Abstract
Cardiovascular CT (CCT) is an important imaging modality in congenital and acquired paediatric heart disease. Technological advances have resulted in marked improvements in spatial and temporal resolution of CCT with a concomitant increase in speed of data acquisition and a decrease in radiation dose. This has elevated CCT from being sparingly used to an essential diagnostic tool in the daily multimodality imaging practice alongside echocardiography, cardiovascular MR and invasive angiography. The application of CCT in paediatric congenital and acquired heart disease can be both technically and diagnostically challenging. This review highlights important considerations for current state of the art CCT across the spectrum of heart disease encountered in children.
Introduction
Cardiovascular CT (CCT) is an increasingly important imaging modality in congenital and acquired paediatric heart disease. Technological advances have resulted in marked improvements in spatial and temporal resolution with a concomitant increase in speed of data acquisition and a decrease in radiation dose.1–3 This has elevated CCT to an essential diagnostic tool in the daily multimodality imaging practice alongside echocardiography, cardiovascular MR (CMR) and invasive angiography.4 The application of CCT in paediatric congenital and acquired heart disease can be both technically and diagnostically challenging. This review highlights important considerations relating to technique and applications in current state-of-the-art CCT across the spectrum of paediatric heart disease. The review is based on a literature review and on our imaging experience as a tertiary and quaternary centre for paediatric cardiac imaging, paediatric cardiology and paediatric cardiac surgery.
Technique
Careful consideration of the clinical question along with the patient-specific physiology and anatomy are crucial steps that along with assessment of the age and maturity of the child help ensure that high quality CCT is obtained. These considerations must commence before the child enters the scanner room, and should continue while the child is in the room to ensure that the study is safe, diagnostic and performed at the lowest possible radiation dose.
Preparation
Awake neonates and very young children can often be made to lie still on the table following the use of an oral sucrose solution, a pacifier or a feed-and-wrap approach. For the youngest children, light immobilisation with an inflatable cushion and a belt is both helpful and safe. The need for sedation and general anaesthetic is declining with increasing speed of scanning.5 However, these measures may still be needed in the pre-school aged or non-compliant children who struggle to remain still during the scan or who are likely to fail to comply with breath holding instructions for longer acquisitions. School aged children tend to aptly comply with instructions but the maturity of each child should be assessed on a case-by-case basis, balancing the likelihood of a diagnostic and safe study against the risks associated with sedation and general anaesthesia.6
Contrast administration
Cannulation should be performed away from the scanner, so that the child is more likely to be calm in the scanner room and during image acquisition. A carefully chosen cannulation and injection site is essential for a diagnostic study by facilitating adequate contrast opacification of key structures. Different injection sites address diagnostic questions according to both the routes taken by the contrast bolus and the endoluminal concentration levels of the contrast medium at the time of imaging. Peripheral cannulas are preferable to central venous catheters. Contrast administration via a lower limb vein is generally preferred in neonates and smaller children. This administration route avoids streak artefact across the upper mediastinum as may occur when dense contrast is present in upper body systemic venous return with upper limb cannulation. This is a particularly important consideration in respect to achieving diagnostic image quality of the branch pulmonary arteries, the aortic root, vascular rings, anomalies of the aortic arch and major branch arteries, and surgical shunts from the aortic branch arteries to the pulmonary arteries (the “Blalock–Taussig”’ shunt). It can be helpful to elongate the contrast bolus in order to facilitate visualisation of the superior vena cava and its tributaries when contrast is administered into the lower limb. This can be achieved with contrast dilution as a single elongated bolus or by using an initial relatively slow contrast injection followed by a denser and/or more rapid contrast bolus. In some cases, it can be advantageous to add a saline chaser, and possibly a pause, between the two contrast boluses. The strategy depends on the total contrast dose available, the intravascular contrast levels needed, the route taken by the contrast bolus, and the estimated recirculation time. In the larger and older child when cannulation of the lower limb can be difficult to tolerate, a right hand cannula (as opposed to left hand cannulation) tends to minimise streak artefact across the mediastinal structures, and streak artefact across adjacent structures is less of a risk in the context of larger mediastinal structures and more mediastinal fat. A left upper limb cannula is sometimes preferred such as when there is a solitary left-sided superior vena cava with suspected unroofed coronary sinus.
A biphasic injection protocol will in general be appropriate, deploying a neat contrast bolus (1–3 ml kg−1) followed by saline chaser. A dose less than 2 ml kg−1 may give suboptimal intravascular opacification in the small child, especially when the venous access necessitates a low injection rate. Conversely, it can be considered to reduce the contrast dose below 2 ml kg−1 when the child weighs around 40 kg or more. A non-ionic low-osmolar iodinated contrast agent is preferable and the lowest possible total contrast dose should be used that achieves diagnostic levels of endoluminal opacification. Adverse reactions to contrast medium are infrequent and tend to be mild.7 Power injectors rather than hand injection are preferred for contrast administration because they can control the injection rate and have an excellent safety profile with the a pre-programmable maximum allowable pressure level according the cannula size.8 Particular care must be taken to avoid accidental injection of air along with contrast and saline solutions. This is due to the high incidence of intracardiac shunt lesions that may lead to paradoxical air emboli. The risk of contrast-induced nephropathy needs consideration when there is renal impairment, and hydration before and after contrast administration is recommended.9 A lower contrast dose may be considered in the newborn owing to a theoretically increased risk of contrast-induced nephropathy in an immature renal system.10 In neonates and low bodyweight children with small absolute contrast doses, a short transit time increases the risk of suboptimal opacification of essential structures. Using the full available contrast dose, reducing the injection rate, and mixing the contrast bolus with saline all increase the transit time. These initiatives reduce the risk of non-diagnostic studies. Empirically, dilution with saline to a 70–80% contrast concentration gives good opacification in most cases whilst extending the transit time of the contrast bolus and reducing the tendency to streak artefact. A biphasic protocol can also be adapted to a triphasic variant to extend the bolus further, by using either a lower injection rate or further dilution for part of the bolus prior to the saline chaser. This approach facilitates opacification of more systemic venous structures.
Simultaneous injection at two peripheral injection sites can be considered for systemic venous imaging, such as in children with total cavopulmonary connections or transposition of the great arteries following the atrial switch procedure.11 The lower limb injection should, particularly in larger children, start slightly earlier or have a relatively faster injection rate than the upper limb injection due to a longer central transit time from the injection site. Different injection strategies are needed for central venous lines due to early arrival of neat contrast into the cardiac chambers. This carries a higher risk of both central streak artefact and poor cardiovascular opacification due to rapid delivery of high concentrations and rapid transit of a concentrated contrast bolus. Depending on the size of the child, contrast dilution down to as much as 50% contrast may along with a lower injection rate be helpful. Similarly, injection strategies should be adapted to the altered physiologies, anatomies and distribution volumes such as will be encountered in ventricular assist and extracorporeal membranous oxygenation devices.12
Injection technique, contrast agent, scanner settings and patient specific factors are all important determinants of intravascular contrast levels (Table 1). Care should be taken with techniques such as dilution and lowering of injection rates when high intravascular opacification is required. High endovascular contrast levels also reduce the radiation dose needed for diagnostic quality imaging. “Packing” of the end of bolus with a saline chaser can be helpful to achieve higher intravascular concentration of particular vascular segments, but in complex cardiovascular connections and anatomies care should be taken to avoid saline causing non-diagnostic opacification of crucial vascular segments.
Table 1.
Factors impacting intravascular contrast opacification
| Contrast delivery factors |
| Disease specific factors |
| Technical factors |
Timing of acquisition
Automated bolus tracking is challenging in neonates and younger children where small vascular structures combined with a potential for patient movement increases the risk of erroneous triggering.13 A pre-scan timing bolus is often avoided because it utilises part of the contrast bolus available and increases the radiation dose. Some imagers prefer pre-determined timing of acquisition relative to the end of injection, but prediction of transit times is challenging in conditions such as venous obstruction, complex connections with shunting and perturbed cardiac output states. Visual bolus triggering by means a low-resolution monitoring scan is our favoured approach because it more reliably ensures opacification of the appropriate cardiac structures at the time of imaging. This does incur a small additional radiation dose, but this can be minimised by delaying monitoring as far as possible towards the end of the injection (as near to the likely timing of start of the scan as possible) and reducing the frequency of monitoring scans. Care should be taken to always have sufficient monitoring scans available for cases where central delivery of contrast medium is delayed. The timing of triggering of the acquisition must take into account the expected delay caused by table movement and breath-holding instruction to the actual start of data acquisition. Data acqusition must also be timed for optimal enhancement of all essential components for longer acquisitions when the data acquired over multiple heartbeats, at lower scan pitch or with extended z-axis coverage.
Scan protocol
Most studies are fully diagnostic without electrocardiography (ECG)-gating. ECG-triggered and ECG-gated acquisitions only need to be utilised when cardiac motion may result in non-diagnostic images.14 Newer generation scanners with high pitch spiral and volumetric scan modes permit rapid image acquisition at high temporal resolution with minimal artefact from cardiac and breathing motion, and they have reduced the need for ECG-gated scans, breath-holding and general anaesthesia.5, 15 Retrospective and prospective sequential ECG-gated protocols not only increase the radiation dose but also increase the acquisition time. Where possible, scans should be carried out at end-inspiration to completely eliminate non-cardiac motion of the cardiovascular structures and to maximise visualisation of the airways and lungs. Multiheartbeat acquisitions in the form of retrospective and prospective sequential scan protocols result in longer scan time for the same z-axis coverage. In our experience, this increases the risk of breathing artefact in children who struggle to breath-hold, and this becomes an increasing challenge as the z-axis scan length is extended. It has, however, been hypothesised that breathing artefact does not cause significant degradation of image quality for larger cardiovascular structures due to reduced diaphragmatic excursion in young children.16 Irrespective, general anaesthesia may still be needed to control breathing when detailed imaging of smaller structures such as the coronary arteries is required.
