Abstract
Radiotherapy treatment planning of complex radiotherapy techniques, such as intensity modulated radiotherapy and volumetric modulated arc therapy, is a resource-intensive process requiring a high level of treatment planner intervention to ensure high plan quality. This can lead to variability in the quality of treatment plans and the efficiency in which plans are produced, depending on the skills and experience of the operator and available planning time. Within the last few years, there has been significant progress in the research and development of intensity modulated radiotherapy treatment planning approaches with automation support, with most commercial manufacturers now offering some form of solution. There is a rapidly growing number of research articles published in the scientific literature on the topic. This paper critically reviews the body of publications up to April 2018. The review describes the different types of automation algorithms, including the advantages and current limitations. Also included is a discussion on the potential issues with routine clinical implementation of such software, and highlights areas for future research.
Introduction
Radiotherapy is a major non-surgical technique in the treatment of cancer. A technology that was once expected to become obsolete in the face of chemotherapy and biological therapy is now used in around 40–60% of all cancer patient cases.1–3 This is partly due to technological advances, such as fixed- and rotational-field intensity modulated radiotherapy (IMRT) techniques and image-guided radiotherapy (IGRT), leading to more accurate radiotherapy.4 The field has entered an exciting era with rapidly evolving developments such as image-guided four-dimensional adaptive radiotherapy (ART), integration of novel & advanced quantitative imaging and future developments on the horizon such as stratified or personalised radiotherapy.5–10 At the heart of these developments is the central role of optimised, high quality and efficient treatment planning. Complex IMRT techniques have to be inversely planned; this means that a computerised treatment plan is generated by defining a set of objectives and constraints for tumour coverage and healthy tissue sparing and the software uses these to generate a large number of radiation segments to deliver the required dose. Even though the inverse planning process is highly computerised, it is still human resource intensive, and typically a high level of treatment planner intervention is required to ensure a high-quality plan is produced. A typical inverse IMRT planning pathway is shown schematically in Figure 1 and described in detail in the figure legend. Especially, the depicted interactive feedback loop can lead to variability in inter- and intracentre plan quality depending on the skills and experience of the operator, which could affect clinical outcome11 and the efficiency in which a plan is produced. Such resource requirements may also limit access to advanced IMRT and emerging treatments such as adaptive radiotherapy, or to suboptimal usage of these techniques.
Figure 1.
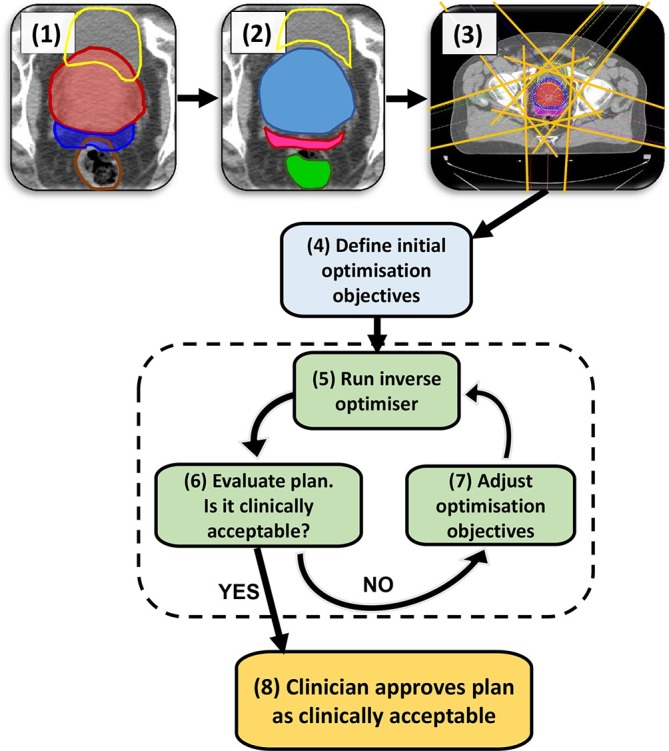
A typical manual IMRT treatment planning pathway. The example shown is for a prostate + seminal vesicle case. The steps are as follows: (1) CT scan with PTVs and OARs delineated; here the colours of ROIs are red: Prostate PTV, dark blue: SV PTV, yellow: bladder OAR, brown: rectum OAR. (2) create a range of “helper” (ROI) to aid the optimiser; e.g. the part of an OAR not overlapping with the PTV, PTVs overlapping with each other, ring structures to control dose spillage. In the example in Step 2, the ROIs shown are yellow: bladder cropped from prostate PTV, green: rectum cropped from seminal vesicle PTV, magenta: SV PTV cropped from prostate PTV, blue: prostate PTV unedited as it is the higher dose prescription than SV PTV. Step (3) set-up beam geometry. (4) Define the initial optimisation objectives either from scratch or from a class solution. (5) Run the inverse optimiser until it converges to a solution, calculate dose distribution. (6) Evaluate the resulting plan, if it is clinically acceptable proceed to Step 8, otherwise go to Step 7 to adjust the optimisation objectives. The part shaded in green (steps 5, 6, 7) is the iterative process of optimisation required by the planner to arrive at a clinically acceptable treatment plan to be approved by the clinician in Step 8. After this step, the plan will go through the quality control process and preparation for treatment, not shown on the flow chart. IMRT, intensity modulated radiotherapy; OAR, organ at risk; ROIs, regions of interest; PTV, planning target volume; SV, seminal vesicle.
Efforts to streamline and standardise the treatment planning process are ongoing. In the last few years, there has been significant progress into research and development of automated inverse treatment planning approaches, with most commercial manufacturers now offering some form of solution. There is a rapidly growing body of research published in the literature. These algorithms could significantly improve the efficiency, consistency, and quality of treatment planning, leading potentially to improved patient access and improved patient outcome through maintaining and improving high-quality radiotherapy. In 2014, the National Health Service in England and Cancer Research UK published a 10 year Vision for Radiotherapy in the UK to allow patients to receive advanced and innovative radiotherapy that is cost-effective, and one suggestion to facilitate this is through the implementation of software that automate parts of the planning process.1
This paper critically reviews the body of publications up to April 2018. The review describes the different types of automation algorithms for IMRT planning, including the advantages and current limitations. Also included is a discussion on the potential issues with routine clinical implementation of such software, and highlights areas for future research.
Literature search methodology
The literature was searched using Elsevier Scopus®, MEDLINE, Web of Science™ using the following keywords and logic statements: (“automated planning” OR “automatic planning” OR “automation planning” OR “automate planning” OR “knowledge-based” OR “Pareto” OR “multicriteria optimisation” OR “multicriteria optimization” OR “template based optimization” OR “template based optimisation” OR “interactive optimization” OR “interactive optimisation” OR “artificial intelligence” OR “AI” OR “artificial neural network” OR “dose prediction” OR “machine learning” OR “RapidPlan” OR “AutoPlan” OR “rayNavigator”) AND (“radiotherapy treatment planning” OR “radiation therapy treatment planning” OR “IMRT treatment planning” OR “intensity modulated radiotherapy treatment planning” OR “VMAT treatment planning” OR “volumetric modulated arc therapy treatment planning” OR “Tomotherapy treatment planning” OR “radiotherapy planning” OR “radiation therapy planning” OR “IMRT planning” OR “intensity modulated radiotherapy planning” OR “VMAT planning” OR “volumetric modulated arc therapy planning” OR “Tomotherapy planning”).
The search was made on the 7 May 2018 and included articles published up until the end of April 2018. Only full peer-reviewed original research articles written in English were included. There was no specific limit set on the date of earliest publication. After filtering to remove journals unrelated to healthcare and merging the searches from the different databases, 342 eligible records remained. These records were manually scanned based on the title to highlight articles for inclusion. The criteria were to retain articles that clearly described either the idea, development or clinical application of automated inverse treatment planning for IMRT, VMAT, or tomotherapy. Articles that only described automatic selection of beam angles, and did not also describe subsequent automation of the inverse IMRT or VMAT treatment planning, were excluded from this review. While these studies are interesting, the decision was made to apply this criterion to focus the review on the current topical issue of automated inverse planning for IMRT, VMAT or tomotherapy. In articles where the title was deemed ambiguous as to whether it fit the criteria for inclusion, the abstract was read. In total, 171 peer-reviewed papers in scientific journals were included up until the end of April 2018. The earliest publication on the topic of automated planning in IMRT was in 2003; there were other publications pre-2003 related to three-dimensional conformal radiotherapy, however while important, these were excluded from this critical review. Henceforth, we will succinctly refer to automated inverse IMRT planning as “automated planning”.
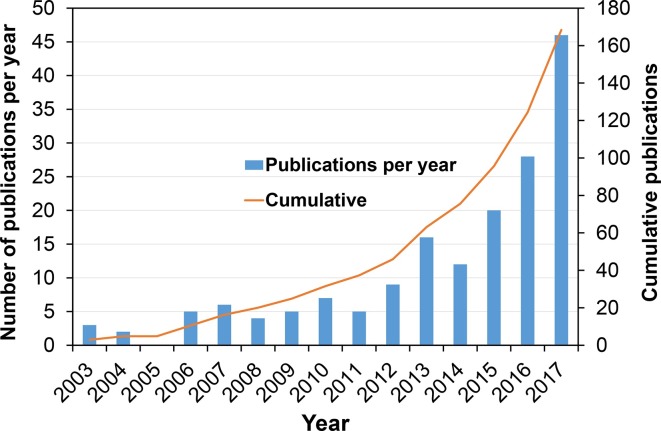
Figure 2 shows the number of publications per year and the cumulative number of papers over the years. The curve exhibits some of the characteristics described by Rogers.12 There is the initial “lag” period with a steady trend until 2011. After this, there is a sharper uplift in the number of papers published per year; described as the “take-off” phase. Around 2008, major radiotherapy manufacturers began releasing commercial systems and this upward trend represents the effect of the early innovations of automated treatment planning being translated into widely available software. There is no evidence yet of the tail-off phase where the innovation has been so widely adopted that new research is limited. This graph, of course, does not demonstrate the rate of clinical adoption as some of the papers are multiple publications from the same group. Moreover, not all papers related to clinical application.
Figure 2.
Trend showing the number of peer-reviewed publications on innovations in automated planning software per year, and the cumulative number of publications. The graph shows a significant increase from 2011.
Through the literature search, three different paradigms of automated planning that were employed in clinical practice are apparent. These are: knowledge-based planning (KBP),13–85 protocol-based automatic iterative optimisation (PB-AIO)86–110 and multicriteria (or also called multiobjective) optimisation (MCO).111–183
Background on the different automated planning algorithms
Knowledge-based planning
An approach to improving the speed, efficiency and reducing variability in treatment planning is using so-called KBP approach. KBP is defined as any approach which directly utilises prior knowledge and experience to either predict an achievable dose in a new patient of a similar population or to derive a better starting point for further trial-and-error optimisation by a planner. There are two distinct approaches to this: the atlas-based approach and the model-based approach.
In the atlas-based method,13,14,25,36,47,58 the knowledge base is used to select the closest matching patient(s) to give a better starting point for the inverse optimisation than would be provided by conventional template-based approaches. Chanyavanich et al investigated an approach of predicting the starting treatment machine parameters based on a database of prior prostate cancer fixed-field IMRT plans.58
Dose-volume histogram (DVH)-guidance is one of the approaches of model-based KBP.15–24,26–32,69,80,84,85 In this approach, a large number of clinically accepted treatment plans and contours are used to characterise the relationships between anatomical and geometric features for a given anatomical site to build a predictive DVH model for that site. For any new patient treated in the same anatomical site, this knowledge can be used to predict the achievable DVH based on the features of similar contours and quality of treatment plan; see an example in Figure 3. A range of different implementations of DVH-guided KBP has been proposed and developed. Commercially, the DVH-guidance KBP approach is utilised by the Varian Eclipse® Treatment Planning System as RapidPlan™ (Varian Medical Systems, Palo Alto, CI).
Figure 3.
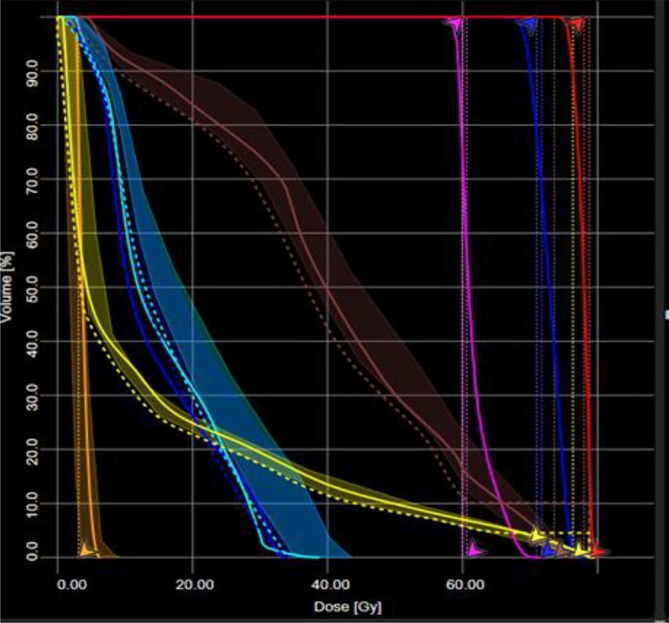
An example of DVH prediction KBP in a 3-dose level localised prostate cancer case. The shaded lines are the predicted range of achievable DVHs for the different OARs. The solid lines are the actual achieved DVH in the plan. This example is from Varian RapidPlan and the dashed lines and arrows are the optimisation objectives that have been generated by RapidPlan. Courtesy: Royal Surrey County Hospital NHS Foundation Trust, Guildford, UK. DVH, dose-volume histogram; KBP, knowledge-based planning; OAR, organ at risk.
