariate logistic analysis according to tumor size, ER status, trastuzumab use and HIF-1α expression revealed that HIF-1α expression was an independent significant predictor of pCR (Table 3).
Table 2.
Associations between clinicopathological characteristics and pCR.

Table 3.
Multivariate logistic regression model for pathological complete response according to tumor size, ER, Trastuzumab use, and HIF-1α expression.
3.4. Survival and prognostic factors for RFS in LABC who received NCT
In our study, population of 220 subjects of local advanced breast cancer, the median follow-up time was 30 months. Among the 41 LABC patients who received pCR, only 1 developed recurrences of liver metastasis. However, 39 of the 179 non-pCR patients had recurrences within the follow-up period including local recurrence and distant metastasis. Failure to achieve a pCR was associated with worse long-term outcomes for RFS than those who received pCR.
In non-pCR breast cancer cohort, univariate survival analysis was performed to assess the prognostic value of variables for RFS including primary tumor size, primary nodal involvement, primary ER status, primary PR status, primary Her-2 status, primary HIF-1a expression, primary tumor Ki-67, residual tumor size, residual node involvement, residual tumor Ki-67, and vascular invasion. Univariate survival analysis demonstrated that primary nodal involvement (P = .034), residual tumor size (P = .003), residual node involvement (P < 0.001), residual tumor Ki-67 (P = .007), and HIF-1a expression (P = .021) were significant predictors of RFS and were entered into the multivariate Cox regression model with forward analysis. In the multivariate Cox model, the HIF-1a expression before NCT showed an independent prognostic value for RFS (HR = 4.168, 95% CI: 1.012-17.170, P = .048) (shown in Table 4). Kaplan–Meier survival curve revealed that patients with HIF-1a negative expression showed a favorable outcome for RFS (Fig. 2).
Table 4.
Multivariate logistic regression model for RFS.
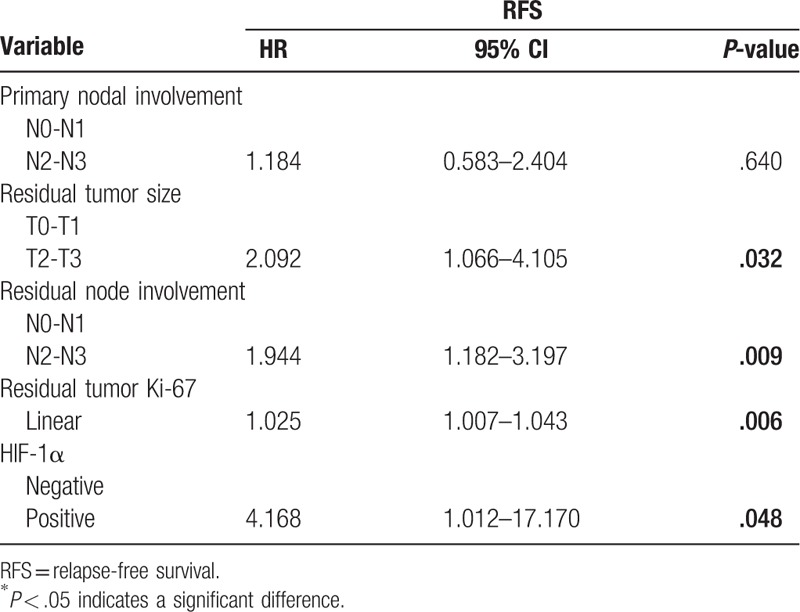
Figure 2.
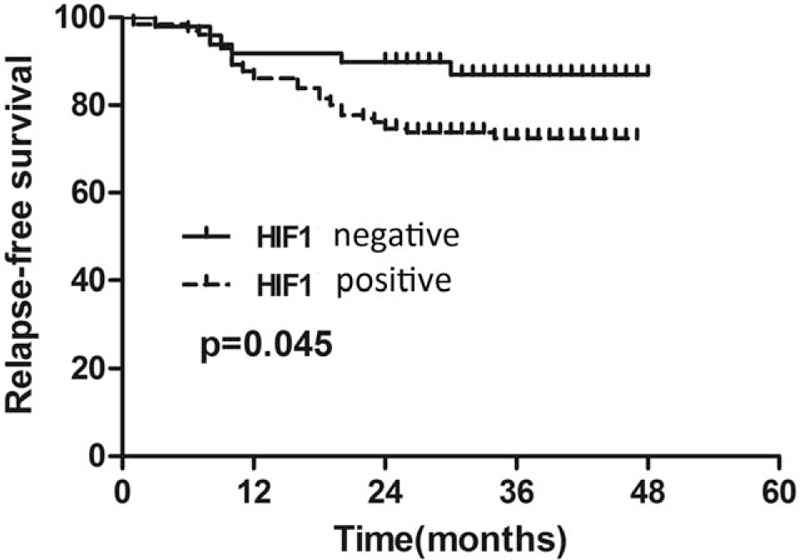
Kaplan–Meier survival curve of patients with HIF-1a positive and negative expression. HIF-1a = hypoxia inducible factor 1a.