All scans must give the lowest possible radiation dose for a diagnostic scan.17 For many paediatric cardiac applications, the newest generation scanners reach effective radiation doses of less than 1 mSv.18 Automated dose modulation functions and iterative reconstruction algorithms reduce radiation doses whilst maintaining diagnostic image quality. However, even when automated measures are in place the principal determinants of radiation dose must always be critically appraised prior to each study, including tube current, tube potential, pitch, slice collimation and scan length.19–21 Besides using the scanner specific automatic technologies, imagers should focus on both reducing radiation doses and eliminating the risk of non-diagnostic studies through careful attention to technical factors (Table 2).17, 22 The highest possible pitch and table movement speed should always be used. A weight-based algorithm should be adapted for tube current settings where possible, and automatic exposure control techniques reduce doses significantly. A tube potential in the range of 70–80 kVp is appropriate for most paediatric body sizes.20 Higher kVp levels are only needed in the largest children, for metallic foreign bodies or with dense contrast delivery when streak artefacts may otherwise occur. Importantly, high kVp imaging not only increases radiation dose but also results in smaller relative contrast differences between tissues.23–25 Familiarity with the scanner settings is important because both ECG-gated and ECG-triggered protocols may have adaptive settings that expand the exposure beyond the set intervals when RR-intervals vary. This may be minimised by over-riding or restricting any such padding, which will of course also restrict the ability to eliminate cardiac motion through reconstructions for different time points in the cardiac cycle.26 ECG-triggered prospective sequential and retrospective ECG-gated imaging should always be performed with the narrowest possible acquisition window and padding. On newer generation scanners, the padding may even be avoided and with this approach, radiation doses start to approach those of non-ECG-gated CCT studies whilst minimising cardiac motion artefact. Heart rate reduction may reduce radiation dose by enabling lower dose ECG-triggered and ECG-gated protocols.
Table 2.
Technical and procedural factors that ensure diagnostic quality studies at the lowest possible radiation dose
| Preparation |
| Centre the child with their arms above the head |
| Cannulation site: according to indication, physiology and anatomy |
| Heart rate reduction: if cardiac motion is likely to give a non-diagnostic study |
| Avoid motion: practice table movement and breath holds (awake older children), gentle immobilisation (awake younger children), or consider sedation and general anesthesia (non-compliant children) |
| Remove foreign bodies where possible |
| Protocol |
| Essential z-axis scan coverage only |
| Non-ECG-gated high pitch and high table speed scanning wherever possible |
| Enable automated dose modulation strategies, including adjustment of kVp and mAs according to body size |
| Prospective ECG-gated scan: apply minimum ECG padding |
| Retrospective ECG-gated scan: apply ECG-gated tube current modulation and narrowest possible acquisition window |
| Use iterative reconstruction |
| Acquisition |
| Optimise contrast bolus to visualise all relevant structures |
| Visual bolus triggering: latest possible monitor start and lowest possible frequency |
| Avoid multiphase scanning |
ECG, electrocardiography.
Applications
CCT has become increasingly used in paediatric heart disease over the last decades. The shift in practice has been driven by improvements in temporal and spatial resolution that have coincided with an improved radiation exposure profile and ultrarapid acquisition of isotropic volumetric data sets with submillimetre spatial resolution. This progress has improved the diagnostic accuracy of CCT for paediatric heart disease, whilst making CCT available to a larger cohort of both ambulatory and critically ill children who may now safely have diagnostic studies performed without sedation or general anaesthesia.
The comprehensive three-dimensional (3D) nature of CCT makes it an important complement to echocardiography, which remains the first-line imaging modality in children with suspected congenital or acquired heart disease. CCT does not face the limitations of echocardiography in which acoustic windows, as defined by the bony thoracic cage and lung cover over the heart, or metallic devices and implants may hinder adequeate visualisation of cardiovascular structures. Restictions by acoustic windows become more prnounced as the child grows. CCT is less affected than CMR by metallic objects that may limit visualisation of a device itself and the surrounding structures, and some devices are not compataible with the CMR environment. Invasive angiography has long been the gold-standard for imaging of the pulmonary arterial and venous system. CCT has, however, become the modality of choice over invasive angiography in many cases owing to a less invasive nature, low radiation dose and high diagnostic accuracy,. This is, especially the case when the radiation free CMR is not tolerated and/or high spatial resolution anatomical data is crucial. Of course, neither CCT nor CMR offer the opportunity to measure pressures or intervene such as can be done at invasive angiography with cardiac catheterisation. Finally, CCT provides a unique opportunity for comprehensive assessment of the mediastinum, lungs, airways, pleural space, and thoracic cage, which is important in a cohort where extracardiac abnormalities are common and may both contribute to symptoms and define outcomes.
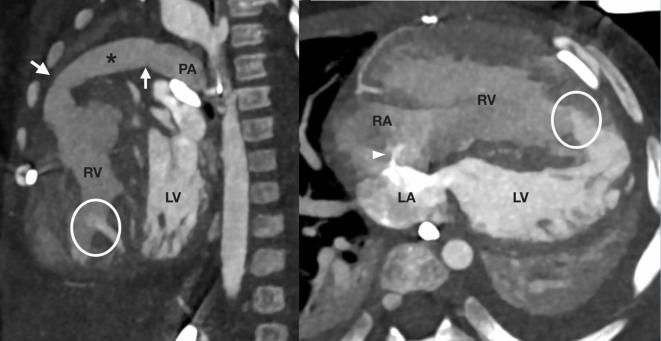
CCT can provide functional imaging of the cardiac chambers and valves. However, the temporal resolution is inferior to both echocardiography and CMR in the often fast heart rates encountered in children with a short peak systolic phase. In addition, the volumetric data obtained with CCT cannot be internally validated by flow measurement as can be done in CMR. CCT may also visualise native and implanted cardiac valves but the temporal resolution is, again, inferior to both echocardiography and CMR at oten high heart rates. CCT also cannot visualise the flow jets that occur with stenosis and regurgitation, which aid in the non-invasive severity grading of valvular heart disease with echocardiography and CMR. Furthermore, functional imaging with CCT needs retrospectively ECG-gated protocols with a relatively high radiation dose. CCT should therefore only be used as a last resort for functional assessment. However, even though no dynamic data is usually acquired, assessment of each study should factor in understanding of cardiovascular physiology and function in order to extract as much information as possible from each study. Knowledge of contrast administration pathways and physiology is crucial to understanding shunts and other anomalous cardiovascular connections (Figure 1).
Figure 1.
A 12-month-old infant with a failing RV after a truncus arteriosus repair referred for assessment of pulmonary blood flow. The surgically placed and purposely restrictive conduit from the RV to the PAs has mild proximal and distal calibre reduction (arrows). The presence of left to right shunting across multiple muscular ventricular septal defects (circles) supports an only mild degree of functional restriction to PA flow in the context of an unobstructed left ventricular outflow tract. A fenestrated interatrial septum shunts in the left to right direction (arrowhead). Contrast medium was administered into the left-sided chambers via a left superior vena cava to an unroofed coronary sinus, leading to higher contrast in the LA and LV compared with the RA and RV. This facilitated directional assessment of the atrial and ventricular level shunts. Note left lower lobe collapse and left pleural effusion with less severe right-sided changes. LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle.
Complex heart disease
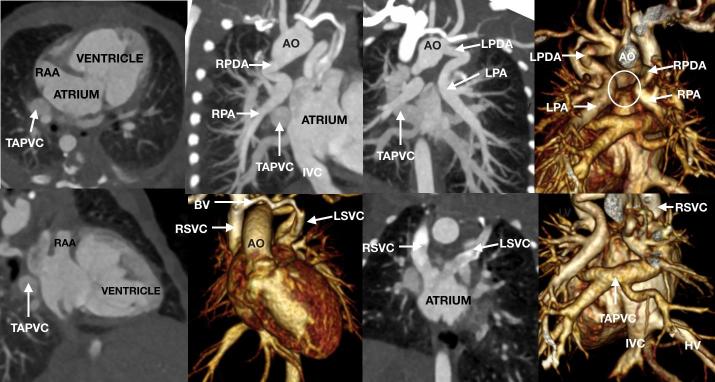
It is often challenging to gain a complete anatomical overview using echocardiography in neonates and children with complex congenital heart disease—in particular, when it comes to the extracardiac vascular anatomy. CCT provides comprehensive 3D assessment that can guide clinical decision-making. A non-ECG-gated high-pitch protocol provides excellent delineation of extracardiac vascular structures and may also provide useful information regarding the intracardiac anatomy (Figure 2). The cannulation site is an essential consideration prior to starting the scan, and a relatively long bolus should be utilised that provides opacification of all essential structures with adequate contrast levels for diagnostic purposes. The upper abdomen should be included in complex congenital heart disease such as atrial isomerism when the systemic venous return and positioning of abdominal organs are important for complete diagnosis. If ultrasound has already reliably identified the upper abdominal structures then a more limited z-axis scan length may be considered to reduce the radiation dose.
