Abstract
Objective
To analyze times of occurrence and identify risk factors (RFs) for technical and clinical failure and mortality of transcatheter arterial embolization (TAE) of acute bleeding in a major hospital.
Methods
All TAEs performed at our hospital from 2006 to 2013 (n = 327) were retrospectively analyzed.
Results
TAEs were performed during regular weekday hours in 165 (50%) and during off-hours in 162 (50%) cases. With 40 regular and 128 off-hours/week, 3.25 times more TAEs were performed during regular hours. There was an even distribution across weekdays (Mon-Fri:16.9 ± 1.5%), while fewer TAEs were performed on weekends (Sat: 8.3%, Sun: 7.3%). Technical success of TAEs was 93.9% with a clinical success of 79.2% and a 30-day mortality of 18.4%. Shock was an RF for technical failure (p = 0.022). RFs for clinical failure were low hemoglobin (Hb) (p = 0.021) and transfusion of ≥6 units packed cells (p = 0.009). Independent RFs for mortality were clinical failure (p < 0.001), coagulopathy (p = 0.005), and shock (p < 0.001).
Conclusion
Our results provide no evidence for a subjectively perceived increase in TAEs during off-hours but rather appear to show that most TAEs are performed during regular hours. Prompt TAE to control acute bleeding is crucial to prevent a drop in Hb with shock and the need for transfusion, which may promote coagulopathy and rebleeding, all of which are risk factors for a negative outcome.
Advances in knowledge
The presented analysis provides insights of occurrences and risk factors for success of transcatheter arterial embolization in acute bleeding in a large study population.
Introduction
Despite advances in diagnosis and treatment, acute arterial bleeding continues to pose a challenge in routine clinical practice.1–3 Besides endoscopic treatment of pulmonary or gastrointestinal hemorrhage and surgical repair, transcatheter arterial embolization (TAE) has evolved into a mainstay in the management of acute bleeding.4–7 TAE is minimally invasive and combines excellent technical and clinical success rates with a low risk of complications.2, 3 With currently available catheter technology and embolic agents, it is possible to safely use TAE in all body regions and control nearly any form of acute bleeding.8–10 Nevertheless, performing TAE requires some technical skills, experience, and serenity of the interventional radiologist, especially when patients have profuse bleeding and/or are in poor condition. Knowing the different types of hemorrhages and their frequencies and awareness of risk factors are crucial in establishing the indication for TAE and making a reasonable estimate of the chances of success (both technically and clinically). The outcome of TAE also depends on being able to honestly assess one’s own skills and those of beginning interventionalists before they can be relied on to perform TAE during off-hours.
The retrospective analysis presented here was conducted to obtain an overview of the spectrum of TAEs performed to control acute bleeding and their temporal distribution across regular workday hours and off-hours including weekends in a major hospital with the aim of investigating how the temporal distribution affects outcome and of identifying other factors affecting technical and clinical success and 30-day mortality.
methods and materials
Patients
All patients who underwent (super-)selective TAE to control acute arterial bleeding in the Department of Radiology and Nuclear Medicine of the University of Magdeburg from Jan. 2006 through June 2013 were retrospectively included. TAE was performed in patients with acute arterial bleeding not manageable surgically or endoscopically and/or subacute non-varicose bleeding. Following the institutional protocol patients with clinical suspicion for sub/acute bleeding [e.g. hemoglobin (Hb) drop, decrease in blood pressure, tachycardia, shock, hematemesis, rectal or vaginal bleeding] are transferred to the radiology department to receive contrast-enhanced three-phase (native, arterial, and venous) CT of the region of interest (contrast agent: Immeron 300, Braco, Milano, Italy; 2 ml kg−1 body weight, maximum dose of 150 ml). Exceptions are made in case of polytrauma (mixed arterial-venous phase whole body CT), if bleeding is directly documented by visual means (e.g. endoscopy) or if patient is transferred from other hospital with documented bleeding in external CT. After verification of bleeding or if unclear in CT or if clinically highly suspicious but not visible in CT (e.g. vaginal bleeding, temporarily not visible due to tamponade, etc.) patients are directly transferred to the angio suite for angiography (see below). Time from documentation (CT or endoscopy) to angio (ready to puncture) is less than 45 min during regular hours and off-hours (during off-hours interventional radiologist is consulted shortly after CT by phone -images can be assessed at home and if necessary angiography nurse (in house) and intensive care staff prepare patient for intervention till arrival of the interventional radiologist). Different times apply to patients that are transferred from external hospitals. The institutional protocol was not changed since 2006. Heads of the department of surgery, gastroenterology, gynecology, and radiology did not change between 2006 and 2013 (period of patient recruitment). This retrospective study was approved by the Ethics Committee of the Medical Faculty of the University of Magdeburg (reference: RAD243).