4. Discussion
Neoadjuvant chemotherapy (NCT), also known as preoperative chemotherapy, induction chemotherapy, initial therapy, etc., refers to the treatment of systemic chemotherapy before surgery, which downstages the disease stage and enables surgery to be performed in those initially inoperable,[12] has been widely used as a standard treatment option for local advanced breast cancer (LABC). Locally advanced breast cancer (LABC) represents the most advanced stage breast cancer that is still potentially curable with surgery, radiation, and systemic therapy.[13] Similar therapeutic strategies could evoke diverse responses to neoadjuvant chemotherapy within local advanced breast cancer, however there still lacks effective predictive biomarkers for responses to NCT in LABC. Although gene expression profiling has led to a better understanding of the biological behavior of breast cancer, high costs have limited its conventional application. Therefore, it is urgent to develop novel biomarkers to improve response prediction to NCT and optimize therapy choice for LABC.
Tumor microenvironmental factors have an important effect on tumorigenesis, growth and metastasis. Hypoxia is prevalent in most malignancies and is associated with tumor biological behavior and chemotherapy resistance.[14] The oxygen-induced hypoxia inducible factor 1a (HIF-1a) protein is the main regulator of hypoxia,[15] which is overexpressed in a variety of cancers, including breast cancer.[16] Most ductal carcinoma in situ and almost all of the poorly differentiated invasive breast cancer overexpress HIF-1a. Overexpression of HIF-1a protein in primary tumors is associated with poor prognosis in breast cancer patients.[17] As a factor, tumor hypoxia is associated with aggressive biological behavior of the tumor, chemotherapy resistance, and then leading to treatment failure, including breast cancer.[18]
Many studies have documented the usefulness of HIF-1a in breast cancer. However, the characteristics of HIF-1a in LABC to NAC have not been elucidated to date. In this present study, the expression of HIF-1a in 220 cases of local advanced breast cancer patients who were treated with neoadjuvant chemotherapy was investigated. So far, this is the first large study to report the expression patterns of HIF-1a in local advanced breast cancer patients.
Our study demonstrated that HIF-1a positive accounts for 68.2% of all breast carcinoma (150/220, 68.2%), the positive rate of HIF-1a in local advanced breast cancer patients was significantly higher than those in primary operable breast cancer. As previous researches demonstrate that HIF-1a has been linked to aggressive phenotypes, we think that the main cause of high positive rate in local advanced breast cancer attributes to advanced tumor stage of local advanced breast cancer.[19,20] This explanation is supported by the findings that HIF-1α expression was significantly associated with lymph node status (P = .022), HIF-1α positive expression cases were more common in patients with high tumor stage and positive lymph node status. Previous evidence shows that HIF-1α is involved in breast tumorigenesis, growth, and metastasis.[21,22] In experimental models, hypoxia can independently stimulate the epithelial–epithelial–mesenchymal transition (EMT) program in breast cancer.[23–25] Our present study confirmed the significant over-expression of HIF-1α in local advanced breast cancer with positive lymph node status. This finding is very informative because it is crucial to identify the clinicopathological factors that may influence metastases to the lymph nodes in clinical.
In our study, the definition of pCR was determined according to the criterion established by M.D. Anderson trial: no invasive residual in breast or nodes; noninvasive breast residuals (ypT0/is ypN0). According to this criterion, the pCR rate was 18.6% in all patients in our study, which is relatively lower than reported in the literature. We think the main cause is that all the patients enrolled in our present study are local advanced breast cancer; stage III breast cancer accounts for 75.5% and 51.8% cases were N2 or N3 nodal status. This explanation is supported by the findings that the positive rate of HIF-1a in the present study was significantly higher than those in primary operable breast cancer. In many recent studies, pCR rates associated with NAC have been regarded as a surrogate endpoint for DFS and OS. Therefore, it is vitally important to identify intrinsic biomarkers not only in predicting the pCR rate of local advanced breast cancer populations but also in refining our understanding of breast cancer biology.
In the breast cancer phenotype system, pCR varied among tumor subtypes classified by the status of HR, HER2 and Ki-67.[26,27] In our study, the pCR rate according to subtypes were 0% (0/8) in the luminal A type, 15.7% (20/127) in the luminal B type, 26.0% (13/50) in the HER2 enrich type, 22.9% (8/35) in the triple negative type. The pCR rate of HER2 enrich and triple negative type is higher than luminal type breast cancer; however, there were no statistically significant differences. Most patients received 4 to 8 cycles, previous studies have also suggested that the number of chemotherapy cycles has an effect on pCR, so the number of established chemotherapy cycles is completed as much as possible in the study.