Figure 2.
A 4-day-old neonate referred for anatomical assessment of suspected complex congenital heart disease. The heart is in the left hemithorax with the apex to the left. There is right atrial isomerism with bilateral RAAs. The atrium is effectively common due to a large interatrial communication. A single atrioventricular valve connects the atria to a single ventricle of indeterminate morphology. The single ventricle gives rise to the AO and a right-sided aortic arch that has a mirror image branching pattern. There is pulmonary atresia with an absent central pulmonary arterial confluence (circle). Both the LPA and RPA were unobstructed and independently supplied by a LPDA and RPDA, respectively. There is unobstructed supracardiac total anomalous pulmonary venous return via a common confluence (TAPVC) to a RSVC. The RSVC goes to the right-sided atrium and a LSVC goes to the left-sided atrium via an unroofed coronary sinus. A small BV is present. The IVC and HV drain to right-sided atrium. The liver was midline, the stomach was on the right, and there was bronchial situs inversus. AO, aorta; BV, bridging vein; CCT, cardiovascular CT; HV, hepatic veins; IVC, inferior vena cava; LPA, left pulmonary artery; LPDA, left patent arterial duct; LSVC, left superior vena cava; RAA, right atrial appendage; RPA, right pulmonary artery; RPDA, right patent arterial duct; RSVC, right superior vena cava.
Thoracic aortic disease
A major indication for CCT is the diagnosis and monitoring of thoracic aortic disease. A biphasic protocol is suitable for anomalies of the thoracic aorta and the major branch arteries (Figures 3–5),27 and a relatively narrow contrast bolus triggered for maximum aortic opacification may be used. However, opacification of both the aorta and pulmonary arterise should be sought when the assessment is for a vascular ring. A lower limb or right arm injection is often preferred in order to avoid streak artefact across the thoracic aorta and the major branch arteries from high concentration contrast in SVC and its tributary veins. For detailed aortic root assessment, it is often necessary to ECG-trigger or ECG-gate to avoid cardiac motion that may simulate dissection or hinder accurate diameter measurements (Figure 6). Cardiac motion is very limited beyond the aortic root and rapid scanning with high temporal resolution will suffice for the remaining thoracic aorta.28
Figure 3.
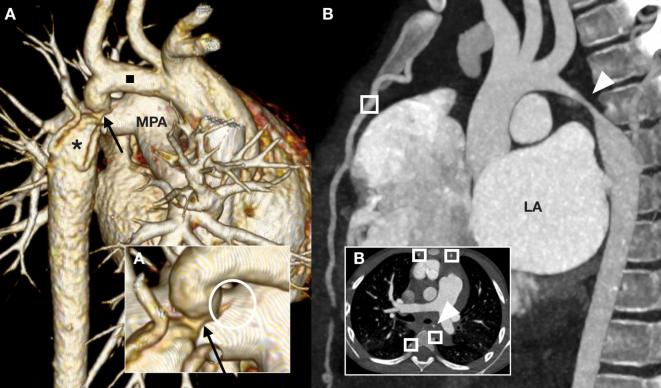
A 23-month-old toddler (A) referred for anatomical assessment prior to coarctation repair. The left-sided transverse aortic arch is hypoplastic (black square) with severe juxtaductal coarctation (arrows), post-stenotic dilation (asterisk) and collaterals formation. At the coarctation site the ligamentous arterial duct (circle) tethers the isthmic aorta towards the MPA. A 5-year-old child (B) referred for vascular, mediastinal and pulmonary assessment after presenting in heart failure with fever and raised inflammatory markers. Severe long segment narrowing is present at the aortic isthmus (arrowhead) with wall thickening and collaterals (squares). Note the severely dilated LA. Surgical biopsy established the diagnosis of Takayasu arteritis. LA, left atrium; MPA, main pulmonary artery.
Figure 4.
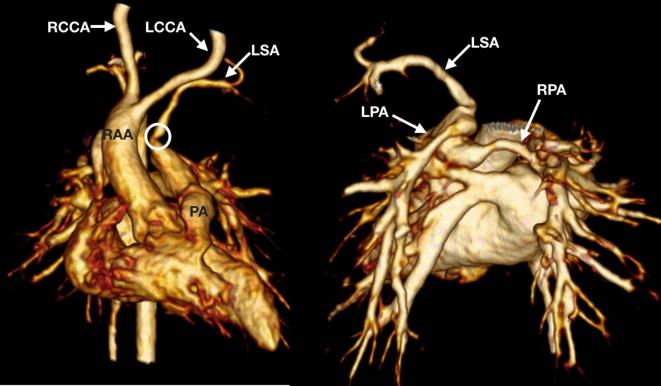
A 3-month-old infant with 22q11 microdeletion awaiting complete repair for tetralogy of Fallot referred for assessment of a possible left-sided patent arterial duct. An isolated LSA arises from the distal pulmonary trunk and there is a RAA. The isolated LSA is narrowed at the origin (circle). The LCCA, RCCA and right subclavian arteries arise seperately from the transverse aortic arch. The origins of the confluent branch pulmonary arteries are abnormal with a rotated and superioinferior orientation—the LPA originates above the RPA consistent with a pattern often seen in 22q11 mircodeletion. Both branch pulmonary arteries were small calibre. LCCA, left common carotid artery; LPA, left pulmonary artery; LSA, left subclavian artery; RAA, right-sided transverse aortic arch; RCCA, right common carotid artery; RPA, right pulmonary artery.
Figure 5.
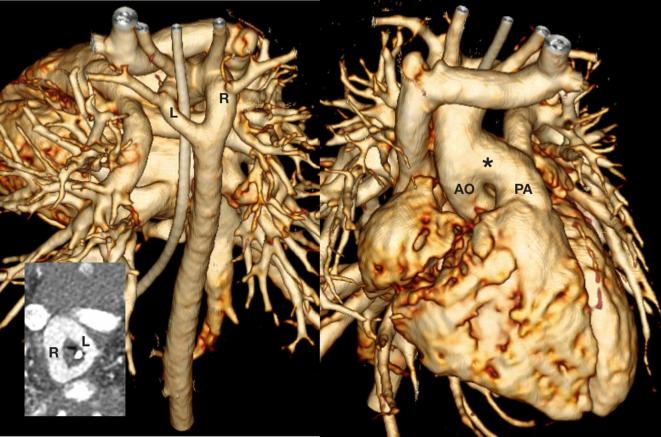
An 8-day-old neonate referred for surgical planning for a known double aortic arch. There was a large aortopulmonary window (asterisk) connecting the AO to the PA. The double aortic arch has a dominant right arch (R) and a smaller left (L) transverse arch. There is severe tracheal compression from the vascular ring (insert). AO, aorta; PA, pulmonary artery.
Figure 6.
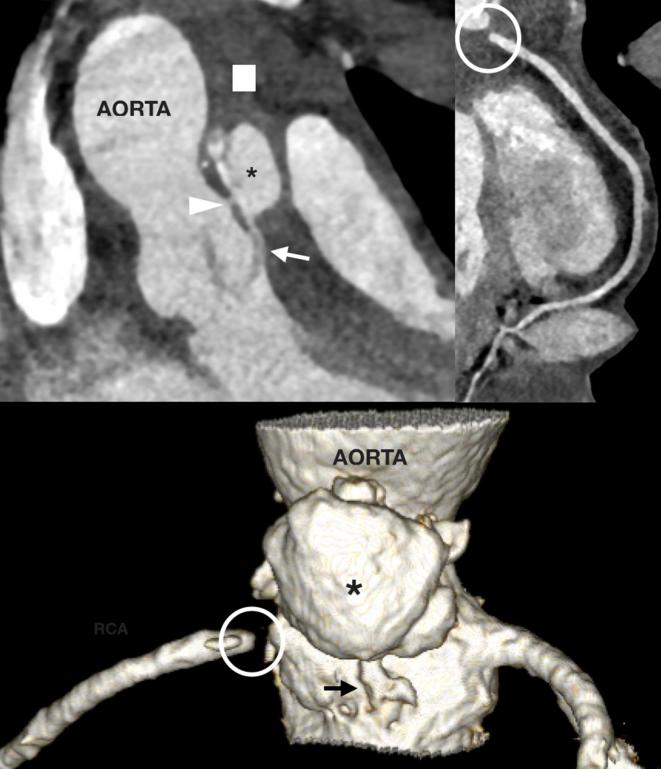
A 16-year-old teenager with prior aortic valve surgery referred for aortic root and mediastinal assessment due to new sepsis. A large aortic root abscess (asterisk) associates with extensive fat stranding and lymph node enlargement (square). The abscess has fistulous connections to the aortic sinus (arrowhead) and subvalvar left ventricular outflow tract (arrow). The RCA was proximally obstructed (circle) due to a tethered and thickened right coronary leaflet of the aortic valve. RCA, right coronary artery.