Transcatheter arterial embolization (TAE)
TAE was most commonly performed via a transfemoral approach and, in rare cases, via a transbrachial access (5 F introducer sheath). Angiographic overviews were obtained with automated contrast agent injection (Imeron 300, Bracco Imaging, Milano, Italy) through a 5 F Omni Flush catheter (Angiodynamics, Latham, NY). 5 F selective catheters (Cobra, Sos, Aachen, or Roberts catheter; Angiodynamics or Boston Scientific, Natick, MA) were used to catheterize side branch vessels (first or second order). Microcatheters (2.4/3.0 F MicroFerret®, Cook Medical, Bloomington, IN) were used and coaxially introduced into the target artery to search for the site of bleeding or when the site was known. The choice of embolic agent depended on vascular anatomy, pathomechanism of bleeding, and the interventionalist’s experience with the different agents. The embolic agents used in the study patients included 0.018” microcoils (Cook Medical), Contour® PVA embolization particles (250–355, 355–500, or 710–1000 microns, Boston Scientific), Histoacryl® glue (B.Braun, Melsungen, Germany), Embozene® microspheres (Boston Scientific), Onyx® liquid embolic system (Covidien, Dublin, Ireland), Amplatzer vascular plugs (8–12 mm) (St. Jude Medical, Saint Paul, MN), covered stents (GORE® VIABAHN® Newark, DE), and Gelaspon® sponge (Bausch + Lomb, Rochester, NY). These embolic agents were used either alone or in various combinations. Contrast extravasation on digital subtraction angiography (DSA) was defined as positive evidence of bleeding. In patients with intermittently visible escape of blood from a vessel, empirical embolization was performed taking into account the patient’s clinical history, CT angiography findings, and possible prior placement of hemoclips during endoscopy, or indirect bleeding signs (e.g. tumor blush or vessel discontinuity).11 Radiologists with at least 5 years of experience in transarterial therapeutic interventions performed (off-hours) or supervised (regular hours) all TAEs.
Outcome parameters
The times when TAEs were performed were extracted from the department‘s RIS/PACS system. Regular hours are from 7:30 am to 3:30 pm on weekdays (Monday through Friday); off-hours include the period from 3:30 pm to 7:30 am the next day during weekdays and full 24 h periods during weekends and public holidays. Outcome parameters were defined in accordance with the Guidelines of the Society of Interventional Radiology.12 Primary endpoints were technical and clinical success/failure and 30-day mortality. Technical success was defined as complete occlusion of the target vessel(s) for first-time interventions and absence of contrast extravasation. Clinical failure of TAE was defined as rebleeding during the 30-day follow-up period. The mortality rate was calculated from deaths occurring within 30 days of TAE. Other parameters considered were defined as follows: concomitant diseases: arterial hypertension, renal failure, diabetes mellitus, cardiac arrhythmia, coronary heart disease, liver cirrhosis, pulmonary embolism, and chronic obstructive pulmonary disease (COPD). Anticoagulant treatment: phenprocoumon, acetylsalicylic acid, heparin, and clopidogrel. Shock: RRsyst <100 and heart rate >100 Hz. Coagulopathy: INR ≥1.5, PTT ≥ 45 s, or thrombocyte count ≤50 gigaparticles per liter (Gpt l–1).
Statistical analysis
Encoded and anonymized primary data were collected and used for statistical analysis with SPSS Statistics, v. 22.0 (IBM Corp., Armonk, NY). Results for continuous variables are given as means (M) and standard deviations (SD) with ranges. The results of univariate analysis were tested for significance using the Chi-square test, Fisher’s exact test, and the Mann-Whitney U-test. Multivariate analysis was performed using binary logistic regression. For all statistical tests, p-values < 0.05 were considered to indicate significant differences. Factors with p < 0.05 in univariate analysis were included in multivariate analysis. Patients for whom no clinical follow-up data were available were excluded from clinical analysis.
Results
Table 1 summarizes basic patient characteristics (age, sex) and the underlying causes of bleeding in the 327 TAEs performed in this retrospective analysis. Latrogenic bleeding (n = 153) and spontaneous tumor bleeding (n = 78) were by far the main causes in our study population.
Table 1.