A known limitation of the DVH-guidance approach is that the DVHs are only predicted for the regions of interest that are delineated. This means that regions of tissue outside of delineated regions of interest (ROIs), which a human planner may also optimise to reduce dose, may not be taken into account, e.g. to enhance conformality or avoid hot spots. Additionally, DVHs do not provide any spatial information. An interesting approach that has been investigated to overcome these issues is voxel-based dose prediction. Rather than predicting DVHs, the idea is to use knowledge from prior plans to build a model that can predict doses to individual voxels within the patient’s image.33–35,38,39 A limitation of the model-based dose prediction approach is that the plan quality for new patients strongly depends on the quality of plans generated in the past.
Protocol-based automatic iterative optimisation
The challenge with manually optimising a plan is that it is sometimes a time-consuming process to arrive at a clinically acceptable plan. Moreover, it is often not clear if the plan could be better if further adjustments of the optimisation criteria were made. The clinically optimal plan is one where there is the best trade-off between normal tissue sparing and target coverage, taking into account the clinical requirements and priorities regarding sparing of the various tissues. For example, one may consider a head and neck cancer where the PTV abuts the spinal cord. In this case, it is typically the highest clinical priority to keep the spinal cord within tolerance and therefore, requires compromise of PTV coverage. It is relatively straightforward to achieve a plan that meets the spinal cord tolerance (and may. e.g. still achieve the PTV D95% objective). However, a better plan may be one that keeps the spinal cord just within tolerance while maximising the coverage of the PTV, as well as pushing the lower doses to other healthy tissue as low as possible. Achieving this better plan manually would require significant time, effort, and planner experience, as it requires iterative adjustment of optimisation criteria to keep challenging the optimiser to achieve a progressively better plan until no improvement in plan quality is possible. The success of performing this process manually and efficiently will depend on the skill and experience of the treatment planner, and the time available to plan.
One approach to solve this is to automate the iterative adjustment of the optimisation objectives and constraints.86–88,98,104–110 The basic idea is to start with a user-defined template which has the required clinical objectives. The user can then input the priorities for mandatory (hard) constraints. The optimiser then generates a plan that meets all the objectives, at which point the high priority constraints are locked down and become hard constraints. A script is then put in motion which iteratively pushes the DVH of all of the structures to the point where the hard constraints were just breached, and then a step is taken back to the point where the breach did not occur. At this point, the plan cannot be pushed further and could be considered the optimum plan.
Tol et al107 developed an interface to move the mouse cursor on the computer screen automatically and thus adapt the optimisation objectives in the Varian Eclipse VMAT optimiser. The interface detects the position of the DVH line for each ROI on the screen and iteratively moves fixed objectives to ones more challenging during the optimisation and was shown to be able to automate VMAT planning of head and neck cancer giving improved dosimetric results.107
Various authors have developed artificial intelligence (AI) systems which simulate the reasoning behaviour of a human planner to automatically adjust the optimisation parameters during the optimisation process.98,104,105,109 Such AI systems have been based on fuzzy logic theory whereby the trial-and-error actions of expert human planners were converted into binary “IF-THEN” logic statements.
A commercially available solution is AutoPlanning within the Philips Pinnacle3 TPS (Philips Radiation Oncology Systems, Fitchburg, WI). The user initially generates a template (“Treatment Technique”) which has the target prescriptions and the goals for organ at risk (OAR) sparing according to the required clinical protocol. For OARs, the user also specifies their clinical importance, from those that have low significance to those that have hard constraints. Based on the PTV(s) and OARs defined, the software automatically generates “dummy” optimisation structures such as those that take into account overlap between OAR and PTV, PTV ring structures to control dose fall, and various other “help” structures to control target uniformity and dose spillage to the rest of the body. There are also additional “advanced” settings to control dose fall-off, homogeneity and managing cold/hotspots, which initially have default factory set values or could be fine-tuned by the user. Based on the optimisation contours and the settings used, the software automatically generates the starting optimisation criteria. The software then enters into a 5-loop iterative optimisation cycle to gradually fine-tune the plan to achieve a solution based on the clinical protocol as defined by the user.89,103
Multicriteria optimisation
Another approach which seeks to overcome the issue of finding the optimal trade-offs between target coverage and sparing of all normal tissues is called MCO (also sometimes called multiobjective optimisation).121,131,140,143,146,184 Central to MCO is the concept of the “pareto optimal solution”; which is a plan that cannot be improved in any of the objectives without degrading at least one of the other objectives. There are two approaches to MCO, a posteriori and a priori approach. In the a posteriori approach, rather than the optimiser generating a single plan, multiple plans are automatically generated where each criterion is optimised to the extent where it cannot be improved upon without affecting at least one other criterion; each of these plans is a so-called pareto optimal solution. The schematic in Figure 4 illustrates this concept with a graph of two competing criteria. The graph shows a large number of different feasible planning solutions, representing a variety of different permutations for criterion 1 and 2. The solid line represents the pareto front where improving one criterion inevitably leads to the worsening of the other and vice versa. Plans that lie on this front are the “pareto optimal solutions”; shown as blue circles in the schematic. The plans shown as diamonds are referred to as “dominated” because there is always a solution on the pareto front where at least one criterion can be improved. Pareto optimality by itself does not imply clinical optimality and pareto optimal plans can be clinically highly undesirable. On the other hand, the best clinically acceptable plan is pareto optimal. Therefore, in the a posteriori approach the database of pareto optimal plans is interactively (a posteriori) navigated by the treatment planner to choose a clinically optimal plan.127,131,140,144 The automation in this process is that the database of pareto optimal plans is automatically generated. The main issue with this approach is the number of plans that are generated, since mathematically there is an infinite number of pareto optimal plans, and the intensive computing resource required can be a limiting factor. Moreover, especially in the case of a large number of clinical objectives, selection of a plan may be difficult and operator-dependent. The a posteriori approach has been implemented in the commercial RayStation TPS, and recently as an option in the Varian Eclipse TPS. In this approach, the dimensionality of the pareto fronts is dictated by the number of objectives, and thus also the number of plans that are required to construct the fronts. Craft and Bortfield describe a method to estimate the number of plans that are sufficient128 and suggest that N+1 plans are sufficient, where N is the number of objectives. At the time of writing, this recommendation is the basis of the number of plans generated in RayStation rayNavigator. Different methods were described in the literature for interactively navigating the pareto fronts, using a navigation star127,131,140,144 or sliders, however, the premise is still the same. The lowest and highest values for each objective is displayed visually along with a starting dose distribution and DVH line. The user then uses the mouse to drag the objective of interest and in near real-time, the software finds the plans in the database that are better in the selected criterion, and then, via fast interpolation, the dose distribution and DVH are updated.
Figure 4.
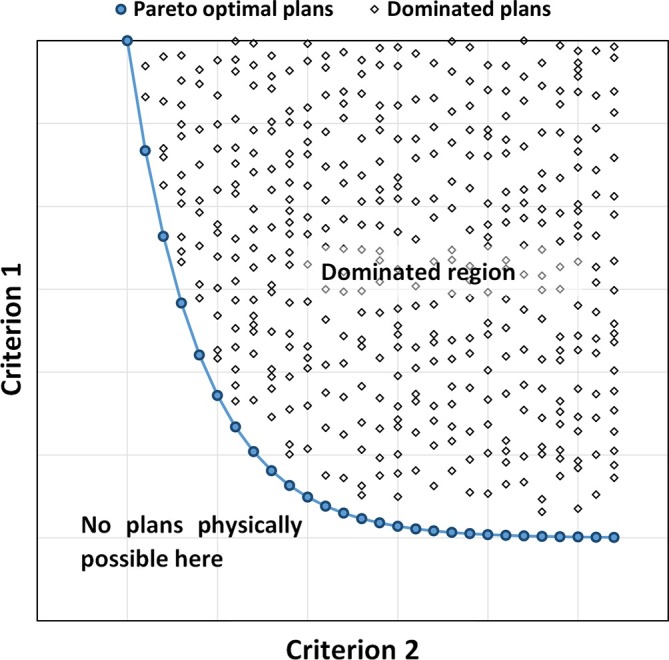
Schematic diagram of two competing criteria. The graph shows a large number of different feasible planning solutions, representing a variety of different permutations for criterion 1 and 2. The solid line represents the pareto front where improving one criterion inevitably leads to the worsening of the other and vice versa. Plans that lie on this front are the “pareto optimal solutions”, shown as blue circles in the schematic. The plans shown as diamonds are referred to as “dominated” because there is always a solution on the pareto front where at least one criterion can be improved.
In the a priori MCO approach, for each patient, only a single pareto-optimal plan is fully automatically generated. This plan has clinically desired trade-offs between all objectives, in line with the institution’s clinical protocol and treatment tradition.143,146,185 Optimization is based on a treatment site-specific protocol, a so-called “wish-list”, containing the objective functions with assigned priorities and hard constraints that should never be violated. An example wish-list for automated plan generation with iCycle for localised prostate cancer patients is in Table 1. In an automatic multiobjective optimisation approach, the objectives are sequentially minimized according to their priorities to obtain a pareto optimal plan with favourable balances between all objectives. Wish-lists are treatment site specific, i.e. no patient-specific adaption is applied. They are generated in an iterative tuning process, involving the multidisciplinary planning team. In this process, a first estimate of the wish-list is made based on a review of plans of recently treated patients, the clinical protocol, and initial team discussions. This wish-list is then improved in several iterations consisting of (1) use of current wish-list to automatically generate treatment plans for CT-scans of a small group of previously treated patients (typically five), (2) evaluate the automatically generated plans (including comparison with clinically applied plan), (3) update the current wish-list (new estimate), (4) go back to (1). This iterative improvement of the wish-list is stopped when further improvements of the wish-list are deemed not possible. This iterative wish-list improvement has an intrinsic drive to improve the clinical plan quality. This a priori MCO approach has been developed and implemented in the Erasmus MC Cancer Institute in their “Erasmus-iCycle” software.129,143,146,162 Apart from beam profile optimisation, the system also features automated beam angle optimisation.161 As well as optimisation for regular linacs, Erasmus-iCycle has separate models for optimization of Cyberknife treatments186 and proton treatments (intensity modulated proton therapy, IMPT).187 Currently, Elekta AB (Stockholm, Sweden) is preparing a commercial implementation of the system for photon beams.150
Table 1.
An example wish-list for automated plan generation with Erasmus-iCycle for localised prostate cancer patients
| Constraints | ||||
| Volume | Type | Limit | ||
| PTV | Max dose | 105% of DPx | ||
| PTV | Mean dose | 101% of DPx | ||
| Rectum & anus | Max dose | 102% of DPx | ||
| PTV shell 50mm | Max dose | 50% of DPx | ||
| Unspecified tissues | Max dose | 105% of DPx | ||
| Objectives | ||||
| Priority | Volume | Type | Goal | Parameters |
| 1 | PTV | ↓LTCP | 0.8 | DPx = 78Gy, α = 0.8 |
| 2 | Rectum | ↓EUD | 20Gy | k = 12 |
| 3 | OAR 2 | ↓EUD | 10Gy | k = 8 |
| 4 | PTV shell 5mm Skin ring 20mm |
↓Max dose ↓Max dose |
80% of DPx 20% of DPx |
|
| 5 | Rectum | ↓Mean dose | 5Gy | |
| 6 | Anus | ↓Mean dose | 5Gy | |
| 7 | Bladder | ↓Max dose | 5Gy | |
| 8 | PTV shell 15mm PTV shell 25mm |
↓Max dose | 50% of DPx 30% of DPx |
|
| 9 | Left & right femoral heads | ↓Max dose | 50% of DPx | |
α, cell sensitivity;EUD, equivalent uniform dose;k, volume effect; LTCP, logarithmic tumour control probability; PTV, planning target volume;DPx, prescribed dose.
The priorities assigned to the objectives are used in the a priori MCO, guaranteeing for each patient generation of a pareto-optimal plan with clinically favorable balances between all treatment objectives. (Courtesy: A.W. Sharfo).
Clinical evaluation and implementation of automated planning techniques
There are many studies in the literature which have clinically implemented commercial and in-house implementations of PB-AIO, KBP, and MCO automated planning.42–46,48–57,59–68,70–79,81–83,89–97,99–103,150–155,157–166,168–177,179–181 Most of these articles tackle the current commercial implementations of Varian RapidPlan (KBP), Pinnacle AutoPlan (PB-AIO), Raystation (a posteriori MCO), and Erasmus-iCycle (a priori MCO).