Coronary arterial disease
Coronary arterial imaging is the principal paediatric indication for ECG-triggered and ECG-gated protocols. The clinical indication and heart rate together determine the optimal protocol. CCT provides high-quality images for anomalies of origin and course in both congenital and acquired coronary arterial disease, and following surgical manipulation of the coronary arteries.29–31 CCT avoids potential issues relating in cannulation of anomalous coronary arterial origins at invasive angiography and spasm at vessel cannulation, and CCT rapidly provides 3D images that map out entire diseased or anomalous coronary arterial anatomies.18, 32,33 Pharmaceutical heart rate reduction improves coronary artery visualisation through reduced cardiac motion artefact, and also reduces radiation dose by enabling less radiation heavy protocols.34, 35 The scan can, however, still be adversely affected by frequent occurring sinus arrhythmia or a reflex rise in heart rate during contrast injection. Single heartbeat scanning tends to suffice for surgical planning, when anatomical information regarding origin and course of the coronary arteries is required (Figure 7). This applies especially to high temporal resolution scanners.i
Figure 7.
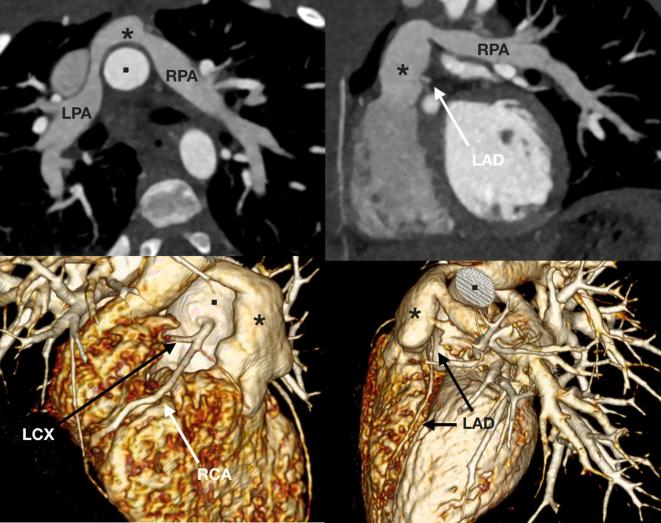
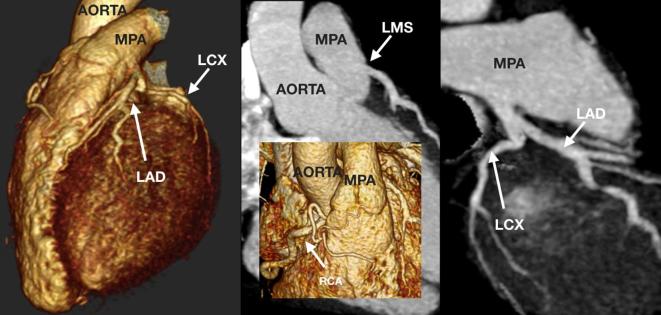
A 22-month-old toddler referred for assessment of the branch pulmonary arteries with signs suggestive of high afterload on the right ventricle. The child was born with transposition of the great arteries for which an arterial switch procedure was performed, and following the Lecompte maneuver the branch pulmonary arteries (LPA and RPA) course on either side of the neo-aortic root. The neopulmonary trunk (asterisk) is unobstructed with moderate bilateral branch pulmonary artery stenosis. There is also an anomalous LCX from the proximal RCA, and the LAD arises in the space between the neopulmonary trunk (asterisk) and aortic root (square). LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LPA, left pulmonary artery; RCA, right coronary artery; RPA, right pulmonary artery.
The heart rate determines the scan protocol when detailed luminal coronary arterial imaging is needed. The three available scan modes are in order of increasing ability to isolate cardiac motion and increasing radiation dose: ECG-triggered “single heartbeat”, ECG-triggered prospective “sequential”, and ECG-gated retrospective protocols. Heart rates often remain relatively high in the paediatric cohort, despite pharmacological heart rate reduction, and the retrospective scan mode may be needed. However, for elevated heart rates the retrospective scan protocol may not provide sufficient temporal resolution—and non-diagnostic imaging at high radiation doses should always be avoided. Sinus arrhythmia is an issue in children, but this may be minimised through initiating early breath-holding when contrast is in the pulmonary arterial system. In very low heart rates, a high pitch scan mode can provide diagnostic ECG-triggered images in a single heartbeat. The scan is acquired from a pre-defined time of the cardiac cycle as a one continuous acquisition along the z-axis. The continuous mode means that cardiac motion becomes an issue with increasing heart rate. On some scanners, this scan mode can be combined with non-gated whole thorax imaging so that the part of the z-axis that covers the coronary arteries is acquired starting from a pre-defined phase of the RR-interval. The sequential scan protocol is preferred at relatively higher heart rates.36 As opposed to the single heartbeat scan, the sequential scan acquires a segment of the z-axis that corresponds to the coverage of the detectors before moving to acquire the next z-axis segment. Each segment is acquired at the same pre-defined phase of the cardiac cycle. Owing to the necessary movement between acquisitions, this scan is performed every second heartbeat (“step and shoot”). This increases the acquisition time compared with the single heartbeat approach. An important advantage over the single heartbeat scanning is that all segments of the z-axis are imaged for the same phase, which makes this scan mode better for comparatively higher rates. When using the sequential mode, a systolic imaging window (at 40%of the RR interval) is better for motion-free coronary imagingat, higher heart rates wheras diastole (at 70%of RR-interval) is better for relatively lower heart rates.37 This mainly applies to delineation of origin and course because the systolic phase often has too much motion for high diagnostic quality luminal imaging of the entire artery at higher heart rates. ECG “padding” can be applied to sequential scanning. This means acquiring, for instance 5% of the cardiac cycle before and after the pre-defined time point, which allows image reconstruction for any point within this time interval and is helpful if there is RR interval variability. Padding increases the likelihood of diagnostic data in arrhythmia and at slightly higher heart rates, but also means a higher radiation dose. Similar reconstructions are not possible without “padding” or for the single heartbeat scan mode. If the heart rate is higher, then retrospectively ECG-gated scans are needed (Figures 6, 8 and 9). This mode enables reconstructions for more phases in order identify the phase of minimum cardiac motion.29 In this scan mode, the data is acquired using a relatively low pitch scan with ECG co-registration and oversampling. The data is then retrospectively grouped into the desired phases of the cardiac cycle, and the data can be reconstructed for different phases of the cardiac cycle. The principal consideration of retrospective gating is radiation dose, and ECG dose modulation should be utilised to decrease dose outside the pre-selected ECG scan intervals. Owing to the often slightly different indications for CCT of the coronary arteries, the heart rate thresholds that apply to adults for the different protocols may be taken as a guide.38 However, the strategies will often differ from adults because children have different size coronary arteries, are typically affected by other diseases, and have with specific issues relating to breath-holding and arrhythmia that should impact the diagnostic strategies. Thus, it may be preferable for dedicated coronary imaging to be performed at institutions with paediatric experience.
Figure 8.
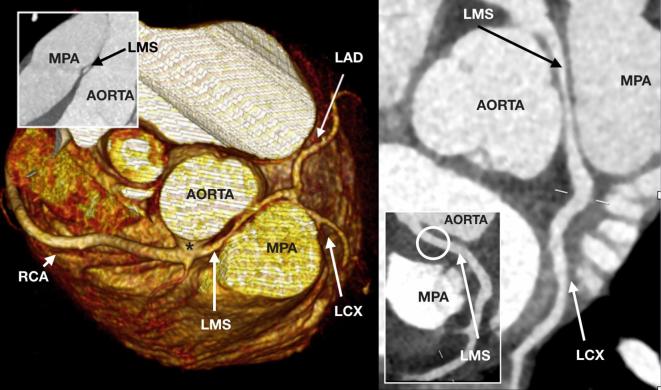
A 15-year-old teenager referred for coronary arterial assessment following out-of-hospital cardiac arrest with ventricular fibrillation. The coronary arteries originate from a single broad ostium (asterisk) on the right coronary sinus. The LMS has long segment narrowing over a long interarterial course between the aorta and MPA. The LMS regains better calibre prior to dividing into the normal LAD and LCX. The RCA is unobstructed. Following surgical translocation of the MPA, the LMS was unobstructed (circle). LMS, left main stem; MPA, main pulmonary artery; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery.
Figure 9.
A 17-year-old teenager referred for coronary arterial assessment following out-of-hospital cardiac arrest with ventricular fibrillation. A left coronary artery originates with a LMS from the MPA, and bifurcates normally into the LAD and LCX. This anomaly is called ALCAPA. The dominant and dilated RCA originated normally. The shunt from the ALCAPA had caused dilation of the left ventricle and left atrium. ALCAPA, anomalous left coronary artery from the left coronary artery; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LMS, left main stem; MPA, main pulmonary artery; RCA, right coronary artery.
A biphasic contrast administration protocol is generally the method of choice for coronary arterial CCT, and it is helpful to use a chaser in which the contrast has been diluted to a 20% contrast and 80% saline solution. This facilitates smooth opacification of the heart chambers and reduces artefact. Compared with imaging in the acquired coronary heart disease, setting a longer contrast bolus is needed for adequate delineation of cardiovascular anatomy and anomalous connections (Figure 8). For suspected anomalous coronary arterial origins the scan range should be expanded to include the ascending aorta and pulmonary arteries (Figure 9). Nitroglycerine may optimise visualisation of the distal coronary arteries, but the value in terms of sensitivity and specificity has not been demonstrated in children.