Basic patient characteristics and causes of bleeding
| All patients/male/female [n (%)] |
327(100)/211 (64.5)/116 (35.5) |
| Age (SD) | 63 ± 15.6 years |
| Cause of bleeding | n (%) |
| Upper GI tract | 39 (11.9) |
| Peptic ulcer | 37 (11.3) |
| Aneurysm rupture | 2 (0.6) |
| Lower GI tract | 10 (3.1) |
| Diverticulitis | 7 (2.1) |
| Ulcer | 1 (0.3) |
| Angiodysplasia | 2 (0.6) |
| Pancreatitis-related bleeding | 23 (7.0) |
| Acute pancreatitis | 5 (1.5) |
| Chronic pancreatitis | 18 (5.5) |
| Traumatic bleeding | 14 (4.3) |
| Tumor-related bleeding | 78 (23.9) |
| Gynecological tumor | 25 (7.6) |
| Gastrointestinal tumor | 23 (7.0) |
| Urological tumor | 8 (2.4) |
| Hepatic tumor | 7 (2.1) |
| Pulmonary tumor | 6 (1.8) |
| Pancreatic tumor | 5 (1.5) |
| Other tumor | 5 (1.5) |
| Latrogenic bleeding | 153 (46.8) |
| Post-operative | 89 (27.2) |
| Post-interventional | 38 (11.6) |
| Anticoagulation | 26 (8.0) |
| Other | 10 (3.1) |
| Total n (%) | 327 (100) |
GI, gastro intestinal; SD, standard deviation.
In our patient population, 50% of TAEs were performed during regular hours and 50% during off-hours. Figure 1A shows the distribution of cases categorized by the cause of bleeding during regular hours and off-hours. Taking into account that a week has 40 regular hours and 128 off-hours, 3.25 times more TAEs were performed during regular hours compared with off-hours [(165/40)/(162/128) = 3.25]. Analysis by time of day identified a peak of TAEs during regular hours with a downward trend beginning after regular hours and continuing through the night (Figure 1B). There was a nearly even temporal distribution of TAEs across weekdays (Mon–Fri: 16.9 ± 1.5%) with a markedly lower number of procedures during weekends (Sat: 8.3%, Sun: 7.3%) (Figure 1C). Distribution through the year also appeared homogeneous (Jan–Dec: 8.3 ± 1.5%) (Figure 1D).
Figure 1.
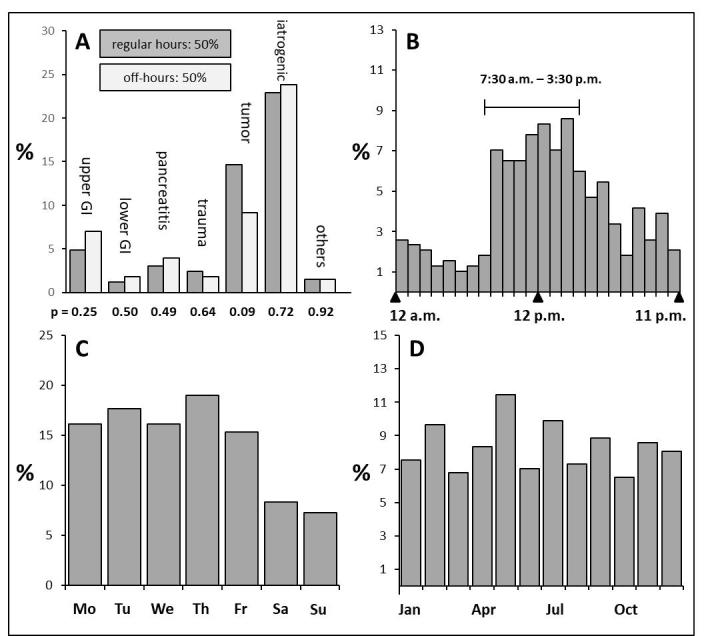
(A) Percentage of TAEs performed during regular hours and off-hours. (B) From Mondays through Fridays, there is a peak in the number of TAEs during regular hours with a drop beginning at the end of regular hours and continuing through the night to the morning of the next day (beginning of regular hours). (C) TAEs are evenly distributed across weekdays (Mondays through Fridays) with a smaller number during weekends. (D) shows the fairly even distribution of TAEs through the year. TAEs, transcatheter arterial embolizations.
Technical success of TAE was 93.9%. The results of univariate analysis of selected potential risk factors (RFs) for technical failure (6.1%) of TAE are summarized in Table 2. Only hemodynamic shock before and during TAE was identified to be associated with technical failure (p = 0.022).
Table 2. .