From the present literature review, 81 (~43%) papers were reporting on the clinical evaluation, implementation, or application of automated planning. It was noted that most studies were retrospective and different methodologies were reported for evaluating automated plans and manual plans. The most popular method employed, in 67 of the 81 papers, was evaluation based on comparing DVH metrics for PTVs and OARs, or deriving other metrics from DVHs such as conformity index, homogeneity, tumour control probability, normal tissue complication probability. Some papers used these in conjunction with qualitative blinded clinician evaluation which took the format of either binary decisions on whether plans were clinically acceptable95,162,169,172 or giving plans a ranking.93,103,164 Most studies are retrospective, comparing already delivered clinical plans with automatic plans. Voet et al performed a prospective study on automated planning of head and neck cancer with Erasmus-iCycle giving, for each new patient, the treating clinician the choice between a plan made with routine trial-and-error planning and an automatically generated plan.162 Hansen et al prospectively compared Pinnacle3 AutoPlan vs manual planning in head and neck cancer with blinded review by three clinicians to select the better plan.89
Knowledge-based planning
KBP has been clinically investigated in various clinical sites, with most studies reporting that KBP is at least as good, or (slightly) better, than manual planning with improved efficiency and consistency, without manual intervention.42–46,48–57,59–68,70–79,81–83 All of the published studies are via the use of the commercial implementation of KBP in the Varian RapidPlan solution. Reports have been published for head and neck cancer,72,79,82 prostate cancer,62,66,78,83,188 cervical cancer,78 lung cancer,75,83,189 spinal metastasis,63 breast cancer,65 upper gastrointestinal (GI) cancers,61,70,76 and lower GI cancers.190 Foy et al reported that KBP could reduce the VMAT planning of stereotactic body radiotherapy of the spine from 1–1.5 h to around 10–15 min.63 Hussein et al reported on the clinical validation and benchmarking of the commercial RapidPlan KBP system for both IMRT and VMAT planning in prostate and cervical cancer. The authors highlighted that using the software “out-of-the-box” with the default settings for training the KBP models lead to automated plans with poor conformity, coverage and plan generation efficiency compared to the original clinical plans, and that an iterative process is required to fine-tune and optimise the model. After this refinement of the model was performed, the authors showed that RapidPlan was able to achieve better or comparable plans when compared to the original clinical plans.78
Typically, a dose prediction KPB model is trained for one particular technique and clinical site, meaning that the model has been characterised for that particular population of patients; take, e.g. a prostate static field IMRT model. Suppose that the treatment technique was changed to VMAT or the model was shared with a centre that does not have the capability for VMAT. An option is to create a VMAT specific model, which requires replanning of a large number of patients followed by the refinement of the model. However, as the KBP model predicts the dose based on the anatomy of the patient and not treatment technique, there is the potential that (in this example) the IMRT model could be used outside of its original scope for VMAT planning. This is an interesting research question to demonstrate how robust a model is to changing techniques and sharing between centres. Additionally, broadening the scope of the model further by including both IMRT and VMAT plans may potentially improve plan quality. Cagni et al investigated the use of helical tomotherapy plans to create KBP models for prostate cancer and found that this could be successfully performed.53 Other areas of interest are whether a model that was trained for a particular clinical site could be used in another site with similar anatomy and similar relative dose levels to the original model. Some of these areas have been addressed in a limited number of studies but is still an area of active research.61,66,68,74,76,78 Wu et al investigated using a RapidPlan model trained on VMAT rectal plans treated in the supine position to create plans in patients treated with IMRT and those who were set up in the prone position. The study found that OAR sparing and plan consistency was improved but that the optimiser needs to be readapted to IMRT planning and that manual hotspot reduction is required.74 Most of the reported studies in the literature focussed on single institution analysis. The performance of a broad scope RapidPlan KBP model for oesophageal cancer was investigated by Fogliata et al,76 whereby the model took into account different dose prescriptions and tumour locations with the ultimate aim of determining whether the model could be shared amongst different centres where variations in clinical protocols can occur. The authors carried out the study across three centres, where one centre did not contribute any data to the model. In the latter centre, KBP resulted in superior plan quality. The study highlights the potential benefit of a heterogeneous data set, and this has also been highlighted by other studies that suggest that keeping statistical (but clinically relevant) outliers may be an advantage to the model strength.57,78
An apparent limitation of the KBP approach is that the models can only be as good as the training data that has been input in the first instance. Strictly speaking, the plans can be clinically acceptable but not the optimal plan. RapidPlan attempts to get around this by always placing the optimisation objectives for OARs lower than the predicted DVH such that it always tries to improve on the prediction.
Protocol-based automatic iterative optimisation
Papers in the literature have generally reported that PB-AIO, commercially implemented within the Pinnacle TPS, is either equivalent to or superior to manual planning regarding plan quality and efficiency in automatically generating IMRT or VMAT plans in various clinical sites.89–97,99–103 Hazell et al compared PB-AIO with manual planning of 26 IMRT head and neck cancer plans and evaluated 2 types of plans through DVH metrics and clinician-blinded reviews. They found comparable target coverage and better sparing of normal tissues, with all plans clinically acceptable without manual intervention.103 Hansen et al extended the analysis to VMAT and found similar results and reported that planner time was halved from 64 min using PB-AIO.89 Speer et al used a quantitative point-based scoring system where treatment plan parameters were scored to objectively judge plan quality of PB-AIO over manual planning in head & neck cancer, where a score of 100 points indicates an optimum plan. They demonstrated automated plans using PB-AIO were better with an average score of 62.3 points compared to manual plans with a score of 59.1 points.100 Similar conclusions about the potential for PB-AIO to efficiently produce clinically acceptable plans for head and neck cancer have been reported.97,101.91 Nawa et al compared PB-AIO with manual planning in 23 prostate cancer cases. Comparison was performed using DVH objectives, and the study found target coverage and rectal dose was comparable between PB-AIO and manual planning. There was a significant reduction of the dose to the bladder and femoral heads with PB-AIO compared to manual plans. This is potentially due to the manual plans not pushing these doses down further as they had already passed the clinical tolerances and more attention was paid to the harder to achieve rectal dose constraints. Additionally, the authors quantified a reduction in interoperator variability with PB-AIO.91 PB-AIO has been evaluated for hippocampal sparing whole brain radiotherapy87,99 and was found to result in comparable or better plans with minimal manual intervention and expedited planning time which is essential in this palliative group of patients. Studies in oesophagus,92,95 and rectal cancer93 cases are also consistent in their conclusions about the potential benefit of PB-AIO over manual planning.
However, whilst the overall message in all of these papers is favourable for PB-AIO, some studies argue that PB-AIO is a tool to improve overall plan quality, but not necessarily to completely remove the need for manual optimisation103 and that for some particular cases, experienced planners performed better than PB-AIO.100 The quality of plans generated by Auto-Planning in Pinnacle has also been reported to be dependent on the input from experience treatment planners to set the initial user settings and define good clinical protocols which are also an important consideration.100
Multicriteria optimization
A posteriori MCO clinical implementations (all in RayStation) have been investigated and validated in different clinical sites including prostate cancer,168,169,171,176,179 head and neck cancer,164,171 brain,168 lung cancer,172 and lower GI cancers.177 All studies report better or comparable plan quality with a reduction of planning time. Wala et al report that MCO using RayStation took approximately 1 h per case and achieved superior plan quality based on blinded review and DVH objective comparisons for localised prostate cancer.169 Other studies also report comparable or superior plan quality of a posteriori plans in prostate cases. Muller et al report a reduction in planning time by around 10 min for post-prostatectomy cases and 45 min for brain tumour cases.168 Chen et al used MCO to generate 20 field IMRT plans for prostate cancer and head and neck cancer to then use the resulting DVH information as the basis for defining optimisation objectives for VMAT plan optimisation. Using this method, they were able to match the quality of single arc VMAT with the quality of a 20 field IMRT plan (where there is high quality due to the large degree of freedom).171 Kamran et al evaluated the potential benefit of a posteriori MCO in 10 patients with non-small cell lung cancer who were eligible for the RTOG 1308 Phase II trial.172 Evaluation of plan quality between MCO and manual planning was performed via a double-blinded review and DVH metrics. While all the MCO plans passed the DVH objectives, it was noted that clinicians preferred 8/10 of the MCO plans. The two manual plans were chosen due to better skin sparing and a lower maximum dose to the spinal cord, even though the oesophagus dose was lower. Planning time improved by a median of 88 min.172
Navigation-based (a posteriori) MCO could potentially avoid the often iterative interaction between planner and physician to arrive at a clinically acceptable plan by involving the clinician at an earlier stage. However, it is not clear as to whether, practically, this results in an improvement in the planning workflow particularly given the often limited clinician time. Müller et al investigated this retrospectively in prostate and brain cancer planning and demonstrated potential time savings, but further prospective studies are required.168 In the context of a technique like a posteriori MCO, how the clinician and planner role will evolve will likely vary between different countries. For example in the UK, the Clinical Oncologists are responsible for administering both radiotherapy and other non-surgical treatments such as chemotherapy whereas in other countries there is a different approach with Radiation Oncologists who are more focused in radiotherapy. Therefore, the practicality of a posteriori MCO in the hands of clinicians in the UK will likely be different. One approach may be treatment planners taking on an extended role, or formalising an existing role, in the decision-making aided by the availability of other relevant clinical data and with the appropriate training and qualifications.
A limitation of current implementations of a posteriori MCO is that the plans optimised are near pareto optimal in the fluence space and do not directly consider the machine parameter optimisation. The final navigated plan is then converted into a deliverable using direct aperture optimisation. However, McGarry et al and Kyroudi et al have shown that dosimetric discrepancies can occur between the conversion of the navigated plan into a deliverable plan, and therefore may not reflect the clinical preferences that resulted in the choice of the navigated plan and this may increase the uncertainty in the plan navigation process.165,179 In most cases, this dosimetric difference may not translate to a clinically significant difference, and the advantage in the ability for navigating the trade-offs was retained. However, for some cases where there are small targets on low-density tissues, the dosimetric difference can be significantly larger such that manual fine-tuning is likely required.165
A priori MCO with Erasmus-iCycle for linacs was validated for head and neck cancer,162 prostate cancer,150,185 cervical cancer,174 lung cancer,180 spinal metastases,175 and gastric cancer.151. By itself, Erasmus-iCycle had fully automated MCO for beam fluence optimization. For the generation of clinically deliverable plans, the system was used as a pre-optimizer for the commercial Monaco TPS (Elekta AB, Stockholm, Sweden), which generates a deliverable, segmented plan that mimics the pre-optimized Erasmus-iCycle dose distribution. In the first evaluation study (on head and neck cancer), this plan reconstruction in Monaco was performed by a planner.162 In all later studies, the reconstruction in Monaco was fully automated, i.e. for each patient the Erasmus-iCycle dose distribution was used to automatically create a patient-specific Monaco planning template, which was then used by Monaco for automated generation of a deliverable VMAT or IMRT plan, mimicking the Erasmus-iCycle plan. In all validation studies, the automated Monaco plans were compared with manually generated Monaco plans. Manual fine-tuning of automatically generated plans was not performed. For all investigated treatment sites, there was a considerable reduction in hands-on planning time, which virtually reduced to zero with fully automated planning. For treatment of the prostate only or prostate with seminal vesicles, plan quality between automated and conventional plan generation was similar.185 For treatment of the prostate with seminal vesicles and elective nodal irradiation and all other sites, the quality of the automatically generated plans was superior. In the prospective head and neck cancer study,162 treating clinicians could, for each patient, choose between an automatically and a conventionally generated plan. In 97% of cases, preference was given to the plan that was generated with Erasmus-iCycle. At the Erasmus MC Cancer Institute, fully automated planning with the combination Erasmus-iCycle/Monaco was in routine use for prostate, cervix, lung, and head and neck cancer patients.
Novel approaches to using automated planning algorithms
Automated planning as a plan quality assessment and checking tool
It was recognised in several studies that the principle of KBP can be used both as an automated planning tool and as a plan quality assessment tool. This is because the first component of KBP is to use prior knowledge to predict the achievable dosimetry for a prospective patient. This information can be used to judge the quality of a plan and has been shown to be effective by several groups.32,36,59,65,71,77,188,191 Additionally, it has also been reported to be a useful tool for training new staff members and improve the quality of manual planning, and also useful in clinical trial QA.61
“Bias-free” comparison of different treatment techniques using automated planning
Comparing different treatment techniques (e.g. VMAT and IMRT) in a treatment planning study can be prone to human subjectivity and bias, particularly there can be questions to what extent there were differences in the optimality of the plans for the compared techniques. An interesting approach is to use automated planning in these studies.163,166,173,181,192 Boylan and Rowbottom developed a PB-AIO approach and applied it in comparing seven fixed-field IMRT with two arc VMAT for nasopharyngeal head and neck cancer patients using a standard protocol, and to investigate two experimental strategies (a parotid-sparing strategy and dose escalation strategy).192 They showed that the IMRT and VMAT techniques were clinically comparable for the standard and dose escalation protocols, whereas VMAT was better in the parotid sparing strategy. Lechner et al, used MCO to objectively compare the quality of flattening filter free IMRT and VMAT vs flattening filter plans in prostate and head and neck cancer patients.163 Sharfo et al used automated planning for bias-free comparisons of IMRT and VMAT techniques for cervical cancer.181 They demonstrated that a 12 field IMRT technique had similar quality as a dual-arc VMAT technique. Sharfo et al also used bias-free automated planning for comparison of liver SBRT with a fully non-coplanar technique, coplanar VMAT, or a new approach called VMAT+.161 The latter was defined as VMAT supplemented with 1–5 computer-optimized non-coplanar beams. Regarding plan quality, they demonstrated that VMAT+ was superior to VMAT, and almost as good as fully non-coplanar. Treatment times with VMAT+ were much shorter than with fully non-coplanar treatment.
In prostate cancer (and other pelvic malignancies), one of the common challenges faced by treatment planners is the scenario where a patient has an artificial metallic hip implant. This presents a challenge to the planner as a typical technique is to avoid beams entering through the implant due to uncertainties in the density, which limits the permissible beam directions. The difficulty is amplified if the patient has bilateral implants. Voet et al, reported on using a priori MCO to automatically investigate different fixed-field IMRT strategies using the iCycle software which was able to optimise both beam angles and fluence profiles.173
Automated planning as a decision support tool for treatment selection and personalised treatment
Studies have reported on the use of automated planning as a useful tool for making informed decisions on a patient’s eligibility for specific novel radiotherapy techniques. The potential advantage of automated planning in this context is the quick production of plans for different techniques and clinical scenarios, where otherwise resource-intensive manual plan generations would be required.