Pulmonary arterial disease
Non-ECG-gated CCT is the standard protocol for imaging of the pulmonary arterial system (Figures 7 and 10). An elongated contrast transit time in a biphasic injection protocol is often needed, balancing intravascular contrast levels against a transit time that allows contrast to reach all potential sources of pulmonary blood supply. This is particularly important when the usual forward flow across the pulmonary valve is absent in pulmonary atresia or if there are additional sources of blood flow to the lung (Figures 11 and 12). In these cases, perfusion from systemic sources should be opacified prior at the scan acquisition whilst good opacification of any conventional pulmonary arterial system should be maintained. The scan range should include the upper abdomen for identification of infradiaphragmatic aortopulmonary collaterals along with the lower neck for the proximal head and neck arteries for collaterals arising from here. CCT has good diagnostic accuracy for aortopulmonary collaterals compared with invasive angiography.39 When attempting to exclude pulmonary thromboembolic disease, it is important for the contrast bolus to be delivered relatively rapidly and without dilution to the pulmonary arteries.
Figure 10.
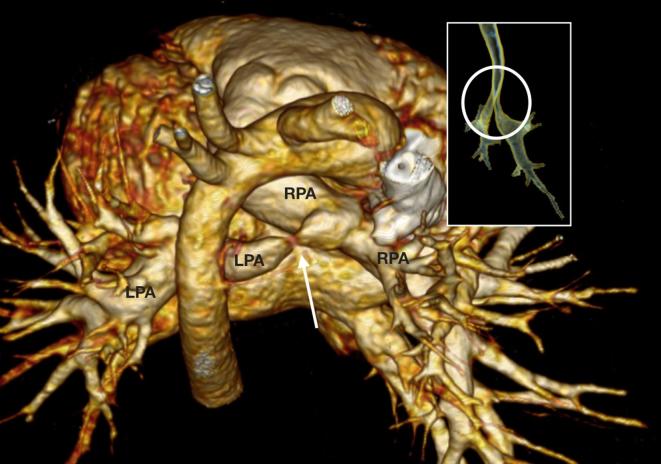
A 27-month-old toddler referred for assessment of the pulmonary arteries following repeated chest infections with suspected airways disease. There is a LPA sling in which the LPA originates from the RPA and courses behind the distal trachea towards the left hilum. There is severe proximal narrowing of the LPA sling (arrow) and mild calibre reduction of the distal trachea and proximal main bronchi (circle). LPA, left pulmonary artery; RPA, right pulmonary artery.
Figure 11.
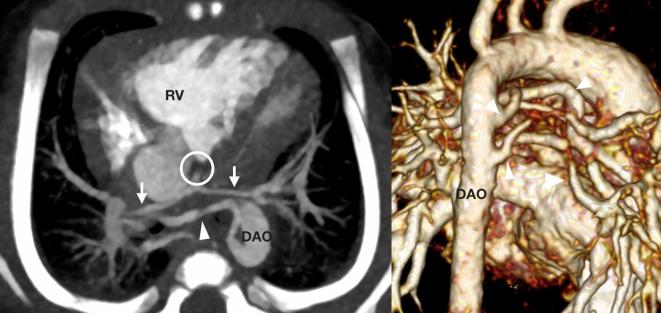
A 27-day-old infant with a pre-natal diagnosis of pulmonary atresia referred for assessment of the pulmonary arteries and the source of pulmonary blood flow. There was pulmonary atresia with an absent connection from the RV to the central pulmonary arterial system (circle). The central pulmonary arteries were present but severely hypoplastic (arrows). There were multiple tortuous and stenosed major aortopulmonary collaterals (arrowheads) to both lungs from the DAO. These collaterals communicated with the central pulmonary arterial system. DAO, descending aorta; RV, right ventricle.
Figure 12.
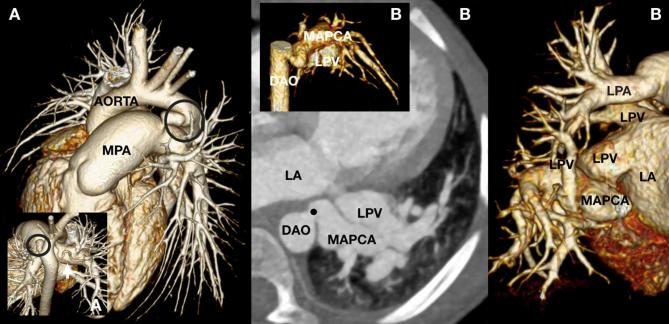
An 8-year-old child (A) referred for baseline investigation of known pulmonary hypertension. There is a patent arterial duct (circle) with a restrictive segment at the MPA end. The duct was deemed suitable for percutaneous stenting in order to promote right to left shunting. Consistent with severe pulmonary hypertension the pulmonary arteries are severely dilated and there is collateral pulmonary blood supply via hypertrophied bronchial arteries (arrow). A 27-month-old toddler (B) with a dilated LA and left ventricle referred for diagnosis of potential left to right shunting lesions. A severely dilated MAPCA (dot) arises from the distal DAO to supply the left lower lobe. The left lower lobe of lung has normally draining LPV to the LA and a conventionally connected bronchial tree. The LPA, LPV and large airways of the left upper lobe iwere conventionally connected. DAO, descending aorta; LA, left atrium; LPA, left pulmonary artery; LPV, left pulmonary vein; MAPCA, major aorto pulmonary collateral artery; MPA, main pulmonary artery.
Pulmonary venous disease
CCT is useful for assessment of anomalous pulmonary venous connections and for the diagnosis of obstruction to native or surgically reimplanted pulmonary venous pathways. Mostly, non-ECG-triggered or non-ECG-gated protocols are fully diagnostic (Figure 13) but cardiac motion may lead to erroneous diagnosis of pulmonary venous obstruction. If there is cardiac motion on CCT, then it is important to look for ancillary signs of pulmonary venous obstruction such as dilation of the vein downstream from a suspected stenosis, interlobular septal thickening in the drained lung and collateral venous drainage pathways. Correlation with echocardiography is also important. Prospective ECG-triggered sequential CCT can be considered when there is diagnostic doubt and invasive angiography is not justified. All CCT for pulmonary venous should ensure that there has been ample time for delayed filling of venous collaterals and slow flow segments—a protracted bolus with a biphasic contrast injection often suffices. Demonstration of anomalous pulmonary venous connections are reliably performed with CCT (Figures 13 and 14) and 3D volume-rendered reconstructions are helpful.40 When planning the contrast injection, care should be taken to avoid very dense contrast causing streak artefact and obscuring anomalous entry points into the systemic venous system. This may be achieved by delivering contrast through a lower limb in supracardiac types and an upper limb in infracardiac types of anomalous pulmonary venous connections. Contrast dilution can also assist in minimising streak artefact. The scan should include the liver and hepatic inferior vena cava in suspected infradiaphragmatic anomalous pulmonary connections (Figure 14).
Figure 13.
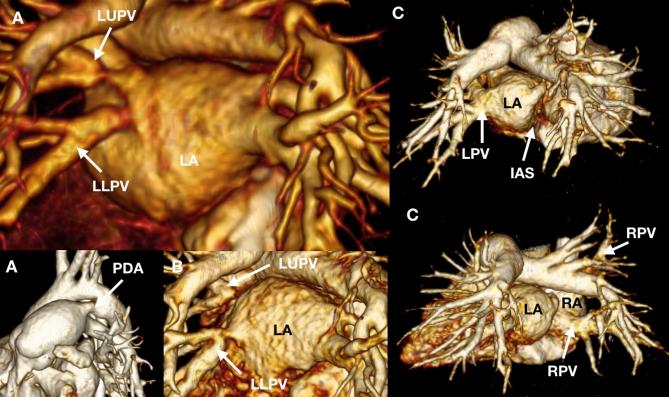
A 6-month-old infant (A) referred for assessment of the optimal closure strategy for a known PDA. The PDA is suitable for percutaneous device closure. There is also moderate LUPV and mild LLPV stenosis at the LA confluence. A follow-up CCT (B) shows progressive subtotal LUPV and moderate LLPV stenosis. A 4-month-old infant (C) referred for assessment of pulmonary venous anatomy. The pulmonary venous drainage is abnormal with an ipsilateral drainage pattern of the LPVs to the LA and hemi-anomalous return of the RPVs to the RA. Consistent with this, the RPVs do not cross the plane of the interatrial septum (IAS) to go to the LA. LA, left atrium; LLPV, left lower pulmonary vein; LPV, left pulmonary vein; LUPV, left upper pulmonary vein; PDA, patent arterial duct; RA, right atrium; RPV, right pulmonary vein.
Figure 14.