Risk factors for technical failure-univariate analysis
| Variable | Success | Failure | p value |
| All cases = 327 | 93.9% (307) | 6.1% (20) | |
| Age in yearsa | 63.3 ± 15.4 | 63.5 ± 20.0 | 0.671 |
| Sex (male) | 65.8% (202/307) | 45.0% (9/20) | 0.060 |
| ≥ 2 concomitant diseases | 41.7% (120/288) | 27.8% (5/18) | 0.245 |
| Hb level in mmol/la | 5.7 ± 1.3 | 6.1 ± 1.5 | 0.310 |
| FFPa | 0.2 ± 1.3 | 0.0 | 0.351 |
| Red blood cell concentratesa | 2.5 ± 6.0 | 1.6 ± 2.8 | 0.648 |
| ≥ 6 units of red blood cell concentrates | 12.9% (37/286) | 17.6% (3/17) | 0.478 |
| No bleeding evidence (CT) | 23.6% (42/178) | 12.5% (2/16) | 0.532 |
| No bleeding evidence (DSA) | 2.0% (6/307) | 5.0% (1/20) | 0.360 |
| TAE during off-hours including weekend | 49.5% (152/307) | 50.0% (10/20) | 0.966 |
| Selective catheterization only | 20.2% (62/307) | 20.0% (4/20) | 1.000 |
| Sole use of coils for embolization | 51.5% (158/307) | 55.0% (11/20) | 0.759 |
| > 1 vessel embolized | 13.0% (40/307) | 25.0% (5/20) | 0.132 |
| Anticoagulation | 30.6% (88/288) | 44.4% (8/18) | 0.218 |
| Corticosteroids | 6.6% (19/288) | 5.6% (1/18) | 1.000 |
| Shock | 16.3% (47/289) | 36.8% (7/19) | *0.022 |
| Coagulopathy | 16.7% (43/257) | 33.3% (6/18) | 0.075 |
| Referral from other hospital | 15.4% (45/292) | 15.8% (3/19) | 1.000 |
| Referral from ICU | 30.2% (87/288) | 27.8 (5/18) | 0.827 |
DSA, digital subtraction angiography; FFP, fresh frozen plasma; Hb, hemoglobin; TAE, transcatheter arterial embolization;
Mean and standard deviation; concomitant diseases: arterial hypertension, chronic renal failure, diabetes mellitus, cardiac arrhythmia, coronary heart disease, liver cirrhosis, pulmonary embolism, chronic obstructive pulmonary disease (COPD); *P-values <0.05 were assumed to be significant.
Clinical success of TAE was 79.2%. Univariate analysis of potential factors for clinical failure (20.8%) revealed three RFs— namely reduced Hb level before or during embolization, transfusion of six or more units of red blood cell concentrates, and TAEs performed in patients referred from other hospitals (time to angio) (Table 3). Multivariate analysis revealed reduced Hb and transfusion of ≥6 units as the only independent RFs (Table 3).
Table 3.
Risk factors for clinical failure (rebleeding within 30 days after TAE)
| Univariate analysis | |||
| Variable | Success | Failure | p value |
| Cases included in analysis = 289 | 79.2% (229) | 20.8% (60) | |
| Age in yearsa | 62.3 ± 15.7 | 64.6 ± 14.9 | 0.313 |
| Sex (male) | 65.5% (150/229) | 66.7% (40/60) | 0.866 |
| ≥2 concomitant diseases | 41.0% (94/229) | 44.1% (26/59) | 0.675 |
| Hb level in mmol/la | 5.8 ± 1.3 | 5.3 ± 1.2 | *0.025 |
| FFPa | 0.2 ± 1.1 | 0.4 ± 1.8 | 0.444 |
| Red blood cell concentratesa | 1.9 ± 4.0 | 5.0 ± 10.4 | 0.061 |
| ≥6 units of red blood cell concentrates | 9.7% (22/227) | 25.4% (15/59) | *0.001 |
| No bleeding evidence (CT) | 23.0% (31/135) | 24.3% (9/37) | 0.862 |
| No bleeding evidence (DSA) | 2.2% (5/229) | 1.7% (1/60) | 1.000 |
| TAE during off-hours including weekends | 47.6% (109/229) | 60.0% (36/60) | 0.087 |
| Selective catheterization only | 19.2% (44/229) | 25.0% (15/60) | 0.322 |
| Sole use of coils for embolization | 52.4% (120/229) | 55.0% (33/60) | 0.720 |
| >1 vessel embolized | 11.8% (27/229) | 20.0% (12/60) | 0.098 |
| Anticoagulation | 31.0% (71/229) | 28.8% (17/59) | 0.745 |
| Corticosteroids | 7.4% (17/229) | 3.4% (2/59) | 0.382 |
| Shock | 14.4% (33/229) | 23.3% (14/60) | 0.095 |
| Coagulopathy | 16.8% (33/196) | 15.8% (9/57) | 0.852 |
| Referral from other hospital | 12.7% (29/229) | 25.0% (15/60) | *0.018 |
| Referral from ICU | 27.9% (64/229) | 39.0% (23/59) | 0.100 |
| Multivariate analysis | |||||
| Variable | B | p-value | Exp(B) | 95% confidence interval for Exp(B) | |
| Lower | Upper | ||||
| Hb level (in mmol l–1) | 0.30 | *0.021 | 1.35 | 1.00 | 1.74 |
| ≥6 units of red blood cell concentrates | –1.01 | *0.009 | 0.37 | 0.17 | 0.78 |
| Referral from other hospital | –0.77 | 0.051 | 0.46 | 0.21 | 1.00 |
| Constant | 0.03 | 0.449 | 1.79 | ||
DSA, digital subtraction angiography; FFP, fresh frozen plasma; Hb, hemoglobin; TAE, transcatheter arterial embolization.