One application of this is patient selection for proton therapy. Automated planning is used to select patients suitable for whom a proton treatment plan may be suitable and to promote that only patients that would clinically benefit from the treatment are selected and to thus avoid using this limited resource in patients where potentially a faster photon-based plan would be equally or more clinically useful. Delaney et al found that a KBP model based only on prior photon VMAT plans was able to predict proton DVHs and therefore, may be used in identifying patients for proton therapy.64 Bijman et al used MCO to generate photon and proton plans, and while the primary focus of their study was in the context of analyzing the uncertainty of using normal tissue complication probability models for patient selection, the use of MCO for fast plan generation was still demonstrated.193
In patients with liver tumours (such as from hepatocellular carcinoma or oligometastatic disease) and who are contraindicated for surgical intervention, stereotactic ablative radiotherapy (SABR) is a promising treatment modality. However, there are some limiting factors and criteria which dictate whether a patient is eligible for SABR. The most significant of these is the dose to the healthy liver tissue, which will vary depending on the tumour volume and liver volume. In practice, the decision on eligibility can only be determined once the trial-and-error planning has been attempted, which is an inefficient use of resources. Tran et al, reported on using KBP as a tool for predicting patient eligibility for liver SABR and to also determine whether the patient would benefit from a more complex non-coplanar technique than a standard coplanar VMAT technique.24 Rønde et al investigated the feasibility of using MCO for shared decision-making in anal cancer and conclude that patient–clinician preference-informed plan selection is feasible.177 Smith et al described a novel approach to personalized treatment planning by integrating a model of radiotherapy outcome with MCO for prostate cancer treatment.170 The MCO model generates the set of pareto optimal plans which are then integrated into a Bayesian network to model the probabilities of outcomes such as toxicity, recurrence, distant metastasis. To predict these probabilities, the model uses information from expert opinion and published data, and patient characteristics such as clinical staging, Gleason score and PSA. The final step is use these probabilities in a Markov model then to predict Quality Adjusted Life Expectancy which is then the final basis for ranking and selecting the best plan. This approach appears to be promising; however, the authors point out that further work is required to validate the accuracy of the predictions of outcomes. A similar approach has also been reported for glioblastoma.139 Valdes et al describe an AI approach which identifies previously approved treatment plans which are achievable for a prospective patient to aid decision made on a personalized level.40
Automated planning for plan library in plan-of-the-day (PotD) adaptive radiotherapy
Due to various challenges in modern radiotherapy, such as limitations in accuracy in automated image segmentation and automated planning speed, daily online replanning based on daily reimaging has not been routinely applied. A simpler, but more feasible approach is PotD ART, which has been reported for bladder cancer,194 cervical cancer195 and rectum cancer.196 In this approach, the patient is imaged daily and then treated with a plan selected from a pre-treatment established patient-specific plan library. The library contains plans for various patient anatomies. The PotD is the library plan that best fits with the anatomy-of-the-day. PotD ART involves generation of multiple plans for each patient, increasing the planning workload for a department. Heijkoop et al have avoided this problem by applying automated plan generation.195 For each cervical patient, they created a library with up to three plans.
Discussion
Over the last few years, innovations in automated treatment planning software have led to the potential to improve the efficiency and quality of radiotherapy treatment planning. The gain in plan quality and reduced interplanner variation may have clinical benefit for patients by removing the low-quality outliers and therefore potentially cure more patients, however, these need to be demonstrated with clinical evidence.
There has been a rapid increase in the number of papers published in this field and there were a variety of approaches and commercial implementations. In general, most papers in the literature showed improvements over manual planning across a variety of clinical sites. This review covered innovations in automated planning, but the patient’s treatment planning pathway also involves the contouring of ROIs and the quality assurance procedures to ensure safe delivery of plans. Automation of these areas is also being addressed but have not been covered in this review.197–204
Real-time interactive planning is a new paradigm for IMRT planning that is not based on using traditional optimisation algorithms. Instead, the goal is to be able to give the treatment planner the ability to perform real-time and interactive manipulation of the isodose or DVH lines and ultra-fast (automated) reoptimisation and dose estimation to update the user on the impact of interactive modifications. The main potential for this type of semi-automated planning is (along with advancements in imaging, auto-segmentation, and fast plan verification) to realise dose-guided, fast and intuitive adaptive replanning. This approach was still in the very early stages in the research domain, and as such there were limited proof-of-principle papers that were published,205–207 but it is anticipated that these systems may become commercially available within the next few years. Real-time interactive planning and other innovations in fast reoptimisation will play an essential role in the topic of on-table adaptation which was being made possible due to developments such as MR-radiotherapy machines.208,209 This is an area of active research, and some preliminary publications have been published, some of which use similar principles for automation of the plan reoptimisation as those discussed in this review.210–214 These developments were also in parallel with necessary developments in automation of contouring and QA which are also central to this concept.
There were a few research areas that require further study, which we highlight here. Some studies suggested that cross-institutional sharing of knowledge may improve the quality of the automated plan generation;66,68,76 however, further research on the impact of sharing of KBP models and a priori MCO wish-lists for more clinical sites was needed. Furthermore for KBP, the size and heterogeneity of the dataset required and the robustness of the resulting model needs further investigation. An area of automation where there were limited papers is in IMPT, and future developments will be needed as the number of centres that will be treating with IMPT is gradually increasing.64,193,215 Also, automated plan generation including (non-coplanar) beam angle optimization (or beam arc optimization for VMAT) was only explored with “bias-free” comparisons in a limited number of studies, however showing promising results.163,166,173,181,192 The use of automated planning in randomised clinical trials offers the opportunity to reduce the level of variability of plan quality which may affect the clinical outcomes, and the reduced variability may also lead to the possibility of reducing the required sample sizes meaning trials could potentially recruit quicker. This use of automation may also facilitate randomised trials testing two extreme treatment techniques which may be manually challenging to achieve at the desired plan quality. A possible example of this could be comparing treatment with a maximum focus on sparing of OAR1 with another treatment that maximally spares the competing OAR2. The endpoint of such a study could be the patients’ Quality of Life. Therefore, the role of automated planning within clinical trials is an important area of future investigations. Sharing of automated techniques between centres may lead to improved consistency between different centres (perhaps even worldwide), particularly in rare diseases where adequate patient numbers to develop expertise may only be possible across a handful of centres. However, a significant barrier was that the different types of automated planning implementations presents a challenge for sharing of different experiences between centres and commercial vendors. Furthermore, the equivalence between automated planning techniques was not well-known. Of potential interest is to investigate whether one automated planning technique could be ported to another. The role of automated planning for shared decision-making and personalised radiotherapy has been briefly discussed in this review but this research was in the early stages and it is anticipated that further studies will follow. For this to become more feasible, plan quality should ideally be linked to patient outcome and not DVH-like metrics. Finally, guidelines and recommendations on how to perform planning studies, agreed by planning experts and endorsed by professional bodies, are also highly needed. Planning studies can have a focus on development and evaluation of novel strategies/algorithms for automated planning, or automated planning may be used for investigating clinical questions, e.g. are protons better than photons. For both types of studies, statistical power and avoidance of bias (e.g. related to diversity in human planner experience) has to be considered. In the technical studies, clinical deliverability of generated plans may not always have the highest urgency, whereas this seems a must when using planning for comparing different treatment strategies in a clinical setting. Other factors that have to be considered in guidelines are quality of dose calculation algorithms, adequate coping with geometrical uncertainties (e.g. using margins, and appropriate metrics for comparing plans).
While there were some papers in the literature highlighting the technical feasibility of automated planning over manual planning, there were limited studies that describe the practicalities of implementing automated planning, including more detail on how the treatment planner’s role may evolve. One of the common elements that impact the successful adoption of any innovation is “interconnection” as described by Euchner,216 where it is argued that “innovation happens when ideas collide”. This is true for automated planning techniques where there may be mixed viewpoints within a hospital organisation regarding its advantage and disadvantage amongst the multidisciplinary staff groups, which presents a challenge for the successful clinical adoption. Is it an efficiency saving meaning potentially less dedicated staff are required and therefore, a mean for cost saving or is it an essential tool to be used by a finite staff resource? Is it an evolution in treatment planning requiring modified treatment planning skills or a route to deskilling staff? As such there are the “human factors” that need to be considered for implementing automated planning. There are known questions and challenges that need to be rationalised, such as: what will happen to the role of a treatment planner? Can one fully automate a plan and trust the software? Is it possible to automatically generate a plan for every patient? How does one ensure people retain their skills and is this even necessary? Is there a balanced approach to implementing automated planning? For example, it may be best practice to prioritise implementing automated planning algorithms to handle the most routine cases that have a high workload (such as prostate cancer or breast cancer) to re-route expertise to focus more efforts on tackling complex cases (which may otherwise be referred elsewhere) and be able to spend more time on innovative new treatment techniques and research. Potentially, this could mean more difficult cases could be treated closer to a patient’s home, assuming adequate case numbers are available to maintain clinical expertise. Sharing of DVH prediction models (RapidPlan), Treatment Techniques (Pinnacle Auto-Planning) and wish-lists (Erasmus-iCycle) between centres may also address this issue, particularly in rare diseases where adequate patient numbers to develop a model may only be possible across several centres. Alternatively, should such techniques be initially focussed on those sites where significant time savings may be made? Experienced treatment planners, in particular, may not see the benefits in the use of more routine planning where time savings are modest. As such, it can be argued that there should always be a role for a multidisciplinary team consideration. There may be a clash of cultures amongst different staff groups concerning automated planning, and this may require addressing before the adoption of such techniques into clinical practice.
Furthermore, the actual benchmarking and validation of automated planning is not straightforward and requires expert physics resource, as highlighted in the literature it is not merely possible to use “out-of-the-box”.78 It is important to realize that all current implementations of automated planning require a high-level of manual planning knowledge for configuration. It is questionable that this will change in the coming years as no mathematical formulas exist for balancing trade-offs, and dose–response relationships have significant uncertainties. The impact of a suboptimal configuration of an automated planning algorithm may be similar to an overall geometric error in the treatment preparation process: it will introduce an overall systematic error in the treatments, i.e. for all patients (of a particular tumour type), the plan quality is lower than feasible. Treatment planning can be a complicated process, and achieving the best radiotherapy treatment with the optimal trade-offs between good tumour coverage and healthy tissue sparing requires expert knowledge; replicating this with automated software is a difficult achievement and there will often be scepticism that the software will do as good a job as a human, regardless of how good the published results are.
Guidance by professional bodies on implementation of automated planning and possible redefining of treatment planner roles could provide rationalisation. Algorithms for (semi-)automation of the configuration of automated planning software could also be highly beneficial in facilitating better implementation, and the development of simple metrics in plan comparison may facilitate this.
Conclusion
Recent innovations in automated treatment planning software have given rise to the potential to broadly improve the efficiency of radiotherapy planning and to enhance the overall quality of generated treatment plans, which is expected to result in higher quality treatments. Automated planning can also facilitate better access to high-quality advanced treatments, and harmonize radiotherapy treatments between treatment centres. Automated planning is now available in different commercial packages, each with a different technical approach, and the initial clinical reports are promising. The field is currently still rapidly developing and in a steep upward trend, and there are various areas of future research required that have been highlighted in this review. Much work is still needed to explore practical issues related to clinical implementation, including staffing, and their changing roles. In a resource-limited world, disruptive innovative technology is essential to meet future healthcare needs, and the rapid adoption of automated planning is one area that should be embraced and also possibly supported by professional body recommendations.
Footnotes
Acknowledgements: MH would like to acknowledge funding from the UK National Measurement System.
Disclosures: MH has received speaker’s honoraria from Varian Medical Systems and AN has received travel and speaker’s honoraria from Varian Medical Systems. The Royal Surrey County Hospital has a research agreement with Varian Medical Systems. MH is funded by the UK National Measurement System. BJMH, and Erasmus MC Cancer Institute, has received research grants from Elekta AB, Stockholm, Sweden and also has a research collaboration with Accuray Inc, Sunnyvale, USA.
Contributor Information
Mohammad Hussein, Email: mohammad.hussein@npl.co.uk.
Dirk Verellen, Email: dirk.verellen@gza.be.
Andrew Nisbet, Email: andrew.nisbet@nhs.net.