In this study, there were significant differences in HIF-1a positive and negative cohorts. Remarkably, the pCR rate was approximately 27.1% in HIF-1α negative expression patients, which is almost twice the pCR rate in HIF-1α positive expression cases. HIF-1α showed an important predictive value and has been associated with likelihood of good response to neoadjuvant chemotherapy. We also confirmed that loss of HIF-1α was correlated with better treatment response in local advanced breast cancer in multivariate logistic analysis according to tumor size, ER status, trastuzumab use, and HIF-1α expression. Our findings indicate that HIF-1α could further differentiate local advanced breast cancer into subtypes with different chemo-sensitivities.
Furthermore, we demonstrated the prognostic value of pretreatment HIF-1α expression in a cohort of breast cancer patients. Given that the survival of pCR responders had a favorable outcome than those who not received pCR, we specifically emphasized on the prognostic value of HIF-1α on the survival of patients who did not achieve pCR afte‘r neoadjuvant chemotherapy. Multivariate analysis demonstrated that the HIF-1a expression before NCT showed an independent prognostic value for RFS. Many previous studies have emphasized strong expression of HIF-1α is associated with aggressive features and poor prognosis of breast cancer.[28] These findings may be explained by the highly proliferative tumor biology of HIF-1a positive residual lesions of breast cancer after neoadjuvant chemotherapy.
The main limitations of using HIF-1α are the lack of standard measurement methods and consistent cut-off values. Other limitations of the present study are that it is a retrospective study from a single institute, and the patient number is small, especially the HIF-1a negative, further researches are needed to confirm the value of HIF-1a in larger patient cohort. Despite these limitations, HIF-1α measurement by IHC is still a low-cost method suitable for responses and prognosis prediction in local advanced breast cancer patients who are treated with NCT. Further prospective studies are needed to establish HIF-1α assessment as a reliable biomarker in daily clinical practice.
5. Conclusions
In conclusion, our study has demonstrated that HIF-1α expression is an important predictive biomarker for pCR in local advanced breast cancer who are treated with NCT. Loss of HIF-1α protein expression could indicate a favorable response to neoadjuvant chemotherapy. The HIF-1α expression status might help in further classifying local advanced breast cancer into subtypes with different responses to chemotherapy, and absent expression of HIF-1α could indicate a favorable prognosis in non-pCR patients. Further prospective studies aimed at determining whether HIF-1α expression can add additional value in tailoring additional systemic treatment strategies for HIF-1α positive local advanced breast cancer on a larger scale is worthwhile.
Author contributions
Conceptualization: Caiyun Nie, Xiaobing Chen.
Data curation: Caiyun Nie, liangyu bie, honglin hou.
Formal analysis: Caiyun Nie, Huifang Lv, Xiaobing Chen.
Funding acquisition: Xiaobing Chen.
Investigation: Caiyun Nie, Huifang Lv, liangyu bie, honglin hou.
Methodology: Caiyun Nie, Huifang Lv, honglin hou.
Project administration: Xiaobing Chen.
Resources: Caiyun Nie.
Supervision: Xiaobing Chen.
Writing - original draft: Caiyun Nie, Xiaobing Chen.
Writing - review & editing: Caiyun Nie, Xiaobing Chen.
Footnotes
Abbreviations: FISH = fluorescence in situ hybridization, HIF-1a = hypoxia inducible factor 1a, LABC = locally advanced breast cancer, NCT = neoadjuvant chemotherapy, pCR = pathological complete response, RFS = relapse-free survival.
This work was supported by the National Natural Science Foundation of China (No. 81472714).
Competing Interests: The authors have declared that no competing interests exist.
The authors have no conflicts of interest to disclose.