Mean and standard deviation; concomitant diseases: arterial hypertension, chronic renal failure, diabetes mellitus, cardiac arrhythmia, coronary heart disease, liver cirrhosis, pulmonary embolism, chronic obstructive pulmonary disease (COPD); *P-values <0.05 were assumed to be significant.
Regarding 30-day mortality (18.4%), univariate analysis identified five RFs: an Hb below 5.4 ± 1.3 mmol l−1, transfusion of >6 units, shock before and during TAE, coagulopathy, and rebleeding within 30 days of TAE (clinical failure) (Table 4). Independent RFs in multivariate analysis were coagulopathy, rebleeding, and shock (Table 4).
Table 4.
Risk factors for mortality (death within 30 days after TAE)
| Univariate analysis | |||
| Variable | Success | Failure | p value |
| Cases included in analysis = 309 | 81.6% (252) | 18.4% (57) | |
| Age in yearsa | 62.2 ± 16.0 | 65.8 ± 14.4 | 0.128 |
| Sex (male) | 65.5% (195/252) | 59.6% (34/57) | 0.407 |
| ≥2 concomitant diseases | 38.8% (97/250) | 50.0% (28/56) | 0.123 |
| Hb level in mmol l–1a | 5.8 ± 1.3 | 5.4 ± 1.3 | *0.045 |
| FFPa | 0.2 ± 1.2 | 0.2 ± 1.3 | 0.274 |
| Red blood cell concentratesa | 2.2 ± 5.0 | 3.8 ± 8.7 | 0.374 |
| ≥6 units of red blood cell concentrates | 11.3% (28/247) | 21.4% (12/56) | *0.045 |
| No bleeding evidence (CT) | 23.8% (36/151) | 16.2% (6/37) | 0.318 |
| No bleeding evidence (DSA) | 2.4% (6/252) | 1.8% (1/57) | 0.774 |
| TAE during off-hours including weekend | 48.8% (123/252) | 59.6% (34/57) | 0.139 |
| Selective catheterization only | 20.6% (52/252) | 19.3% (11/57) | 0.821 |
| Sole use of coils for embolization | 54.0% (136/252) | 47.4% (27/57) | 0.367 |
| >1 vessel embolized | 13.1% (33/252) | 19.3% (11/57) | 0.226 |
| Anticoagulation | 30.0% (75/250) | 37.5% (21/56) | 0.274 |
| Corticosteroids | 7.6% (19/250) | 1.8% (1/56) | 0.112 |
| Shock | 13.1% (33/251) | 35.7% (20/56) | *<0.001 |
| Coagulopathy | 14.3% (32/223) | 33.3% (16/48) | *0.002 |
| Referral from other hospital | 15.9% (40/252) | 14.3% (8/56) | 0.767 |
| Referral from ICU | 28.8% (72/250) | 35.7% (20/56) | 0.308 |
| Clinical success/recurrent bleeding | 15.1% (36/239) | 48.0% (24/50) | *<0.001 |
| Multivariate analysis | |||
| Variable | B | p-value | Exp(B) | 95% confidence interval for EXP(B) | |
| Lower | Upper | ||||
| Hemoglobin level | –0.247 | 0.111 | 0.781 | 0.576 | 1.059 |
| ≥6 units of red blood cell concentrates | –0.066 | 0.896 | 0.936 | 0.351 | 2.499 |
| Clinical success/rebleeding | 2.046 | *<0.001 | 7.738 | 3.568 | 16.779 |
| Shock | 1.25 | *<0.001 | 3.480 | 1.695 | 7.150 |
| Coagulopathy | 1.239 | *0.005 | 3.453 | 1.445 | 8.250 |
| Constant | –1.215 | 0.174 | 0.297 | ||
DSA, digital subtraction angiography; FFP, fresh frozen plasma; Hb, hemoglobin; TAE, transcatheter arterial embolization.
Mean and standard deviation; concomitant diseases: arterial hypertension, chronic renal failure, diabetes mellitus, cardiac arrhythmia, coronary heart disease, liver cirrhosis, pulmonary embolism, chronic obstructive pulmonary disease; *P-values <0.05 were assumed to be significant.