REFERENCES
- 1. Cancer Research, U. K, and NHS England . Vision for radiotherapy 2014-2024. London: Available at:https://www.cancerresearchuk.org/sites/default/files/policy_feb2014_radiotherapy_vision2014-2024_final.pdf (Accessed 02/07/2018). 2014. [Google Scholar]
- 2.Slotman BJ, Cottier B, Bentzen SM, Heeren G, Lievens Y, van den Bogaert W. Overview of national guidelines for infrastructure and staffing of radiotherapy. ESTRO-QUARTS: Work package 1. Radiother Oncol 2005; 75: 349.E1–349.E6. doi: 10.1016/j.radonc.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 3.Sullivan R, Peppercorn J, Sikora K, Zalcberg J, Meropol NJ, Amir E, et al. . Delivering affordable cancer care in high-income countries. Lancet Oncol 2011; 12: 933–80. doi: 10.1016/S1470-2045(11)70141-3 [DOI] [PubMed] [Google Scholar]
- 4.Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol 2011; 84: 967–96. doi: 10.1259/bjr/22373346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghilezan M, Yan D, Martinez A. Adaptive Radiation Therapy for Prostate Cancer. Semin Radiat Oncol 2010; 20: 130–7. doi: 10.1016/j.semradonc.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castadot P, Lee JA, Geets X, Grégoire V. Adaptive radiotherapy of head and neck cancer. Semin Radiat Oncol 2010; 20: 84–93. doi: 10.1016/j.semradonc.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz DL, Garden AS, Shah SJ, Chronowski G, Sejpal S, Rosenthal DI, et al. . Adaptive radiotherapy for head and neck cancer-dosimetric results from a prospective clinical trial. Radiother Oncol 2013; 106: 80–4. doi: 10.1016/j.radonc.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 8.Caudell JJ, Torres-Roca JF, Gillies RJ, Enderling H, Kim S, Rishi A, et al. . The future of personalised radiotherapy for head and neck cancer. Lancet Oncol 2017; 18: e266–e273. doi: 10.1016/S1470-2045(17)30252-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirst DG, Robson T. Molecular biology: the key to personalised treatment in radiation oncology? Br J Radiol 2010; 83: 723–8. doi: 10.1259/bjr/91488645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georg D, Thwaites D. Medical physics in radiation Oncology: New challenges, needs and roles. Radiother Oncol 2017; 125: 375–8. doi: 10.1016/j.radonc.2017.10.035 [DOI] [PubMed] [Google Scholar]
- 11.Peters LJ, O'Sullivan B, Giralt J, Fitzgerald TJ, Trotti A, Bernier J, et al. . Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol 2010; 28: 2996–3001. doi: 10.1200/JCO.2009.27.4498 [DOI] [PubMed] [Google Scholar]
- 12.Rogers EM. Diffusion of innovations. 5th Edition New York: The British Institute of Radiology.; 2003. [Google Scholar]
- 13.Petrovic S, Khussainova G, Jagannathan R. Knowledge-light adaptation approaches in case-based reasoning for radiotherapy treatment planning. Artif Intell Med 2016; 68: 17–28. doi: 10.1016/j.artmed.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 14.McIntosh C, Purdie TG. Contextual Atlas Regression Forests: Multiple-Atlas-Based Automated Dose Prediction in Radiation Therapy. IEEE Trans Med Imaging 2016; 35: 1000–12. doi: 10.1109/TMI.2015.2505188 [DOI] [PubMed] [Google Scholar]
- 15.Skarpman Munter J, Sjölund J. Dose-volume histogram prediction using density estimation. Phys Med Biol 2015; 60: 6923–36. doi: 10.1088/0031-9155/60/17/6923 [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Xing L. Clinical knowledge-based inverse treatment planning. Phys Med Biol 2004; 49: 5101–17. doi: 10.1088/0031-9155/49/22/006 [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Ford EC, Wu B, Pinkawa M, van Triest B, Campbell P, et al. . An overlap-volume-histogram based method for rectal dose prediction and automated treatment planning in the external beam prostate radiotherapy following hydrogel injection. Med Phys 2013; 40: 011709. doi: 10.1118/1.4769424 [DOI] [PubMed] [Google Scholar]
- 18.Petit SF, van Elmpt W. Accurate prediction of target dose-escalation and organ-at-risk dose levels for non-small cell lung cancer patients. Radiother Oncol 2015; 117: 453–8. doi: 10.1016/j.radonc.2015.07.040 [DOI] [PubMed] [Google Scholar]
- 19.Ahmed S, Nelms B, Gintz D, Caudell J, Zhang G, Moros EG, et al. . A method for a priori estimation of best feasible DVH for organs-at-risk: Validation for head and neck VMAT planning. Med Phys 2017; 44: 5486–97. doi: 10.1002/mp.12500 [DOI] [PubMed] [Google Scholar]
- 20.Chau RM, Leung SF, Kam MK, Cheung KY, Kwan WH, Yu KH, et al. . A broadly adaptive array of dose-constraint templates for planning of intensity-modulated radiation therapy for advanced T-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2009; 74: 21–28. doi: 10.1016/j.ijrobp.2008.07.041 [DOI] [PubMed] [Google Scholar]
- 21.Sheng Y, Ge Y, Yuan L, Li T, Yin F-F, Wu QJ. Outlier identification in radiation therapy knowledge-based planning: A study of pelvic cases. Med Phys 2017; 44: 5617–26. doi: 10.1002/mp.12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreibmann E, Fox T. Prior-knowledge treatment planning for volumetric arc therapy using feature-based database mining. J Appl Clin Med Phys 2014; 15: 19–27. doi: 10.1120/jacmp.v15i2.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayo CS, Yao J, Eisbruch A, Balter JM, Litzenberg DW, Matuszak MM, et al. . Incorporating big data into treatment plan evaluation: Development of statistical DVH metrics and visualization dashboards. Adv Radiat Oncol 2017; 2: 503–14. doi: 10.1016/j.adro.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran A, Woods K, Nguyen D, Yu VY, Niu T, Cao M, et al. . Predicting liver SBRT eligibility and plan quality for VMAT and 4π plans. Radiat Oncol 2017; 12. doi: 10.1186/s13014-017-0806-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng Y, Li T, Zhang Y, Lee WR, Yin F-F, Ge Y, et al. . Atlas-guided prostate intensity modulated radiation therapy (IMRT) planning. Phys Med Biol 2015; 60: 7277–91. doi: 10.1088/0031-9155/60/18/7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang HH, Meyer RR, Shi L, D'Souza WD. The minimum knowledge base for predicting organ-at-risk dose–volume levels and plan-related complications in IMRT planning. Phys Med Biol 2010; 55: 1935–47. doi: 10.1088/0031-9155/55/7/010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zawadzka A, Nesteruk M, Brzozowska B, Kukołowicz PF. Method of predicting the mean lung dose based on a patient׳s anatomy and dose-volume histograms. Med Dosim 2017; 42: 57–62. doi: 10.1016/j.meddos.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 28.Zarepisheh M, Long T, Li N, Tian Z, Romeijn HE, Jia X, et al. . A DVH-guided IMRT optimization algorithm for automatic treatment planning and adaptive radiotherapy replanning. Med Phys 2014; 41(6Part1): 061711. doi: 10.1118/1.4875700 [DOI] [PubMed] [Google Scholar]
- 29.Zhu X, Ge Y, Li T, Thongphiew D, Yin F-F, Wu QJ. A planning quality evaluation tool for prostate adaptive IMRT based on machine learning. Med Phys 2011; 38: 719–726. doi: 10.1118/1.3539749 [DOI] [PubMed] [Google Scholar]
- 30.Good D, Lo J, Lee WR, Wu QJ, Yin F-F, Das SK. A knowledge-based approach to improving and homogenizing intensity modulated radiation therapy planning quality among treatment centers: an example application to prostate cancer planning. Int J Radiat Oncol Biol Phys 2013; 87: 176–81. doi: 10.1016/j.ijrobp.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 31.Lee T, Hammad M, Chan TC, Craig T, Sharpe MB. Predicting objective function weights from patient anatomy in prostate IMRT treatment planning. Med Phys 2013; 40: 121706. doi: 10.1118/1.4828841 [DOI] [PubMed] [Google Scholar]
- 32.Nwankwo O, Sihono DSK, Schneider F, Wenz F. A global quality assurance system for personalized radiation therapy treatment planning for the prostate (or other sites). Phys Med Biol 2014; 59: 5575–91. doi: 10.1088/0031-9155/59/18/5575 [DOI] [PubMed] [Google Scholar]
- 33.Lu R, Radke RJ, Hong L, Chui CS, Xiong J, Yorke E, et al. . Learning the relationship between patient geometry and beam intensity in breast intensity-modulated radiotherapy. IEEE Trans Biomed Eng 2006; 53: 908–20. doi: 10.1109/TBME.2005.863987 [DOI] [PubMed] [Google Scholar]
- 34.Shiraishi S, Moore KL. Knowledge-based prediction of three-dimensional dose distributions for external beam radiotherapy. Med Phys 2016; 43: 378–387. doi: 10.1118/1.4938583 [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Wu QJ, Kirkpatrick JP, Yin F-F, Yuan L, Ge Y. From active shape model to active optical flow model: a shape-based approach to predicting voxel-level dose distributions in spine SBRT. Phys Med Biol 2015; 60: N83–N92. doi: 10.1088/0031-9155/60/5/N83 [DOI] [PubMed] [Google Scholar]
- 36.Lin K-M, Simpson J, Sasso G, Raith A, Ehrgott M. Quality assessment for VMAT prostate radiotherapy planning based on data envelopment analysis. Phys Med Biol 2013; 58: 5753–69. doi: 10.1088/0031-9155/58/16/5753 [DOI] [PubMed] [Google Scholar]
- 37.Campbell WG, Miften M, Olsen L, Stumpf P, Schefter T, Goodman KA, et al. . Neural network dose models for knowledge-based planning in pancreatic SBRT. Med Phys 2017; 44: 6148–58. doi: 10.1002/mp.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McIntosh C, Purdie TG. Voxel-based dose prediction with multi-patient atlas selection for automated radiotherapy treatment planning. Phys Med Biol 2017; 62: 415–31. doi: 10.1088/1361-6560/62/2/415 [DOI] [PubMed] [Google Scholar]
- 39.McIntosh C, Welch M, McNiven A, Jaffray DA, Purdie TG. Fully automated treatment planning for head and neck radiotherapy using a voxel-based dose prediction and dose mimicking method. Phys Med Biol 2017; 62: 5926–44. doi: 10.1088/1361-6560/aa71f8 [DOI] [PubMed] [Google Scholar]
- 40.Valdes G, Simone CB, Chen J, Lin A, Yom SS, Pattison AJ, et al. . Clinical decision support of radiotherapy treatment planning: a data-driven machine learning strategy for patient-specific dosimetric decision making. Radiother Oncol 2017; 125: 392–7. doi: 10.1016/j.radonc.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Dong P, Liu H, Xing L. Development of an autonomous treatment planning strategy for radiation therapy with effective use of population-based prior data. Med Phys 2017; 44: 389–96. doi: 10.1002/mp.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Li T, Xiao H, Ji W, Guo M, Zeng Z, et al. . A knowledge-based approach to automated planning for hepatocellular carcinoma. J Appl Clin Med Phys 2018; 19: 50-59. doi: 10.1002/acm2.12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueda Y, Fukunaga JI, Kamima T, Adachi Y, Nakamatsu K, Monzen H. Evaluation of multiple institutions' models for knowledge-based planning of volumetric modulated arc therapy (VMAT) for prostate cancer. Radiat Oncol 2018; 13: 46. doi: 10.1186/s13014-018-0994-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu G, Li Y, Feng Z, Tao C, Yu Z, Li B, et al. . Knowledge-based IMRT planning for individual liver cancer patients using a novel specific model. Radiat Oncol 2018; 13: 52. doi: 10.1186/s13014-018-0996-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanhope C, Wu QJ, Yuan L, Liu J, Hood R, Yin FF, et al. . Utilizing knowledge from prior plans in the evaluation of quality assurance. Phys Med Biol 2015; 60: 4873–4891. doi: 10.1088/0031-9155/60/12/4873 [DOI] [PubMed] [Google Scholar]
- 46.Wu B, Pang D, Simari P, Taylor R, Sanguineti G, McNutt T. Using overlap volume histogram and IMRT plan data to guide and automate VMAT planning: a head-and-neck case study. Med Phys 2013; 40: 021714. doi: 10.1118/1.4788671 [DOI] [PubMed] [Google Scholar]
- 47.Liu ESF, Wu VWC, Harris B, Lehman M, Pryor D, Chan LWC. Vector-model-supported approach in prostate plan optimization. Med Dosim 2017; 42: 79–84. doi: 10.1016/j.meddos.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 48.Caine H, Whalley D, Kneebone A, McCloud P, Eade T. Using individual patient anatomy to predict protocol compliance for prostate intensity-modulated radiotherapy. Med Dosim 2016; 41: 70–4. doi: 10.1016/j.meddos.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 49.Moore KL, Schmidt R, Moiseenko V, Olsen LA, Tan J, Xiao Y, et al. . Quantifying unnecessary normal tissue complication risks due to suboptimal planning: A secondary study of RTOG 0126. Int J Radiat Oncol Biol Phys 2015; 92: 228–235. doi: 10.1016/j.ijrobp.2015.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall DC, Trofimov AV, Winey BA, Liebsch NJ, Paganetti H. Predictingpatient-specific dosimetric benefits of proton therapy for skull-base tumors using a geometric knowledge-based method. Int J Radiat Oncol Biol Phys 2017; 97: 1087–1094. doi: 10.1016/j.ijrobp.2017.01.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang F, Wu H, Yue H, Jia F, Zhang Y. Photon Optimizer (PO) prevails over Progressive Resolution Optimizer (PRO) for VMAT planning with or without knowledge-based solution. J Appl Clin Med Phys 2017; 18: 9–14. doi: 10.1002/acm2.12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chatterjee A, Serban M, Abdulkarim B, Panet-Raymond V, Souhami L, Shenouda G, et al. . Performance of knowledge-based radiation therapy planning for the glioblastoma disease site. Int J Radiat Oncol Biol Phys 2017; 99: 1021–1028. doi: 10.1016/j.ijrobp.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 53.Cagni E, Botti A, Micera R, Galeandro M, Sghedoni R, Orlandi M, et al. . Knowledge-based treatment planning: an inter-technique and inter-system feasibility study for prostate cancer. Phys Medica 2017; 36: 38–45. doi: 10.1016/j.ejmp.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 54.Li N, Carmona R, Sirak I, Kasaova L, Followill D, Michalski J, et al. . Highly efficient training, refinement, and validation of a knowledge-based planning quality-control system for radiation therapy clinical trials. Int J Radiat Oncol Biol Phys 2017; 97: 164–172. doi: 10.1016/j.ijrobp.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kubo K, Monzen H, Ishii K, Tamura M, Kawamorita R, Sumida I, et al. . Dosimetric comparison of RapidPlan and manually optimized plans in volumetric modulated arc therapy for prostate cancer. Phys Medica 2017; 44: 199–204. doi: 10.1016/j.ejmp.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 56.Wu H, Jiang F, Yue H, Li S, Zhang Y. A dosimetric evaluation of knowledge-based VMAT planning with simultaneous integrated boosting for rectal cancer patients. J Appl Clin Med Phys 2016; 17: 78–85. doi: 10.1120/jacmp.v17i6.6410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delaney AR, Tol JP, Dahele M, Cuijpers J, Slotman BJ, Verbakel WFAR. Effect of Dosimetric Outliers on the Performance of a Commercial Knowledge-Based Planning Solution. Int J Radiat Oncol Biol Phys 2016; 94: 469–77. doi: 10.1016/j.ijrobp.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 58.Chanyavanich V, Das SK, Lee WR, Lo JY. Knowledge-based IMRT treatment planning for prostate cancer. Med Phys 2011; 38: 2515–2522. doi: 10.1118/1.3574874 [DOI] [PubMed] [Google Scholar]