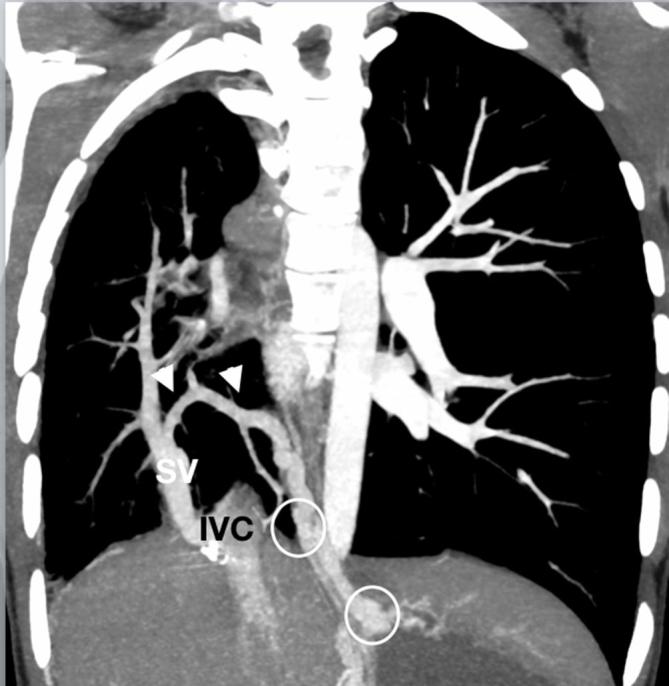
A 16-year-old teenager referred for assessment of the pulmonary vasculature and lung parenchyma after new haemoptysis on a background of scimitar syndrome, hypoplastic right pulmonary artery system and prior coil embolisation of an infradiaphragmatic major aortopulmonary collateral artery to the right lung. The SV drains to the IVC with a venous collateral connection (arrows) from the small right-sided pulmonary veins to the venous plexuses around the distal oesophagus and gastric fundus (circles). Sparse distal pulmonary arterial vasculature is present due to right-sided pulmonary arterial hypoplasia. IVC, inferior vena cava; SV, scimitar vein.
Systemic venous disease
CCT in systemic venous anomalies and diseases is challenging (Figures 2 and 15). Simultaneous dual-site contrast administration or recirculation protocols are often needed and should utilise the highest possible contrast dose and the latest possible imaging in order to reduce the risk of non-visualisation of the systemic venous connections and any venous collaterals. Contrast dilution can be helpful to create homogenous contrast density with a decreased risk of streak artefact for first pass imaging of the systemic venous system, but admixture from veins carrying unopacified blood remains a diagnostic challenge that may simulate endoluminal filling defects. If entire segments of the system venous anatomy needs to be mapped, such as for the diagnosis of interrupted inferior vena cava where both the upper and lower body venous anatomy should be visualised, then multiphase recirculation protocols can be informative. Here, an initial contrast bolus is allowed recirculation time prior to imaging during the delivery of a second contrast bolus. The dead space of the line connector needs consideration especially for smaller total contrast boluses, and a saline chaser should follow the initial contrast bolus to flush all contrast into the circulation. A second contrast bolus and saline chaser then follow after a “recirculation” pause. This technique requires an individualised approach, because the length of the pause depends on factors such as the patient size, injection site, injection rate, and total contrast bolus available. In this type of imaging bolus, monitoring should be performed for a site that sees both the recirculating and first pass volumes. As for any recirculation imaging, the acquisition parameters should take into account that the peak intravascular contrast concentration will inevitably be lower for some parts of the venous system than during first pass imaging.
Figure 15.
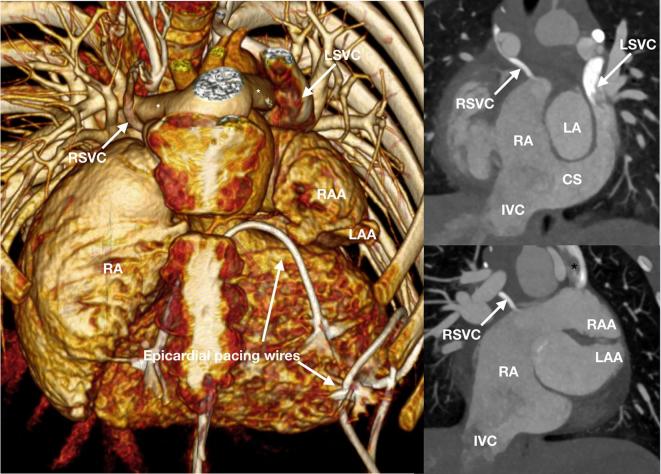
A 7-year-old child referred for assessment of the systemic venous anatomy prior to replacement of epicardial pacing system after a pacing lead fracture. A rudimentary RSVC drains to the RA after receiving a small azygos vein and connecting with a prominent paravertebral collateral venous plexus. A large LSVC receives a dilated hemiazygos vein, draining to the RA via a dilated CS. There is juxtaposition of the RAA that is to the left and on top of the LAA. The branch pulmonary arteries (asterisk) course around aorta following the Lecompte maneuver performed for surgical correction of transition of the great arteries. CS, coronary sinus; IVC, inferior vena cava; LAA, left atrial appendage; LSVC, left superior vena cava; RA, right atrium; RAA, right atrial appendage; RSVC, right superior vena cava;
When the systemic veins are baffled to the pulmonary arteries in univentricular palliation treatments, it can be particularly difficult to achieve homogenous venous and pulmonary contrast opacification.41, 42 Here, the superior vena cava is first connected to a branch pulmonary artery, and this is followed by forming a connection of the inferior vena cava to the branch pulmonary artery. Dual site injections may be helpful using combined upper and lower limb injections.11 Early imaging (even with two cannulas) often produces streaming and it can be difficult to predict recirculation times for delayed imaging in the context of low cardiac output states, impaired ventricular function and collateral circulations. Ultimately, a second delayed scan may be needed, although such scanning with added radiation exposure should generally be avoided. The systemic and venous atrial baffles in atrial switch procedures are also challenging and may require long contrast boluses, dual site injections or recirculation protocols. However, this operation is now infrequent in children and such baffled venous anatomies are mainly encountered in the rare double switch operations for congenitally corrected transposition of the great arteries.
Implants and devices
CCT is particularly helpful for assessment following implantation of devices and stents due to the ability to comprehensively image the cardiovascular system with less extensive artefact when compared with CMR. CCT may also provide better views of and around implants than echocardiography (Figure 16). This includes assessment of complex paediatric patients with ventricular assist or extracorporeal membrane oxygenation devices, where CCT helps diagnose complications relating to both the primary disease and to any procedures and device related complications such as endocarditis, clots and collections (Figure 17).43 Direct assessment of stents and the surrounding vasculature helps diagnose stent fractures, in-stent stenosis and pseudoaneurysms. Smaller stents and structures adjacent to metal implants such as clips and coils can, however, be difficult to assess due to artefacts but higher kVp, edge-enhancing reconstruction kernels and iterative reconstruction all help to improve the diagnostic accuracy of CCT.44, 45 CCT is helpful in children with implanted pacemakers because even when these are deemed conditionally safe in the CMR environment they need to be imaged with low specific absorption rate and may give non-diagnostic images due to artefact.
Figure 16.
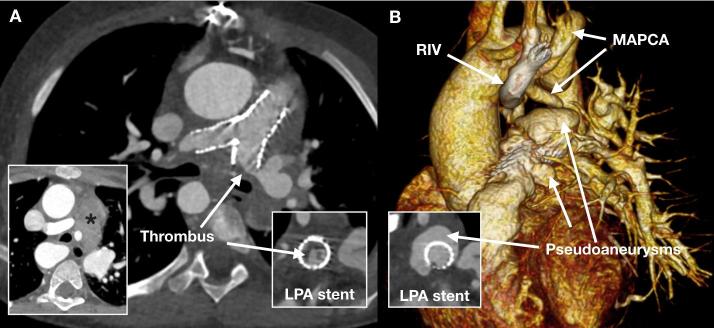
A 6-year-old child with stents in the main and branch pulmonary arteries referred for assessment of the stents, pulmonary arteries and mediastinum due to suspected endocarditis. The first CCT (A) shows extensive laminar thrombus in the LPA stent and marked mediastinal lymph node enlargement (asterisk) but no discrete collection. A subsequent CCT (B) after 5 weeks of antibiotic treatment shows complicated endocarditis with extensive pseudoaneurysms that fill with contrast medium through the stent mesh. There is also Note is made a MAPCA to left lung from the descending aorta and an incidental RIV. CCT, cardiovascular CT; LPA, left pulmonary artery; MAPCA, major aortopulmonary collateral artery; RIV, retroaortic innominate vein.
Figure 17.
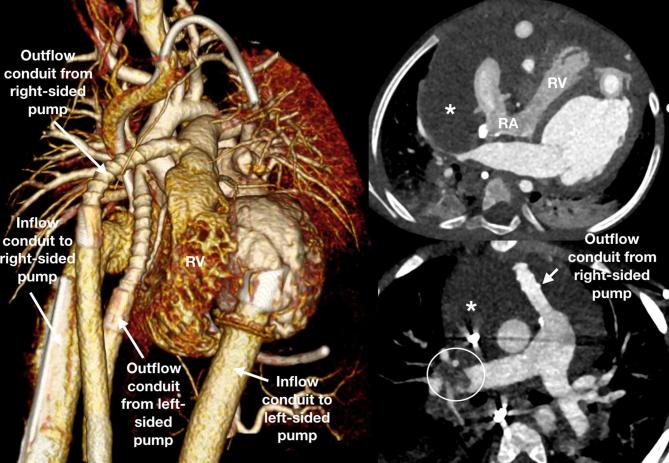
A 3-year-old toddler with biventricular assist device and possible collection adjacent to the right ventricle referred for assessment of the collection and patency of the pathways. The cardiac chambers, great vessels and device components were adequately opacified and unobstructed with no thrombus. The RV and RA are compressed with the right ventricular free wall seen concave. A large pericardial collection (asterisk) was the cause of this with no acute bleeding. A repeat CCT was performed after oxygen requirements suddenly increased and there was now thrombus in the pulmonary arteries (circle). CCT, cardiovascular CT; RA, right atrium; RV right ventricle.