Discussion
The spectrum of bleeding and underlying mechanisms observed in our patient population (Table 1) can be assumed to be representative of the spectrum likely to be encountered in other tertiary care settings. Conversely, the situation in our center may differ from that at other hospitals in terms of the distribution of case numbers, which depends not only on the philosophy of the radiology department (proactive vs reactive) and the available resources but also on the referral policy of the hospital’s clinical specialties and their preferences (conservative/endoscopic/surgical vs interventional treatment). Moreover, the spectrum of acute arterial bleeding encountered in the patients of a hospital also reflects the subspecializations of its clinical departments. Surprisingly, review articles covering the full spectrum of TAEs are rare, and most published studies are limited by the fact that the findings are no longer representative of current practice or are based on the investigation of small patient populations.3, 13
An important result of our analysis is the temporal distribution of TAEs across regular hours and off-hours and across days of the week. In absolute numbers, roughly the same numbers of TAEs in our patient population were performed during regular and off-hours; however, when calculating the proportions taking into account that only 40 h of the 168 h per week are regular hours, this means that roughly three times more TAEs are performed during regular hours compared with off-hours. The larger number of TAEs during regular hours reflects the hospital‘s activity during the day (primarily punctures/biopsies) and the tendency to shift the treatment of subacute bleedings to regular hours even when diagnosed during off-hours. Such bleedings are common in cancer patients.14, 15 Post-operative hemorrhage, on the other hand, tends to be more acute with a longer delay between surgery and the event.16, 17 After pancreatic resection, for example, bleeding occurs with a median delay of 8 days. Such hemorrhages can occur any time and, when diagnosed during off-hours, require immediate management.16 What our data do not confirm is that, contrary to widely held belief, complications do not tend to predominantly occur during off-hours or weekends because their diagnosis is unduly delayed during regular hours. Nor do our results confirm a subjectively perceived summer slump in the number of TAEs.
The technical success rate of 93.9% we found in our study is in the range of 90–100% identified in recent studies investigating the management of bleeding in different body regions in smaller patient populations.18–20 Causes of technical failure reported in the literature include vascular spasm, stenosis of the bleeding artery, and complex anatomy.21 In our patient population, we identified shock before and during TAE to be associated with technical failure. The mechanism is that hypovolemia triggers vasoconstriction, which in turn makes it more difficult to catheterize the bleeding artery. Overall, though, the success rate of TAE is very high, making TAE an excellent approach for controlling various types of acute arterial bleeding.
The clinical success rate (no recurrent bleeding within 30 days) in our patients was 79.2%, which is midway between the lowest rate of 51% and the highest rate of 92% published in the literature.18, 20,22,23 Hb levels of less than 5.3 mmol l−1 and transfusion of six or more units of red blood cell concentrates were identified as negative prognostic factors for clinical success in our analysis. Similar findings regarding transfusion were reported by others,24, 25 and a negative effect of low Hb levels was also observed by Defreyne et al26 and Hongsakul et al27 in patients with gastrointestinal hemorrhage. Overall, these findings suggest that timely control of arterial bleeding before a marked drop in Hb and a need for massive transfusion to occur is crucial for the clinical success of TAE. This assumption is corroborated by the significant negative prognostic impact of referral from other hospitals we found in univariate analysis (Table 3). However, this significance was not confirmed in multivariate analysis, since referral of patients from other hospitals is not an independent factor but merely implies a longer delay before TAE is performed, which in turn means a more marked drop in Hb and greater need for blood transfusion.
Mortality rates after TAE reported in the literature differ widely with the site of bleeding, ranging from 0 to 43%;18, 23,24,28,29 again the rate of 18.4% in our patients is midway between these extremes. The three RFs of mortality identified in our study–shock, rebleeding (clinical failure), and coagulopathy–have also been described as highly relevant by other investigators.23, 26 Similar to its impact on clinical success, prompt control of arterial bleeding (time to angio) appears to be the overarching factor for success of TAE in terms of mortality as both shock and coagulopathy are direct sequelae of uncontrolled blood loss.
Finally, it is important to note that whether TAE is performed during regular hours or off-hours including weekends had no impact on technical and clinical success including mortality in our patient population. Poorer outcome due to weekend or off-hour effects, as reported for other types of acute bleeding, was not observed in our study.30
Our analysis was conducted retrospectively and has some other limitations. While our study is among the largest in this area, inclusion of 327 patients was achieved by pooling patient populations. Another limitation is that no standardized protocol was used for TAE. The interventional approach and choice of embolic agent(s) were at the discretion of the interventional radiologist performing the procedure, who decided based on the clinical presentation and etiology of bleeding.
This means that our insights cannot be generalized but must always be interpreted taking heterogeneity of etiologies and of procedural approaches into account. This limitation is outweighed by the advantage of a comprehensive data analysis in a patient population representative of bleeding management in the routine clinical setting.
Conclusion
In our patient population, roughly half of all TAEs were performed during regular hours and the other half during off-hours. This means that 3.25 times more TAEs were performed during regular hours compared with off-hours and contradicts the widely held subjective assumption that more TAEs are performed during off-hours including weekends. Similar to other reports, our technical success rate of 93.9% in the management of acute bleeding is excellent. However, low Hb and massive blood transfusion, shock, and coagulopathy are associated with lower clinical success and a rather high mortality rate of 18.4%. What is crucial is the time from diagnosis to control of bleeding with a shorter interval reducing the negative effect of these factors on the outcome of TAE. Hence, embolization of arterial bleeding should be performed promptly.