- 59.Tol JP, Dahele M, Delaney AR, Slotman BJ, Verbakel WF. Can knowledge-based DVH predictions be used for automated, individualized quality assurance of radiotherapy treatment plans? Radiat Oncol 2015; 10: 234. doi: 10.1186/s13014-015-0542-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.ESF L, VWC W, Harris B, Foote M, Lehman M, Chan LWC. Vector-model-supported optimization in volumetric-modulated arc stereotactic radiotherapy planning for brain metastasis. Med Dosim 2017; 42: 85–9. [DOI] [PubMed] [Google Scholar]
- 61.Habraken SJM, Sharfo AWM, Buijsen J, Verbakel WFAR, Haasbeek CJA, Öllers MC, et al. . The TRENDY multi-center randomized trial on hepatocellular carcinoma – Trial QA including automated treatment planning and benchmark-case results. Radiother Oncol 2017; 125: 507–13. doi: 10.1016/j.radonc.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, Li T, Yuan L, Ge Y, Yin F-F, Lee WR, et al. . Quantitative comparison of automatic and manual IMRT optimization for prostate cancer: the benefits of DVH prediction. J Appl Clin Med Phys 2015; 16: 241–50. doi: 10.1120/jacmp.v16i2.5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foy JJ, Marsh R, Ten Haken RK, Younge KC, Schipper M, Sun Y, et al. . An analysis of knowledge-based planning for stereotactic body radiation therapy of the spine. Pract Radiat Oncol 2017; 7: e355–e360. doi: 10.1016/j.prro.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 64.Delaney AR, Dahele M, Tol JP, Kuijper IT, Slotman BJ, Verbakel WFAR. Using a knowledge-based planning solution to select patients for proton therapy. Radiother Oncol 2017; 124: 263–70. doi: 10.1016/j.radonc.2017.03.020 [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Hu W, Yang Z, Chen X, Wu Z, Yu X, et al. . Is it possible for knowledge-based planning to improve intensity modulated radiation therapy plan quality for planners with different planning experiences in left-sided breast cancer patients? Radiat Oncol 2017; 12: 85. doi: 10.1186/s13014-017-0822-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schubert C, Waletzko O, Weiss C, Voelzke D, Toperim S, Roeser A, et al. . Intercenter validation of a knowledge based model for automated planning of volumetric modulated arc therapy for prostate cancer. The experience of the German RapidPlan Consortium. PLoS One 2017; 12: e0178034. doi: 10.1371/journal.pone.0178034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ziemer BP, Shiraishi S, Hattangadi-Gluth JA, Sanghvi P, Moore KL, automated F. Fully automated, comprehensive knowledge-based planning for stereotactic radiosurgery: Preclinical validation through blinded physician review. Pract Radiat Oncol 2017; 7: e569–e578. doi: 10.1016/j.prro.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 68.Wu B, Kusters M, Kunze-busch M, Dijkema T, McNutt T, Sanguineti G, et al. . Cross-institutional knowledge-based planning (KBP) implementation and its performance comparison to Auto-Planning Engine (APE. Radiother Oncol 2017; 123: 57–62. doi: 10.1016/j.radonc.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 69.Long T, Chen M, Jiang S, Lu W. Threshold-driven optimization for reference-based auto-planning. Phys Med Biol 2018; 63: 04NT01. doi: 10.1088/1361-6560/aaa731 [DOI] [PubMed] [Google Scholar]
- 70.Fogliata A, Wang P-M, Belosi F, Clivio A, Nicolini G, Vanetti E, et al. . Assessment of a model based optimization engine for volumetric modulated arc therapy for patients with advanced hepatocellular cancer. Radiat Oncol 2014; 9: 236. doi: 10.1186/s13014-014-0236-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Heijmen BJM, Petit SF. Prospective clinical validation of independent DVH prediction for plan QA in automatic treatment planning for prostate cancer patients. Radiother Oncol 2017; 125: 500–6. doi: 10.1016/j.radonc.2017.09.021 [DOI] [PubMed] [Google Scholar]
- 72.Fogliata A, Reggiori G, Stravato A, Lobefalo F, Franzese C, Franceschini D, et al. . RapidPlan head and neck model: the objectives and possible clinical benefit. Radiat Oncol 2017; 12. doi: 10.1186/s13014-017-0808-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boutilier JJ, Craig T, Sharpe MB, Chan TCY. Sample size requirements for knowledge-based treatment planning. Med Phys 2016; 43: 1212–21. doi: 10.1118/1.4941363 [DOI] [PubMed] [Google Scholar]
- 74.Wu H, Jiang F, Yue H, Zhang H, Wang K, Zhang Y. Applying a RapidPlan model trained on a technique and orientation to another: a feasibility and dosimetric evaluation. Radiat Oncol 2016; 11. doi: 10.1186/s13014-016-0684-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Delaney AR, Dahele M, Tol JP, Slotman BJ, Verbakel WFAR. Knowledge-based planning for stereotactic radiotherapy of peripheral early-stage lung cancer. Acta Oncol 2017; 56: 490–5. doi: 10.1080/0284186X.2016.1273544 [DOI] [PubMed] [Google Scholar]
- 76.Fogliata A, Nicolini G, Clivio A, Vanetti E, Laksar S, Tozzi A, et al. . A broad scope knowledge based model for optimization of VMAT in esophageal cancer: validation and assessment of plan quality among different treatment centers. Radiat Oncol 2015; 10. doi: 10.1186/s13014-015-0530-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berry SL, Ma R, Boczkowski A, Jackson A, Zhang P, Hunt M. Evaluating inter-campus plan consistency using a knowledge based planning model. Radiother Oncol 2016; 120: 349–55. doi: 10.1016/j.radonc.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hussein M, South CP, Barry MA, Adams EJ, Jordan TJ, Stewart AJ, et al. . Clinical validation and benchmarking of knowledge-based IMRT and VMAT treatment planning in pelvic anatomy. Radiother Oncol 2016; 120: 473–9. doi: 10.1016/j.radonc.2016.06.022 [DOI] [PubMed] [Google Scholar]
- 79.Krayenbuehl J, Norton I, Studer G, Guckenberger M. Evaluation of an automated knowledge based treatment planning system for head and neck. Radiat Oncol 2015; 10: 226. doi: 10.1186/s13014-015-0533-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wall PDH, Carver RL, Fontenot JD. An improved distance-to-dose correlation for predicting bladder and rectum dose-volumes in knowledge-based VMAT planning for prostate cancer. Phys Med Biol 2018; 63: 015035. doi: 10.1088/1361-6560/aa9a30 [DOI] [PubMed] [Google Scholar]
- 81.Nwankwo O, Mekdash H, Sihono DSK, Wenz F, Glatting G. Knowledge-based radiation therapy (KBRT) treatment planning versus planning by experts: validation of a KBRT algorithm for prostate cancer treatment planning. Radiat Oncol 2015; 10: 111. doi: 10.1186/s13014-015-0416-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tol JP, Delaney AR, Dahele M, Slotman BJ, Verbakel WFAR. Evaluation of a Knowledge-Based Planning Solution for Head and Neck Cancer. Int J Radiat Oncol Biol Phys 2015; 91: 612–20. doi: 10.1016/j.ijrobp.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 83.Fogliata A, Belosi F, Clivio A, Navarria P, Nicolini G, Scorsetti M, et al. . On the pre-clinical validation of a commercial model-based optimisation engine: Application to volumetric modulated arc therapy for patients with lung or prostate cancer. Radiother Oncol 2014; 113: 385–91. doi: 10.1016/j.radonc.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 84.Boutilier JJ, Lee T, Craig T, Sharpe MB, Chan TC. Models for predicting objective function weights in prostate cancer IMRT. Med Phys 2015; 42: 1586–1595. doi: 10.1118/1.4914140 [DOI] [PubMed] [Google Scholar]
- 85.Kuo L, Yorke ED, Dumane VA, Foster A, Zhang Z, Mechalakos JG, et al. . Geometric dose prediction model for hemithoracic intensity-modulated radiation therapy in mesothelioma patients with two intact lungs. J Appl Clin Med Phys 2016; 17: 371–9. doi: 10.1120/jacmp.v17i3.6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boylan C, Rowbottom C. A bias-free, automated planning tool for technique comparison in radiotherapy - application to nasopharyngeal carcinoma treatments. J Appl Clin Med Phys 2014; 15: 213–25. doi: 10.1120/jacmp.v15i1.4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krayenbuehl J, Di Martino M, Guckenberger M, Andratschke N. Improved plan quality with automated radiotherapy planning for whole brain with hippocampus sparing: a comparison to the RTOG 0933 trial. Radiat Oncol 2017; 12: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang X, Li X, Quan EM, Pan X, Li Y. A methodology for automatic intensity-modulated radiation treatment planning for lung cancer. Phys Med Biol 2011; 56: 3873–93. doi: 10.1088/0031-9155/56/13/009 [DOI] [PubMed] [Google Scholar]
- 89.Hansen CR, Bertelsen A, Hazell I, Zukauskaite R, Gyldenkerne N, Johansen J, et al. . Automatic treatment planning improves the clinical quality of head and neck cancer treatment plans. Clin Transl Radiat Oncol 2016; 1: 2–8. doi: 10.1016/j.ctro.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang W, Purdie TG, Rahman M, Marshall A, Liu FF, Fyles A. Rapid automated treatment planning process to select breast cancer patients for active breathing control to achieve cardiac dose reduction. Int J Radiat Oncol Biol Phys 2012; 82: 386–393. doi: 10.1016/j.ijrobp.2010.09.026 [DOI] [PubMed] [Google Scholar]
- 91.Nawa K, Haga A, Nomoto A, Sarmiento RA, Shiraishi K, Yamashita H, et al. . Evaluation of a commercial automatic treatment planning system for prostate cancers. Med Dosim 2017; 42: 203–9. doi: 10.1016/j.meddos.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 92.Li X, Wang L, Wang J, Han X, Xia B, Wu S, et al. . Dosimetric benefits of automation in the treatment of lower thoracic esophageal cancer: Is manual planning still an alternative option? Med Dosim 2017; 42: 289–95. doi: 10.1016/j.meddos.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 93.Song Y, Wang Q, Jiang X, Liu S, Zhang Y, Bai S. Fully automatic volumetric modulated arc therapy plan generation for rectal cancer. Radiother Oncol 2016; 119: 531–6. doi: 10.1016/j.radonc.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 94.Tol JP, Dahele M, Delaney AR, Doornaert P, Slotman BJ, Verbakel WFAR. Detailed evaluation of an automated approach to interactive optimization for volumetric modulated arc therapy plans. Med Phys 2016; 43: 1818–28. doi: 10.1118/1.4944063 [DOI] [PubMed] [Google Scholar]
- 95.Hansen CR, Nielsen M, Bertelsen AS, Hazell I, Holtved E, Zukauskaite R, et al. . Automatic treatment planning facilitates fast generation of high-quality treatment plans for esophageal cancer. Acta Oncol 2017; 56: 1495–500. doi: 10.1080/0284186X.2017.1349928 [DOI] [PubMed] [Google Scholar]
- 96.Purdie TG, Dinniwell RE, Fyles A, Sharpe MB. Automation and intensity modulated radiation therapy for individualized high-quality tangent breast treatment plans. Int J Radiat Oncol Biol Phys 2014; 90: 688–95. doi: 10.1016/j.ijrobp.2014.06.056 [DOI] [PubMed] [Google Scholar]
- 97.Gintz D, Latifi K, Caudell J, Nelms B, Zhang G, Moros E, et al. . Initial evaluation of automated treatment planning software. J Appl Clin Med Phys 2016; 17: 331–46. doi: 10.1120/jacmp.v17i3.6167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan H, Yin F-F, Willett C. Evaluation of an artificial intelligence guided inverse planning system: Clinical case study. Radiother Oncol 2007; 83: 76–85. doi: 10.1016/j.radonc.2007.02.013 [DOI] [PubMed] [Google Scholar]
- 99.Wang S, Zheng D, Zhang C, Ma R, Bennion NR, Lei Y, et al. . Automatic planning on hippocampal avoidance whole-brain radiotherapy. Med Dosim 2017; 42: 63–8. doi: 10.1016/j.meddos.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 100.Speer S, Klein A, Kober L, Weiss A, Yohannes I, Bert C. Automation of radiation treatment planning: evaluation of head and neck cancer patient plans created by the Pinnacle3 scripting and Auto-Planning functions. Strahlentherapie Und Onkol 2017; 193: 656–65. [DOI] [PubMed] [Google Scholar]