Surgical and interventional anatomy
The 3D nature of a rapidly acquired and low radiation dose non-ECG-gated CCT study lends itself to mapping of surgical anatomies. This applies to both native and post-surgical anatomies such as when delineating the coronary arterial anatomy in the planning stage for interventions to the right ventricular outflow tract or branch pulmonary arteries in children treated with the arterial switch procedure (Figure 7). CCT is also helpful for also mapping the retrosternal anatomical relationships prior to sternotomy (Figure 18), which is particularly important in redo-sternotomies when adhesions may distort the anatomy.
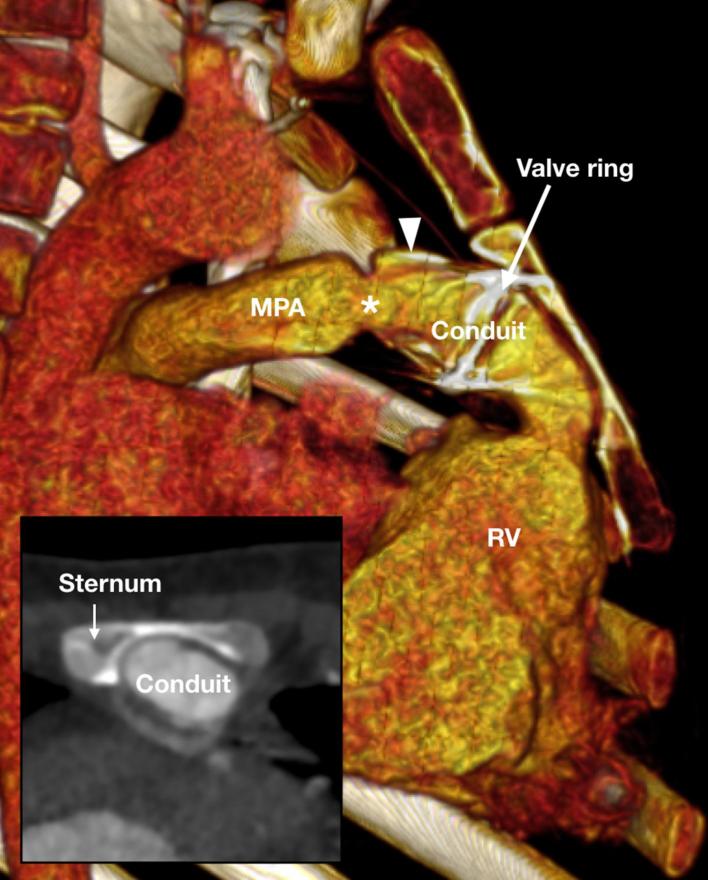
Figure 18.
A 6-year-old child referred for pre-surgical assessment of the retrosternal anatomy prior to replacement of a narrowed Contegra conduit that connects the RV to the MPA. The valved conduit has distal calcification (arrowhead) and narrowing (asterisk). The valve with the metallic ring sits in the mid conduit and has eroded into the thinned sternum. MPA, main pulmonary artery; RV, right ventricle.
Conclusion
CCT is now an important diagnostic tool in the multimodality assessment of congenital and acquired heart disease in children. Performance of diagnostic CCT at the lowest possible radiation dose relies on not just comprehensive knowledge of physiology, anatomy, disease patterns and treatments, but also on detailed technical knowledge about the scanner and protocols. Each scan must be individualised according to patient and disease specific factors with specific thought given to patient preparation, contrast administration and scan protocols.
Contributor Information
Kristian H Mortensen, Email: kristian.mortensen@gosh.nhs.uk.
Oliver Tann, Email: oliver.tann@gosh.nhs.uk.
REFERENCES
- 1.Jadhav SP, Golriz F, Atweh LA, Zhang W, Krishnamurthy R. CT angiography of neonates and infants: comparison of radiation dose and image quality of target mode prospectively ECG-gated 320-MDCT and ungated helical 64-MDCT. AJR Am J Roentgenol 2015; 204: W184–W191. doi: 10.2214/AJR.14.12846 [DOI] [PubMed] [Google Scholar]
- 2.Huang MP, Liang CH, Zhao ZJ, Liu H, Li JL, Zhang JE, et al. Evaluation of image quality and radiation dose at prospective ECG-triggered axial 256-slice multi-detector CT in infants with congenital heart disease. Pediatr Radiol 2011; 41: 858–66. doi: 10.1007/s00247-011-2079-2 [DOI] [PubMed] [Google Scholar]
- 3.Ghoshhajra BB, Lee AM, Engel LC, Celeng C, Kalra MK, Brady TJ, et al. Radiation dose reduction in pediatric cardiac computed tomography: experience from a tertiary medical center. Pediatr Cardiol 2014; 35: 171–9. doi: 10.1007/s00246-013-0758-5 [DOI] [PubMed] [Google Scholar]
- 4.Han BK, Lesser AM, Vezmar M, Rosenthal K, Rutten-Ramos S, Lindberg J, et al. Cardiovascular imaging trends in congenital heart disease: a single center experience. J Cardiovasc Comput Tomogr 2013; 7: 361–6. doi: 10.1016/j.jcct.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 5.Han BK, Overman DM, Grant K, Rosenthal K, Rutten-Ramos S, Cook D, et al. Non-sedated, free breathing cardiac CT for evaluation of complex congenital heart disease in neonates. J Cardiovasc Comput Tomogr 2013; 7: 354–60. doi: 10.1016/j.jcct.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 6.Girshin M, Shapiro V, Rhee A, Ginsberg S, Inchiosa MA. Increased risk of general anesthesia for high-risk patients undergoing magnetic resonance imaging. J Comput Assist Tomogr 2009; 33: 312–5. doi: 10.1097/RCT.0b013e31818474b8 [DOI] [PubMed] [Google Scholar]
- 7.Callahan MJ, Poznauskis L, Zurakowski D, Taylor GA. Nonionic iodinated intravenous contrast material-related reactions: incidence in large urban children’s hospital-retrospective analysis of data in 12,494 patients. Radiology 2009; 250: 674–81. doi: 10.1148/radiol.2503071577 [DOI] [PubMed] [Google Scholar]
- 8.Amaral JG, Traubici J, BenDavid G, Reintamm G, Daneman A. Safety of power injector use in children as measured by incidence of extravasation. AJR Am J Roentgenol 2006; 187: 580–3. doi: 10.2214/AJR.05.0667 [DOI] [PubMed] [Google Scholar]
- 9.Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2011; 21: 2527–41. doi: 10.1007/s00330-011-2225-0 [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurthy R. Neonatal cardiac imaging. Pediatr Radiol 2010; 40: 518–27. doi: 10.1007/s00247-010-1549-2 [DOI] [PubMed] [Google Scholar]
- 11.Greenberg SB, Bhutta ST. A dual contrast injection technique for multidetector computed tomography angiography of Fontan procedures. Int J Cardiovasc Imaging 2008; 24: 345–8. doi: 10.1007/s10554-007-9259-z [DOI] [PubMed] [Google Scholar]
- 12.Lidegran M, Palmér K, Jorulf H, Lindén V. CT in the evaluation of patients on ECMO due to acute respiratory failure. Pediatr Radiol 2002; 32: 567–74. doi: 10.1007/s00247-002-0756-x [DOI] [PubMed] [Google Scholar]
- 13.Han BK, Rigsby CK, Leipsic J, Bardo D, Abbara S, Ghoshhajra B, et al. Computed tomography imaging in patients with congenital heart disease, part 2: technical recommendations. An expert consensus document of the society of cardiovascular computed tomography (SCCT): endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI). J Cardiovasc Comput Tomogr 2015; 9: 493–513. doi: 10.1016/j.jcct.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 14.Goo HW, Yang DH. Coronary artery visibility in free-breathing young children with congenital heart disease on cardiac 64-slice CT: dual-source ECG-triggered sequential scan vs. single-source non-ECG-synchronized spiral scan. Pediatr Radiol 2010; 40: 1670–80. doi: 10.1007/s00247-010-1693-8 [DOI] [PubMed] [Google Scholar]
- 15.Lell MM, May M, Deak P, Alibek S, Kuefner M, Kuettner A, et al. High-pitch spiral computed tomography: effect on image quality and radiation dose in pediatric chest computed tomography. Invest Radiol 2011; 46: 116–23. doi: 10.1097/RLI.0b013e3181f33b1d [DOI] [PubMed] [Google Scholar]
- 16.Pache G, Grohmann J, Bulla S, Arnold R, Stiller B, Schlensak C, et al. Prospective electrocardiography-triggered CT angiography of the great thoracic vessels in infants and toddlers with congenital heart disease: feasibility and image quality. Eur J Radiol 2011; 80: e440–e445. doi: 10.1016/j.ejrad.2011.01.032 [DOI] [PubMed] [Google Scholar]
- 17.Hill KD, Frush DP, Han BK, Abbott BG, Armstrong AK, DeKemp RA, et al. Radiation safety in children with congenital and acquired heart disease: a scientific position statement on multimodality dose optimization from the image gently alliance. JACC Cardiovasc Imaging 2017; 10: 797–818. doi: 10.1016/j.jcmg.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han BK, Rigsby CK, Hlavacek A, Leipsic J, Nicol ED, Siegel MJ, et al. Computed tomography imaging in patients with congenital heart disease part I: rationale and utility. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT): Endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI). J Cardiovasc Comput Tomogr 2015; 9: 475–92. doi: 10.1016/j.jcct.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Wang X, Zhang Y, Lu Y, Wu R, Yuan H. Image quality and required radiation dose for coronary computed tomography angiography using an automatic tube potential selection technique. Int J Cardiovasc Imaging 2014; 30(Suppl 2): 89–94. doi: 10.1007/s10554-014-0526-5 [DOI] [PubMed] [Google Scholar]