Contributor Information
Maciej Powerski, Email: maciej.powerski@med.ovgu.de.
Philipp Meyer-Wilmes, Email: philipp.meyerwilmes@t-online.de.
Jazan Omari, Email: jazan.omari@med.ovgu.de.
Robert Damm, Email: robert.damm@med.ovgu.de.
Max Seidensticker, Email: max.seidensticker@med.uni-muenchen.de.
Björn Friebe, Email: bjoern.friebe@med.ovgu.de.
Frank Fischbach, Email: frank.fischbach@med.ovgu.de.
Maciej Pech, Email: maciej.pech@med.ovgu.de.
REFERENCES
- 1.Walker TG. Acute gastrointestinal hemorrhage. Tech Vasc Interv Radiol 2009; 12: 80–91. doi: 10.1053/j.tvir.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 2.Walker TG, Salazar GM, Waltman AC. Angiographic evaluation and management of acute gastrointestinal hemorrhage. World J Gastroenterol 2012; 18: 1191–201. doi: 10.3748/wjg.v18.i11.1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keeling AN, McGrath FP, Thornton J, Brennan P, Lee MJ. Emergency percutaneous transcatheter embolisation of acute arterial haemorrhage. Ir J Med Sci 2010; 179: 385–91. doi: 10.1007/s11845-009-0400-y [DOI] [PubMed] [Google Scholar]
- 4.Loffroy R, Rao P, Ota S, De Lin M, Kwak BK, Geschwind JF. Embolization of acute nonvariceal upper gastrointestinal hemorrhage resistant to endoscopic treatment: results and predictors of recurrent bleeding. Cardiovasc Intervent Radiol 2010; 33: 1088–100. doi: 10.1007/s00270-010-9829-7 [DOI] [PubMed] [Google Scholar]
- 5.Ramírez Mejía AR, Méndez Montero JV, Vásquez-Caicedo ML, Bustos García de Castro A, Cabeza Martínez B, Ferreirós Domínguez J. Radiological evaluation and endovascular treatment of hemoptysis. Curr Probl Diagn Radiol 2016; 45: 215–24. doi: 10.1067/j.cpradiol.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 6.Sheth R, Someshwar V, Warawdekar G. Treatment of acute lower gastrointestinal hemorrhage by superselective transcatheter embolization. Indian J Gastroenterol 2006; 25: 290–4. [PubMed] [Google Scholar]
- 7.Papakostidis C, Kanakaris N, Dimitriou R, Giannoudis PV. The role of arterial embolization in controlling pelvic fracture haemorrhage: a systematic review of the literature. Eur J Radiol 2012; 81: 897–904. doi: 10.1016/j.ejrad.2011.02.049 [DOI] [PubMed] [Google Scholar]
- 8.Kim PH, Tsauo J, Shin JH, Yun SC. Transcatheter arterial embolization of gastrointestinal bleeding with N-butyl cyanoacrylate: a systematic review and meta-analysis of safety and efficacy. J Vasc Interv Radiol 2017; 28: 522–31. doi: 10.1016/j.jvir.2016.12.1220 [DOI] [PubMed] [Google Scholar]
- 9.Koganemaru M, Nonoshita M, Iwamoto R, Kuhara A, Nabeta M, Kusumoto M, et al. . Ultraselective embolization using a 1.7-Fr catheter and soft bare coil for small intestinal bleeding. Minim Invasive Ther Allied Technol 2016; 25: 345–50. doi: 10.1080/13645706.2016.1192553 [DOI] [PubMed] [Google Scholar]
- 10.Mangini M, Laganà D, Fontana F, Ianniello A, Nicotera P, Petullà M, et al. . Use of Amplatzer Vascular Plug (AVP) in emergency embolisation: preliminary experience and review of literature. Emerg Radiol 2008; 15: 153–60. doi: 10.1007/s10140-007-0696-8 [DOI] [PubMed] [Google Scholar]
- 11.Sos TA, Lee JG, Wixson D, Sniderman KW. Intermittent bleeding from minute to minute in acute massive gastrointestinal hemorrhage: arteriographic demonstration. AJR Am J Roentgenol 1978; 131: 1015–7. doi: 10.2214/ajr.131.6.1015 [DOI] [PubMed] [Google Scholar]
- 12.Angle JF, Siddiqi NH, Wallace MJ, Kundu S, Stokes L, Wojak JC, et al. . Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 2010; 21: 1479–86. doi: 10.1016/j.jvir.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 13.Görich J, Brambs HJ, Allmenröder C, Roeren T, Brado M, Richter GM, et al. . The role of embolization treatment of acute hemorrhage. Rofo 1993; 159: 379–87. doi: 10.1055/s-2008-1032782 [DOI] [PubMed] [Google Scholar]
- 14.Rosen PJ. Bleeding problems in the cancer patient. Hematol Oncol Clin North Am 1992; 6: 1315–28. doi: 10.1016/S0889-8588(18)30277-6 [DOI] [PubMed] [Google Scholar]
- 15.Pereira J, Phan T. Management of bleeding in patients with advanced cancer. Oncologist 2004; 9: 561–70. doi: 10.1634/theoncologist.9-5-561 [DOI] [PubMed] [Google Scholar]