- 101.Kusters JMAM, Bzdusek K, Kumar P, van Kollenburg PGM, Kunze-Busch MC, Wendling M, et al. . Automated IMRT planning in Pinnacle. Strahlentherapie Und Onkol 2017; 193: 1031–8. doi: 10.1007/s00066-017-1187-9 [DOI] [PubMed] [Google Scholar]
- 102.Winkel D, Bol GH, van Asselen B, Hes J, Scholten V, Kerkmeijer LGW, et al. . Development and clinical introduction of automated radiotherapy treatment planning for prostate cancer. Phys Med Biol 2016; 61: 8587–95. doi: 10.1088/1361-6560/61/24/8587 [DOI] [PubMed] [Google Scholar]
- 103.Hazell I, Bzdusek K, Kumar P, Hansen CR, Bertelsen A, Eriksen JG, et al. . Automatic planning of head and neck treatment plans. J Appl Clin Med Phys 2016; 17: 272–82. doi: 10.1120/jacmp.v17i1.5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan H, Yin F-F, Guan H, Kim JH. Fuzzy logic guided inverse treatment planning. Med Phys 2003; 30: 2675–85. doi: 10.1118/1.1600739 [DOI] [PubMed] [Google Scholar]
- 105.Yan H, Yin F-F, Guan H-qun, Kim JH. AI-guided parameter optimization in inverse treatment planning. Phys Med Biol 2003; 48: 3565–80. doi: 10.1088/0031-9155/48/21/008 [DOI] [PubMed] [Google Scholar]
- 106.Xhaferllari I, Wong E, Bzdusek K, Lock M, Chen JZ. Automated IMRT planning with regional optimization using planning scripts. J Appl Clin Med Phys 2013; 14: 176–91. doi: 10.1120/jacmp.v14i1.4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tol JP, Dahele M, Peltola J, Nord J, Slotman BJ, Verbakel WFAR. Automatic interactive optimization for volumetric modulated arc therapy planning. Radiat Oncol 2015; 10. doi: 10.1186/s13014-015-0388-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilkens JJ, Alaly JR, Zakarian K, Thorstad WL, Deasy JO. IMRT treatment planning based on prioritizing prescription goals. Phys Med Biol 2007; 52: 1675–92. doi: 10.1088/0031-9155/52/6/009 [DOI] [PubMed] [Google Scholar]
- 109.Stieler F, Yan H, Lohr F, Wenz F, Yin F-F. Development of a neuro-fuzzy technique for automated parameter optimization of inverse treatment planning. Radiat Oncol 2009; 4: 39. doi: 10.1186/1748-717X-4-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang H, Xing L. Application programming in C# environment with recorded user software interactions and its application in autopilot of VMAT/IMRT treatment planning. J Appl Clin Med Phys 2016; 17: 189–203. doi: 10.1120/jacmp.v17i6.6425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bokrantz R, Miettinen K. Projections onto the Pareto surface in multicriteria radiation therapy optimization. Med Phys 2015; 42: 5862–5870. doi: 10.1118/1.4930252 [DOI] [PubMed] [Google Scholar]
- 112.Schlaefer A, Viulet T, Muacevic A, Fürweger C. Multicriteria optimization of the spatial dose distribution. Med Phys 2013; 40: 121720. doi: 10.1118/1.4828840 [DOI] [PubMed] [Google Scholar]
- 113.Pardo-Montero J, Fenwick JD. An approach to multiobjective optimization of rotational therapy. II. Pareto optimal surfaces and linear combinations of modulated blocked arcs for a prostate geometry. Med Phys 2010; 37: 2606–2616. doi: 10.1118/1.3427410 [DOI] [PubMed] [Google Scholar]
- 114.Craft D, Halabi T, Shih HA, Bortfeld T. An approach for practical multiobjective IMRT treatment planning. Int J Radiat Oncol Biol Phys 2007; 69: 1600–1607. doi: 10.1016/j.ijrobp.2007.08.019 [DOI] [PubMed] [Google Scholar]
- 115.Zhang X, Wang X, Dong L, Liu H, Mohan R. A sensitivity-guided algorithm for automated determination of IMRT objective function parameters. Med Phys 2006; 33: 2935–2944. doi: 10.1118/1.2214171 [DOI] [PubMed] [Google Scholar]
- 116.Zarepisheh M, Uribe-Sanchez AF, Li N, Jia X, Jiang SB. A multicriteria framework with voxel-dependent parameters for radiotherapy treatment plan optimization. Med Phys 2014; 41: 041705. doi: 10.1118/1.4866886 [DOI] [PubMed] [Google Scholar]
- 117.Holdsworth C, Kim M, Liao J, Phillips MH. A hierarchical evolutionary algorithm for multiobjective optimization in IMRT. Med Phys 2010; 37: 4986–4997. doi: 10.1118/1.3478276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen W, Craft D, Madden TM, Zhang K, Kooy HM, Herman GT. A fast optimization algorithm for multicriteria intensity modulated proton therapy planning. Med Phys 2010; 37: 4938–4945. doi: 10.1118/1.3481566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salari E, Unkelbach J. A column-generation-based method for multi-criteria direct aperture optimization. Phys Med Biol 2013; 58: 621-39. doi: 10.1088/0031-9155/58/3/621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van de Water S, Kraan AC, Breedveld S, Schillemans W, Teguh DN, Kooy HM, et al. . Improved efficiency of multi-criteria IMPT treatment planning using iterative resampling of randomly placed pencil beams. Phys Med Biol 2013; 58: 6969–83. doi: 10.1088/0031-9155/58/19/6969 [DOI] [PubMed] [Google Scholar]
- 121.Lahanas M, Schreibmann E, Baltas D. Multiobjective inverse planning for intensity modulated radiotherapy with constraint-free gradient-based optimization algorithms. Phys Med Biol 2003; 48: 2843–71. doi: 10.1088/0031-9155/48/17/308 [DOI] [PubMed] [Google Scholar]
- 122.Salari E, Wala J, Craft D. Exploring trade-offs between VMAT dose quality and delivery efficiency using a network optimization approach. Phys Med Biol 2012; 57: 5587–600. doi: 10.1088/0031-9155/57/17/5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bokrantz R. Multicriteria optimization for volumetric-modulated arc therapy by decomposition into a fluence-based relaxation and a segment weight-based restriction. Med Phys 2012; 39: 6712–6725. doi: 10.1118/1.4754652 [DOI] [PubMed] [Google Scholar]
- 124.Monshouwer R, Hoffmann AL, Kunze-Busch M, Bussink J, Kaanders JHAM, Huizenga H. A practical approach to assess clinical planning tradeoffs in the design of individualized IMRT treatment plans. Radiother Oncol 2010; 97: 561–6. doi: 10.1016/j.radonc.2010.10.019 [DOI] [PubMed] [Google Scholar]
- 125.Redondo JL, Fernández J, Ortigosa PM. FEMOEA: a fast and efficient multi-objective evolutionary algorithm. Math Methods Oper Res 2017; 85: 113–35. doi: 10.1007/s00186-016-0560-2 [DOI] [Google Scholar]
- 126.Clark VH, Chen Y, Wilkens J, Alaly JR, Zakaryan K, Deasy JO. IMRT treatment planning for prostate cancer using prioritized prescription optimization and mean-tail-dose functions. Linear Algebra Appl 2008; 428: 1345–64. doi: 10.1016/j.laa.2007.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Teichert K, Süss P, Serna JI, Monz M, Küfer KH, Thieke C. Comparative analysis of Pareto surfaces in multi-criteria IMRT planning. Phys Med Biol 2011; 56: 3669–84. doi: 10.1088/0031-9155/56/12/014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Craft D, Bortfeld T. How many plans are needed in an IMRT multi-objective plan database? Phys Med Biol 2008; 53: 2785–96. doi: 10.1088/0031-9155/53/11/002 [DOI] [PubMed] [Google Scholar]
- 129.Voet PWJ, Breedveld S, Dirkx MLP, Levendag PC, Heijmen BJM. Integrated multicriterial optimization of beam angles and intensity profiles for coplanar and noncoplanar head and neck IMRT and implications for VMAT. Med Phys 2012; 39: 4858–65. doi: 10.1118/1.4736803 [DOI] [PubMed] [Google Scholar]
- 130.Potrebko PS, Fiege J, Biagioli M, Poleszczuk J. Investigating multi-objective fluence and beam orientation IMRT optimization. Phys Med Biol 2017; 62: 5228–44. doi: 10.1088/1361-6560/aa7298 [DOI] [PubMed] [Google Scholar]
- 131.Monz M, Küfer KH, Bortfeld TR, Thieke C. Pareto navigation—algorithmic foundation of interactive multi-criteria IMRT planning. Phys Med Biol 2008; 53: 985–98. doi: 10.1088/0031-9155/53/4/011 [DOI] [PubMed] [Google Scholar]
- 132.van Haveren R, Ogryczak W, Verduijn GM, Keijzer M, Heijmen BJM, Breedveld S. Fast and fuzzy multi-objective radiotherapy treatment plan generation for head and neck cancer patients with the lexicographic reference point method (LRPM). Phys Med Biol 2017; 62: 4318–32. doi: 10.1088/1361-6560/62/11/4318 [DOI] [PubMed] [Google Scholar]
- 133.Fredriksson A, Bokrantz R. Deliverable navigation for multicriteria IMRT treatment planning by combining shared and individual apertures. Phys Med Biol 2013; 58: 7683–97. doi: 10.1088/0031-9155/58/21/7683 [DOI] [PubMed] [Google Scholar]
- 134.Jee KW, McShan DL, Fraass BA. Lexicographic ordering: intuitive multicriteria optimization for IMRT. Phys Med Biol 2007; 52: 1845–1861. doi: 10.1088/0031-9155/52/7/006 [DOI] [PubMed] [Google Scholar]
- 135.Breedveld S, Storchi PRM, Heijmen BJM. The equivalence of multi-criteria methods for radiotherapy plan optimization. Phys Med Biol 2009; 54: 7199–209. doi: 10.1088/0031-9155/54/23/011 [DOI] [PubMed] [Google Scholar]
- 136.Wang Y, Breedveld S, Heijmen B, Petit SF. Evaluation of plan quality assurance models for prostate cancer patients based on fully automatically generated Pareto-optimal treatment plans. Phys Med Biol 2016; 61: 4268–82. doi: 10.1088/0031-9155/61/11/4268 [DOI] [PubMed] [Google Scholar]
- 137.Bokrantz R. Distributed approximation of Pareto surfaces in multicriteria radiation therapy treatment planning. Phys Med Biol 2013; 58: 3501–16. doi: 10.1088/0031-9155/58/11/3501 [DOI] [PubMed] [Google Scholar]
- 138.Halabi T, Craft D, Bortfeld T. Dose–volume objectives in multi-criteria optimization. Phys Med Biol 2006; 51: 3809–18. doi: 10.1088/0031-9155/51/15/014 [DOI] [PubMed] [Google Scholar]
- 139.Holdsworth CH, Corwin D, Stewart RD, Rockne R, Trister AD, Swanson KR, et al. . Adaptive IMRT using a multiobjective evolutionary algorithm integrated with a diffusion–invasion model of glioblastoma. Phys Med Biol 2012; 57: 8271–83. doi: 10.1088/0031-9155/57/24/8271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thieke C, Küfer K-H, Monz M, Scherrer A, Alonso F, Oelfke U, et al. . A new concept for interactive radiotherapy planning with multicriteria optimization: first clinical evaluation. Radiother Oncol 2007; 85: 292–8. doi: 10.1016/j.radonc.2007.06.020 [DOI] [PubMed] [Google Scholar]
- 141.Romeijn HE, Dempsey JF, Li JG. A unifying framework for multi-criteria fluence map optimization models. Phys Med Biol 2004; 49: 1991–2013. doi: 10.1088/0031-9155/49/10/011 [DOI] [PubMed] [Google Scholar]
- 142.Chen W, Unkelbach J, Trofimov A, Madden T, Kooy H, Bortfeld T, et al. . Including robustness in multi-criteria optimization for intensity-modulated proton therapy. Phys Med Biol 2012; 57: 591–608. doi: 10.1088/0031-9155/57/3/591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Breedveld S, Storchi PRM, Keijzer M, Heemink AW, Heijmen BJM. A novel approach to multi-criteria inverse planning for IMRT. Phys Med Biol 2007; 52: 6339–53. doi: 10.1088/0031-9155/52/20/016 [DOI] [PubMed] [Google Scholar]
- 144.Serna JI, Monz M, Küfer KH, Thieke C. Trade-off bounds for the Pareto surface approximation in multi-criteria IMRT planning. Phys Med Biol 2009; 54: 6299–311. doi: 10.1088/0031-9155/54/20/018 [DOI] [PubMed] [Google Scholar]
- 145.Hu W, Wang J, Li G, Peng J, Lu S, Zhang Z. Investigation of plan quality between RapidArc and IMRT for gastric cancer based on a novel beam angle and multicriteria optimization technique. Radiother Oncol 2014; 111: 144–7. doi: 10.1016/j.radonc.2014.01.024 [DOI] [PubMed] [Google Scholar]
- 146.Breedveld S, Storchi PRM, Voet PWJ, Heijmen BJM. iCycle: integrated, multicriterial beam angle, and profile optimization for generation of coplanar and noncoplanar IMRT plans. Med Phys 2012; 39: 951–63. doi: 10.1118/1.3676689 [DOI] [PubMed] [Google Scholar]
- 147.Craft D, McQuaid D, Wala J, Chen W, Salari E, Bortfeld T. Multicriteria VMAT optimization. Med Phys 2012; 39: 686–96. doi: 10.1118/1.3675601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Craft D, Monz M. Simultaneous navigation of multiple Pareto surfaces, with an application to multicriteria IMRT planning with multiple beam angle configurations. Med Phys 2010; 37: 736–741. doi: 10.1118/1.3292636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hoffmann AL, Siem AY, den Hertog D, Kaanders JH, Huizenga H. Derivative-free generation and interpolation of convex Pareto optimal IMRT plans. Phys Med Biol 2006; 51: 6349–6369. doi: 10.1088/0031-9155/51/24/005 [DOI] [PubMed] [Google Scholar]
- 150.Buschmann M, Sharfo AWM, Penninkhof J, Seppenwoolde Y, Goldner G, Georg D, et al. . Automated volumetric modulated arc therapy planning for whole pelvic prostate radiotherapy. Strahlenther Onkol 2018; 194: 333-342. doi: 10.1007/s00066-017-1246-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sharfo AWM, Stieler F, Kupfer O, Heijmen BJM, Dirkx MLP, Breedveld S, et al. . Automated VMAT planning for postoperative adjuvant treatment of advanced gastric cancer. Radiat Oncol 2018; 13: 74. doi: 10.1186/s13014-018-1032-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Janssen T, van Kesteren Z, Franssen G, Damen E, van Vliet C. Pareto fronts in clinical practice for pinnacle. Int J Radiat Oncol Biol Phys 2013; 85: 873–880. doi: 10.1016/j.ijrobp.2012.05.045 [DOI] [PubMed] [Google Scholar]