References
- [1].Haddad TC, Goetz MP. Landscape of neoadjuvant therapy for breast cancer. Ann Surg Oncol 2015;22:1408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abraham J, Robidoux A, Tan AR, et al. Phase II randomized clinical trial evaluating neoadjuvant chemotherapy regimens with weekly paclitaxel or eribulin followed by doxorubicin and cyclophosphamide in women with locally advanced HER2-negative breast cancer: NSABP Foundation Study FB-9. Breast Cancer Res Treat 2015;152:399–405. [DOI] [PubMed] [Google Scholar]
- [3].Van de Wiel M, Dockx Y, Van den Wyngaert T, et al. Neoadjuvant systemic therapy in breast cancer: challenges and uncertainties. Eur J Obstet Gynecol Reprod Biol 2016;210:144–56. [DOI] [PubMed] [Google Scholar]
- [4].Chen S, Huang L, Chen CM, et al. Progesterone receptor loss identifies luminal-type local advanced breast cancer with poor survival in patients who fail to achieve a pathological complete response to neoadjuvant chemotherapy. Oncotarget 2015;6:18174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dai ZJ, Wang XJ, Li ZF, et al. HER-2 expression in local advanced breast cancer and the efficacy of neoadjuvant chemotherapy regimens. J Southern Med Univ 2007;27:1397–9. [PubMed] [Google Scholar]
- [6].Lee JY, Choi JY, Lee KM, et al. Rare variant of hypoxia-inducible factor-1alpha (HIF-1A) and breast cancer risk in Korean women. Clin Chim Acta 2008;389:167–70. [DOI] [PubMed] [Google Scholar]
- [7].Wang F, Zhang Z, Wang Z, et al. Expression and clinical significance of the HIF-1a/ET-2 signaling pathway during the development and treatment of polycystic ovary syndrome. J Mol Histol 2015;46:173–81. [DOI] [PubMed] [Google Scholar]
- [8].Cai FF, Xu C, Pan X, et al. Prognostic value of plasma levels of HIF-1a and PGC-1a in breast cancer. Oncotarget 2016;7:77793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rodriguez-Enriquez S, Aguilar JL, Villarreal-Garza CM, et al. HIF-1a and target genes as metabolic biomarkers in Mexican women with breast cancer. J Clin Oncol 2011;29(15 Suppl):e21063. [Google Scholar]
- [10].Koukourakis MI1, Giatromanolaki A, Skarlatos J, et al. Hypoxia inducible factor (HIF-1a and HIF-2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res 2001;61:1830–2. [PubMed] [Google Scholar]
- [11].Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bardia A, Baselga J. Neoadjuvant therapy as a platform for drug development and approval in breast cancer. Clin Cancer Res 2013;19:6360–70. [DOI] [PubMed] [Google Scholar]
- [13].Simos D1, Clemons M, Ginsburg OM, et al. Definition and consequences of locally advanced breast cancer. Curr Opin Support Palliat Care 2014;8:33–8. [DOI] [PubMed] [Google Scholar]
- [14].Rockwell S, Dobrucki IT, Kim EY, et al. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med 2009;9:442–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2010;29:625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 1999;59:5830–5. [PubMed] [Google Scholar]
- [17].Chi JT, Wang Z, Nuyten DS, et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med 2006;3:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dales JP, Garcia S, Meunier-Carpentier S, et al. Overexpression of hypoxia-inducible factor HIF-1alpha predicts early relapse in breast cancer: retrospective study in a series of 745 patients. Int J Cancer 2005;116:734–9. [DOI] [PubMed] [Google Scholar]
- [19].Badowska-Kozakiewicz A, Sobol M, Patera J. Expression of hypoxia-inducible factor 1alpha in invasive breast cancer with metastasis to lymph nodes: correlation with steroid receptors, HER2 and EPO-R. Adv Clin Exp Med 2016;25:741–50. [DOI] [PubMed] [Google Scholar]
- [20].Bos R, Zhong H, Hanrahan CF, et al. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst 2001;93:309–14. [DOI] [PubMed] [Google Scholar]
- [21].Kozlova N, Wottawa M, Katschinski DM, et al. Hypoxia-inducible factor prolyl hydroxylase 2 (PHD2) is a direct regulator of epidermal growth factor receptor (EGFR) signaling in breast cancer. Oncotarget 2016;8:9885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang MH, Wu MZ, Chiou SH, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol 2008;10:295–305. [DOI] [PubMed] [Google Scholar]
- [23].Gao T, Li JZ, Lu Y, et al. The mechanism between epithelial mesenchymal transition in breast cancer and hypoxia microenvironment. Biomed Pharmacother 2016;80:393–405. [DOI] [PubMed] [Google Scholar]
- [24].Shiraishi A, Tachi K, Essid N, et al. Hypoxia promotes the phenotypic change of aldehyde dehydrogenase activity of breast cancer stem cells. Cancer Sci 2016;108:362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhou Z, Wang S, Song C, et al. Paeoniflorin prevents hypoxia-induced epithelial-mesenchymal transition in human breast cancer cells. Oncotargets Ther 2016;9:2511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Swisher SK, Vila J, Tucker SL, et al. Locoregional control according to breast cancer subtype and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast-conserving therapy. Ann Surg Oncol 2016;23:749–56. [DOI] [PubMed] [Google Scholar]
- [27].Yang Y, Im SA, Keam B, et al. Prognostic impact of AJCC response criteria for neoadjuvant chemotherapy in stage II/III breast cancer patients: breast cancer subtype analyses. BMC Cancer 2016;16:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li M, Xiao D, Zhang J, et al. Expression of LPA2 is associated with poor prognosis in human breast cancer and regulates HIF-1α expression and breast cancer cell growth. Oncol Rep 2016;36:3479–87. [DOI] [PubMed] [Google Scholar]