- 16.Yekebas EF, Wolfram L, Cataldegirmen G, Habermann CR, Bogoevski D, Koenig AM, et al. . Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg 2007; 246: 269–80. doi: 10.1097/01.sla.0000262953.77735.db [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machado NO, Al-Zadjali A, Kakaria AK, Younus S, Rahim MA, Al-Sukaiti R. Hepatic or cystic artery pseudoaneurysms following a laparoscopic cholecystectomy: literature review of aetiopathogenesis, presentation, diagnosis and management. Sultan Qaboos Univ Med J 2017; 17: 135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hur S, Jae HJ, Lee M, Kim HC, Chung JW. Safety and efficacy of transcatheter arterial embolization for lower gastrointestinal bleeding: a single-center experience with 112 patients. J Vasc Interv Radiol 2014; 25: 10–19. doi: 10.1016/j.jvir.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 19.Comai A, Zatelli M, Haglmuller T, Bonatti G. The role of transcatheter arterial embolization in traumatic pelvic hemorrhage: not only pelvic fracture. Cureus 2016; 8: 722. doi: 10.7759/cureus.722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou CG, Shi HB, Liu S, Yang ZQ, Zhao LB, Xia JG, et al. . Transarterial embolization for massive gastrointestinal hemorrhage following abdominal surgery. World J Gastroenterol 2013; 19: 6869–75. doi: 10.3748/wjg.v19.i40.6869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandi R, Shetty PC, Sharma RP, Burke TH, Burke MW, Kastan D. Superselective arterial embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol 2001; 12: 1399–405. doi: 10.1016/S1051-0443(07)61697-2 [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Shin JH, Yoon HK, Ko GY, Gwon DI, Kim EY, et al. . Endovascular intervention for management of pancreatitis-related bleeding: a retrospective analysis of thirty-seven patients at a single institution. Diagn Interv Radiol 2015; 21: 140–7. doi: 10.5152/dir.2014.14085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yap FY, Omene BO, Patel MN, Yohannan T, Minocha J, Knuttinen MG, et al. . Transcatheter embolotherapy for gastrointestinal bleeding: a single center review of safety, efficacy, and clinical outcomes. Dig Dis Sci 2013; 58: 1976–84. doi: 10.1007/s10620-012-2547-z [DOI] [PubMed] [Google Scholar]
- 24.Poultsides GA, Kim CJ, Orlando R, Peros G, Hallisey MJ, Vignati PV. Angiographic embolization for gastroduodenal hemorrhage: safety, efficacy, and predictors of outcome. Arch Surg 2008; 143: 457–61. doi: 10.1001/archsurg.143.5.457 [DOI] [PubMed] [Google Scholar]
- 25.Loffroy R, Guiu B, D'Athis P, Mezzetta L, Gagnaire A, Jouve JL, et al. . Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: predictors of early rebleeding. Clin Gastroenterol Hepatol 2009; 7: 515–23. doi: 10.1016/j.cgh.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 26.Defreyne L, Vanlangenhove P, De Vos M, Pattyn P, Van Maele G, Decruyenaere J, et al. . Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology 2001; 218: 739–48. doi: 10.1148/radiology.218.3.r01mr05739 [DOI] [PubMed] [Google Scholar]
- 27.Hongsakul K, Pakdeejit S, Tanutit P. Outcome and predictive factors of successful transarterial embolization for the treatment of acute gastrointestinal hemorrhage. Acta Radiol 2014; 55: 186–94. doi: 10.1177/0284185113494985 [DOI] [PubMed] [Google Scholar]
- 28.Sethi H, Peddu P, Prachalias A, Kane P, Karani J, Rela M, et al. . Selective embolization for bleeding visceral artery pseudoaneurysms in patients with pancreatitis. Hepatobiliary Pancreat Dis Int 2010; 9: 634–8. [PubMed] [Google Scholar]
- 29.Lee HJ, Shin JH, Yoon HK, Ko GY, Gwon DI, Song HY, et al. . Transcatheter arterial embolization in gastric cancer patients with acute bleeding. Eur Radiol 2009; 19: 960–5. doi: 10.1007/s00330-008-1216-2 [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Li W, Herath C, Xia J, Hu B, Song F, et al. . Off-hour admission and mortality risk for 28 specific diseases: a systematic review and meta-analysis of 251 cohorts. J Am Heart Assoc 2016; 5: e003102. doi: 10.1161/JAHA.115.003102 [DOI] [PMC free article] [PubMed] [Google Scholar]