- 20.Zhang LJ, Qi L, De Cecco CN, Zhou CS, Spearman JV, Schoepf UJ, et al. High-pitch coronary CT angiography at 70 kVp with low contrast medium volume: comparison of 80 and 100 kVp high-pitch protocols. Medicine 2014; 93: e92. doi: 10.1097/MD.0000000000000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R, Xu XJ, Huang G, Zhou X, Zhang WW, Ma YQ, X-J X, Y-Q M, et al. Comparison of image quality, diagnostic accuracy and radiation dose between flash model and retrospective ECG-triggered protocols in Dual Source Computed Tomography (DSCT) in congenital heart diseases. Pol J Radiol 2017; 82: 114–9. doi: 10.12659/PJR.899876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Udayasankar UK, Toth TL, Seamans J, Small WC, Kalra MK. Automatic patient centering for MDCT: effect on radiation dose. AJR Am J Roentgenol 2007; 188: 547–52. doi: 10.2214/AJR.06.0370 [DOI] [PubMed] [Google Scholar]
- 23.Boas FE, Fleischmann D. CT artifacts: causes and reduction techniques. Imaging Med 2012; 4: 229–40. doi: 10.2217/iim.12.13 [DOI] [Google Scholar]
- 24.Schueller-Weidekamm C, Schaefer-Prokop CM, Weber M, Herold CJ, Prokop M. CT angiography of pulmonary arteries to detect pulmonary embolism: improvement of vascular enhancement with low kilovoltage settings. Radiology 2006; 241: 899–907. doi: 10.1148/radiol.2413040128 [DOI] [PubMed] [Google Scholar]
- 25.Siegel MJ, Schmidt B, Bradley D, Suess C, Hildebolt C. Radiation dose and image quality in pediatric CT: effect of technical factors and phantom size and shape. Radiology 2004; 233: 515–22. doi: 10.1148/radiol.2332032107 [DOI] [PubMed] [Google Scholar]
- 26.Labounty TM, Leipsic J, Min JK, Heilbron B, Mancini GB, Lin FY, et al. Effect of padding duration on radiation dose and image interpretation in prospectively ECG-triggered coronary CT angiography. AJR Am J Roentgenol 2010; 194: 933–7. doi: 10.2214/AJR.09.3371 [DOI] [PubMed] [Google Scholar]
- 27.Li HO, Huo R, Wang XM, Xu GQ, Duan YH, Nie P, et al. High-pitch spiral CT with 3D reformation: an alternative choice for imaging vascular anomalies with affluent blood flow in the head and neck of infants and children. Br J Radiol 2015; 88: 20150005. doi: 10.1259/bjr.20150005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wielandner A, Beitzke D, Schernthaner R, Wolf F, Langenberger C, Stadler A, et al. Is ECG triggering for motion artefact reduction in dual-source CT angiography of the ascending aorta still required with high-pitch scanning? The role of ECG-gating in high-pitch dual-source CT of the ascending aorta. Br J Radiol 2016;: 20160174. doi: 10.1259/bjr.20160174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou P, Celermajer DS, Marini D, Agnoletti G, Vouhé P, Brunelle F, et al. Safety and accuracy of 64-slice computed tomography coronary angiography in children after the arterial switch operation for transposition of the great arteries. JACC Cardiovasc Imaging 2008; 1: 331–9. doi: 10.1016/j.jcmg.2008.02.005 [DOI] [PubMed] [Google Scholar]
- 30.Carbone I, Cannata D, Algeri E, Galea N, Napoli A, De Zorzi A, et al. Adolescent Kawasaki disease: usefulness of 64-slice CT coronary angiography for follow-up investigation. Pediatr Radiol 2011; 41: 1165–73. doi: 10.1007/s00247-011-2141-0 [DOI] [PubMed] [Google Scholar]
- 31.Yu FF, Lu B, Gao Y, Hou ZH, Schoepf UJ, Spearman JV, et al. Congenital anomalies of coronary arteries in complex congenital heart disease: diagnosis and analysis with dual-source CT. J Cardiovasc Comput Tomogr 2013; 7: 383–90. doi: 10.1016/j.jcct.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 32.Chu WC, Mok GC, Lam WW, Yam MC, Sung RY. Assessment of coronary artery aneurysms in paediatric patients with Kawasaki disease by multidetector row CT angiography: feasibility and comparison with 2D echocardiography. Pediatr Radiol 2006; 36: 1148–53. doi: 10.1007/s00247-006-0281-4 [DOI] [PubMed] [Google Scholar]
- 33.Kim JW, Goo HW. Coronary artery abnormalities in Kawasaki disease: comparison between CT and MR coronary angiography. Acta Radiol 2013; 54: 156–63. doi: 10.1258/ar.2012.120484 [DOI] [PubMed] [Google Scholar]
- 34.Rigsby CK, deFreitas RA, Nicholas AC, Leidecker C, Johanek AJ, Anley P, et al. Safety and efficacy of a drug regimen to control heart rate during 64-slice ECG-gated coronary CTA in children. Pediatr Radiol 2010; 40: 1880–9. doi: 10.1007/s00247-010-1711-x [DOI] [PubMed] [Google Scholar]
- 35.Weustink AC, Neefjes LA, Kyrzopoulos S, van Straten M, Neoh Eu R, Meijboom WB, et al. Impact of heart rate frequency and variability on radiation exposure, image quality, and diagnostic performance in dual-source spiral CT coronary angiography. Radiology 2009; 253: 672–80. doi: 10.1148/radiol.2533090358 [DOI] [PubMed] [Google Scholar]
- 36.Duan Y, Wang X, Cheng Z, Wu D, Wu L. Application of prospective ECG-triggered dual-source CT coronary angiography for infants and children with coronary artery aneurysms due to Kawasaki disease. Br J Radiol 2012; 85: e1190–e1197. doi: 10.1259/bjr/18174517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araoz PA, Kirsch J, Primak AN, Braun NN, Saba O, Williamson EE, et al. Optimal image reconstruction phase at low and high heart rates in dual-source CT coronary angiography. Int J Cardiovasc Imaging 2009; 25: 837–45. doi: 10.1007/s10554-009-9489-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography guidelines committee: endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2016; 10: 435–49. doi: 10.1016/j.jcct.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 39.Greil GF, Schoebinger M, Kuettner A, Schaefer JF, Dammann F, Claussen CD, et al. Imaging of aortopulmonary collateral arteries with high-resolution multidetector CT. Pediatr Radiol 2006; 36: 502–9. doi: 10.1007/s00247-006-0143-0 [DOI] [PubMed] [Google Scholar]
- 40.Kim TH, Kim YM, Suh CH, Cho DJ, Park IS, Kim WH, et al. Helical CT angiography and three-dimensional reconstruction of total anomalous pulmonary venous connections in neonates and infants. AJR Am J Roentgenol 2000; 175: 1381–6. doi: 10.2214/ajr.175.5.1751381 [DOI] [PubMed] [Google Scholar]
- 41.Ghadimi Mahani M, Agarwal PP, Rigsby CK, Lu JC, Fazeli Dehkordy S, Wright RA, et al. CT for assessment of thrombosis and pulmonary embolism in multiple stages of single-ventricle palliation: challenges and suggested protocols. Radiographics 2016; 36: 1273–84. doi: 10.1148/rg.2016150233 [DOI] [PubMed] [Google Scholar]
- 42.Park EA, Lee W, Chung SY, Yin YH, Chung JW, Park JH. Optimal scan timing and intravenous route for contrast-enhanced computed tomography in patients after Fontan operation. J Comput Assist Tomogr 2010; 34: 75–81. doi: 10.1097/RCT.0b013e3181ae292c [DOI] [PubMed] [Google Scholar]
- 43.Goodwin SJ, Randle E, Iguchi A, Brown K, Hoskote A, Calder AD. Chest computed tomography in children undergoing extra-corporeal membrane oxygenation: a 9-year single-centre experience. Pediatr Radiol 2014; 44: 750–60quiz 747-9. doi: 10.1007/s00247-014-2878-3 [DOI] [PubMed] [Google Scholar]
- 44.Eichhorn JG, Jourdan C, Hill SL, Raman SV, Cheatham JP, Long FR. CT of pediatric vascular stents used to treat congenital heart disease. AJR Am J Roentgenol 2008; 190: 1241–6. doi: 10.2214/AJR.07.3194 [DOI] [PubMed] [Google Scholar]
- 45.Tan S, Soulez G, Diez Martinez P, Larrivée S, Stevens LM, Goussard Y, et al. Coronary stent artifact reduction with an edge-enhancing reconstruction kernel - a prospective cross-sectional study with 256-slice CT. PLoS One 2016; 11: e0154292. doi: 10.1371/journal.pone.0154292 [DOI] [PMC free article] [PubMed] [Google Scholar]