- 153.Ghandour S, Cosinschi A, Mazouni Z, Pachoud M, Matzinger O. Optimization of stereotactic body radiotherapy treatment planning using a multicriteria optimization algorithm. Z Med Phys 2016; 26: 362–70. doi: 10.1016/j.zemedi.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 154.Hong TS, Craft DL, Carlsson F, Bortfeld TR. Multicriteria optimization in intensity-modulated radiation therapy treatment planning for locally advanced cancer of the pancreatic head. Int J Radiat Oncol Biol Phys 2008; 72: 1208–1214. doi: 10.1016/j.ijrobp.2008.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Holdsworth C, Stewart RD, Kim M, Liao J, Phillips MH. Investigation of effective decision criteria for multiobjective optimization in IMRT. Med Phys 2011; 38: 2964–2974. doi: 10.1118/1.3589128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kierkels RG, Korevaar EW, Steenbakkers RJ, Janssen T, van't Veld AA, Langendijk JA, et al. . Direct use of multivariable normal tissue complication probability models in treatment plan optimisation for individualised head and neck cancer radiotherapy produces clinically acceptable treatment plans. Radiother Oncol 2014; 112: 430–6. doi: 10.1016/j.radonc.2014.08.020 [DOI] [PubMed] [Google Scholar]
- 157.Chen H, Winey BA, Daartz J, Oh KS, Shin JH, Gierga DP. Efficiency gains for spinal radiosurgery using multicriteria optimization intensity modulated radiation therapy guided volumetric modulated arc therapy planning. Pract Radiat Oncol 2015; 5: 49–55. doi: 10.1016/j.prro.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Song T, Li N, Zarepisheh M, Li Y, Gautier Q, Zhou L, et al. . An automated treatment plan quality control tool for intensity-modulated radiation therapy using a voxel-weighting factor-based re-optimization algorithm. PLoS One 2016; 11: e0149273. doi: 10.1371/journal.pone.0149273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Buschmann M, Seppenwoolde Y, Wiezorek T, Weibert K, Georg D. Advanced optimization methods for whole pelvic and local prostate external beam therapy. Phys Medica 2016; 32: 465–73. doi: 10.1016/j.ejmp.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 160.Leinders SM, Breedveld S, Méndez Romero A, Schaart D, Seppenwoolde Y, Heijmen BJ. Adaptive liver stereotactic body radiation therapy: automated daily plan reoptimization prevents dose delivery degradation caused by anatomy deformations. Int J Radiat Oncol Biol Phys 2013; 87: 1016–1021. doi: 10.1016/j.ijrobp.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 161.Sharfo AWM, Dirkx MLP, Breedveld S, Romero AM, Heijmen BJM. VMAT plus a few computer-optimized non-coplanar IMRT beams (VMAT+) tested for liver SBRT. Radiother Oncol 2017; 123: 49–56. doi: 10.1016/j.radonc.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 162.Voet PWJ, Dirkx MLP, Breedveld S, Fransen D, Levendag PC, Heijmen BJM. Toward Fully Automated Multicriterial Plan Generation: A Prospective Clinical Study. Int J Radiat Oncol Biol Phys 2013; 85: 866–72. doi: 10.1016/j.ijrobp.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 163.Lechner W, Kragl G, Georg D. Evaluation of treatment plan quality of IMRT and VMAT with and without flattening filter using Pareto optimal fronts. Radiother Oncol 2013; 109: 437–41. doi: 10.1016/j.radonc.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 164.Kierkels RGJ, Visser R, Bijl HP, Langendijk JA, van ‘t Veld AA, Steenbakkers RJHM, et al. . Multicriteria optimization enables less experienced planners to efficiently produce high quality treatment plans in head and neck cancer radiotherapy. Radiat Oncol 2015; 10. doi: 10.1186/s13014-015-0385-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kyroudi A, Petersson K, Ghandour S, Pachoud M, Matzinger O, Ozsahin M, et al. . Discrepancies between selected Pareto optimal plans and final deliverable plans in radiotherapy multi-criteria optimization. Radiother Oncol 2016; 120: 346–8. doi: 10.1016/j.radonc.2016.05.018 [DOI] [PubMed] [Google Scholar]
- 166.Ottosson RO, Engström PE, Sjöström D, Behrens CF, Karlsson A, Knöös T, et al. . The feasibility of using Pareto fronts for comparison of treatment planning systems and delivery techniques. Acta Oncol 2009; 48: 233–7. doi: 10.1080/02841860802251559 [DOI] [PubMed] [Google Scholar]
- 167.Petersson K, Ceberg C, Engström P, Benedek H, Nilsson P, Knöös T. Conversion of helical tomotherapy plans to step-and-shoot IMRT plans-Pareto front evaluation of plans from a new treatment planning system. Med Phys 2011; 38: 3130–3138. doi: 10.1118/1.3592934 [DOI] [PubMed] [Google Scholar]
- 168.Müller BS, Shih HA, Efstathiou JA, Bortfeld T, Craft D. Multicriteria plan optimization in the hands of physicians: a pilot study in prostate cancer and brain tumors. Radiat Oncol 2017; 12. doi: 10.1186/s13014-017-0903-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wala J, Craft D, Paly J, Zietman A, Efstathiou J. Maximizing dosimetric benefits of IMRT in the treatment of localized prostate cancer through multicriteria optimization planning. Med Dosim 2013; 38: 298–303. doi: 10.1016/j.meddos.2013.02.012 [DOI] [PubMed] [Google Scholar]
- 170.Smith WP, Kim M, Holdsworth C, Liao J, Phillips MH. Personalized treatment planning with a model of radiation therapy outcomes for use in multiobjective optimization of IMRT plans for prostate cancer. Radiat Oncol 2016; 11. doi: 10.1186/s13014-016-0609-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen H, Craft DL, Gierga DP. Multicriteria optimization informed VMAT planning. Med Dosim 2014; 39: 64–73. doi: 10.1016/j.meddos.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kamran SC, Mueller BS, Paetzold P, Dunlap J, Niemierko A, Bortfeld T, et al. . Multi-criteria optimization achieves superior normal tissue sparing in a planning study of intensity-modulated radiation therapy for RTOG 1308-eligible non-small cell lung cancer patients. Radiother Oncol 2016; 118: 515–20. doi: 10.1016/j.radonc.2015.12.028 [DOI] [PubMed] [Google Scholar]
- 173.Voet PWJ, Dirkx MLP, Breedveld S, Heijmen BJM. Automated generation of IMRT treatment plans for prostate cancer patients with metal hip prostheses: Comparison of different planning strategies. Med Phys 2013; 40: 071704. doi: 10.1118/1.4808117 [DOI] [PubMed] [Google Scholar]
- 174.Sharfo AWM, Breedveld S, Voet PWJ, Heijkoop ST, Mens J-WM, Hoogeman MS, et al. . Validation of fully automated VMAT plan generation for library-based plan-of-the-day cervical cancer radiotherapy. PLoS One 2016; 11: e0169202. doi: 10.1371/journal.pone.0169202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Buergy D, Sharfo AWM, Heijmen BJM, Voet PWJ, Breedveld S, Wenz F, et al. . Fully automated treatment planning of spinal metastases – A comparison to manual planning of Volumetric Modulated Arc Therapy for conventionally fractionated irradiation. Radiat Oncol 2017; 12. doi: 10.1186/s13014-017-0767-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ghandour S, Matzinger O, Pachoud M. Volumetric-modulated arc therapy planning using multicriteria optimization for localized prostate cancer. J Appl Clin Med Phys 2015; 16: 258–69. doi: 10.1120/jacmp.v16i3.5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rønde HS, Wee L, Pløen J, Appelt AL. Feasibility of preference-driven radiotherapy dose treatment planning to support shared decision making in anal cancer. Acta Oncol 2017; 56: 1277–85. doi: 10.1080/0284186X.2017.1315174 [DOI] [PubMed] [Google Scholar]
- 178.Craft D. Calculating and controlling the error of discrete representations of Pareto surfaces in convex multi-criteria optimization. Phys Medica 2010; 26: 184–91. doi: 10.1016/j.ejmp.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.McGarry CK, Bokrantz R, O’Sullivan JM, Hounsell AR. Advantages and limitations of navigation-based multicriteria optimization (MCO) for localized prostate cancer IMRT planning. Med Dosim 2014; 39: 205–11. doi: 10.1016/j.meddos.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 180.Della Gala G, Dirkx MLP, Hoekstra N, Fransen D, Lanconelli N, van de Pol M, et al. . Fully automated VMAT treatment planning for advanced-stage NSCLC patients. Strahlentherapie Und Onkol 2017; 193: 402–9. doi: 10.1007/s00066-017-1121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sharfo AWM, Voet PWJ, Breedveld S, Mens JWM, Hoogeman MS, Heijmen BJM. Comparison of VMAT and IMRT strategies for cervical cancer patients using automated planning. Radiother Oncol 2015; 114: 395–401. doi: 10.1016/j.radonc.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 182.van de Schoot AJ, Visser J, van Kesteren Z, Janssen TM, Rasch CR, Bel A. Beam configuration selection for robust intensity-modulated proton therapy in cervical cancer using Pareto front comparison. Phys Med Biol 2016; 61: 1780–1794. doi: 10.1088/0031-9155/61/4/1780 [DOI] [PubMed] [Google Scholar]
- 183.Craft DL, Halabi TF, Shih HA, Bortfeld TR. Approximating convex pareto surfaces in multiobjective radiotherapy planning. Med Phys 2006; 33: 3399–3407. doi: 10.1118/1.2335486 [DOI] [PubMed] [Google Scholar]
- 184.Craft DL, Halabi TF, Shih HA, Bortfeld TR. Approximating convex Pareto surfaces in multiobjective radiotherapy planning. Med Phys 2006; 33: 3399–407. doi: 10.1118/1.2335486 [DOI] [PubMed] [Google Scholar]
- 185.Voet PWJ, Dirkx MLP, Breedveld S, Al-Mamgani A, Incrocci L, Heijmen BJM. Fully Automated Volumetric Modulated Arc Therapy Plan Generation for Prostate Cancer Patients. Int J Radiat Oncol Biol Phys 2014; 88: 1175–9. doi: 10.1016/j.ijrobp.2013.12.046 [DOI] [PubMed] [Google Scholar]
- 186.Rossi L, Breedveld S, Heijmen BJM, Voet PWJ, Lanconelli N, Aluwini S. On the beam direction search space in computerized non-coplanar beam angle optimization for IMRT—prostate SBRT. Phys Med Biol 2012; 57: 5441–58. doi: 10.1088/0031-9155/57/17/5441 [DOI] [PubMed] [Google Scholar]
- 187.van de Water S, Kraan AC, Breedveld S, Schillemans W, Teguh DN, Kooy HM, et al. . Improved efficiency of multi-criteria IMPT treatment planning using iterative resampling of randomly placed pencil beams. Phys Med Biol 2013; 58: 6969–83. doi: 10.1088/0031-9155/58/19/6969 [DOI] [PubMed] [Google Scholar]
- 188.Powis R, Bird A, Brennan M, Hinks S, Newman H, Reed K, et al. . Clinical implementation of a knowledge based planning tool for prostate VMAT. Radiat Oncol 2017; 12. doi: 10.1186/s13014-017-0814-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chin Snyder K, Kim J, Reding A, Fraser C, Gordon J, Ajlouni M, et al. . Development and evaluation of a clinical model for lung cancer patients using stereotactic body radiotherapy (SBRT) within a knowledge-based algorithm for treatment planning. J Appl Clin Med Phys 2016; 17: 263–75. doi: 10.1120/jacmp.v17i6.6429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wu H, Jiang F, Yue H, Li S, Zhang Y. A dosimetric evaluation of knowledge-based VMAT planning with simultaneous integrated boosting for rectal cancer patients. J Appl Clin Med Phys 2016; 17: 78–85. doi: 10.1120/jacmp.v17i6.6410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stanhope C, Wu QJ, Yuan L, Liu J, Hood R, Yin F-F, et al. . Utilizing knowledge from prior plans in the evaluation of quality assurance. Phys Med Biol 2015; 60: 4873–91. doi: 10.1088/0031-9155/60/12/4873 [DOI] [PubMed] [Google Scholar]
- 192.Boylan C, Rowbottom C. A bias-free, automated planning tool for technique comparison in radiotherapy - application to nasopharyngeal carcinoma treatments. J Appl Clin Med Phys 2014; 15: 213–25. doi: 10.1120/jacmp.v15i1.4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bijman RG, Breedveld S, Arts T, Astreinidou E, de Jong MA, Granton PV, et al. . Impact of model and dose uncertainty on model-based selection of oropharyngeal cancer patients for proton therapy. Acta Oncol 2017; 56: 1444–50. doi: 10.1080/0284186X.2017.1355113 [DOI] [PubMed] [Google Scholar]
- 194.Meijer GJ, van der Toorn P-P, Bal M, Schuring D, Weterings J, de Wildt M. High precision bladder cancer irradiation by integrating a library planning procedure of 6 prospectively generated SIB IMRT plans with image guidance using lipiodol markers. Radiother Oncol 2012; 105: 174–9. doi: 10.1016/j.radonc.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 195.Heijkoop ST, Langerak TR, Quint S, Bondar L, Mens JWM, Heijmen BJM, et al. . Clinical Implementation of an Online Adaptive Plan-of-the-Day Protocol for Nonrigid Motion Management in Locally Advanced Cervical Cancer IMRT. Int J Radiat Oncol Biol Phys 2014; 90: 673–9. doi: 10.1016/j.ijrobp.2014.06.046 [DOI] [PubMed] [Google Scholar]
- 196.van de Schoot AJAJ, Visser J, van Kesteren Z, Janssen TM, Rasch CRN, Bel A. Beam configuration selection for robust intensity-modulated proton therapy in cervical cancer using Pareto front comparison. Phys Med Biol 2016; 61: 1780–94. doi: 10.1088/0031-9155/61/4/1780 [DOI] [PubMed] [Google Scholar]
- 197.Ciller C, De Zanet SI, Rüegsegger MB, Pica A, Sznitman R, Thiran J-P, et al. . Automatic segmentation of the eye in 3D magnetic resonance imaging: a novel statistical shape model for treatment planning of retinoblastoma. Int J Radiat Oncol Biol Phys 2015; 92: 794–802. doi: 10.1016/j.ijrobp.2015.02.056 [DOI] [PubMed] [Google Scholar]
- 198.Fortunati V, Verhaart RF, van der Lijn F, Niessen WJ, Veenland JF, Paulides MM, et al. . Tissue segmentation of head and neck CT images for treatment planning: a multiatlas approach combined with intensity modeling. Med Phys 2013; 40: 071905. doi: 10.1118/1.4810971 [DOI] [PubMed] [Google Scholar]
- 199.Wong WKH, Leung LHT, Kwong DLW. Evaluation and optimization of the parameters used in multiple-atlas-based segmentation of prostate cancers in radiation therapy. Br J Radiol 2016; 89: 20140732. doi: 10.1259/bjr.20140732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Martínez F, Romero E, Dréan G, Simon A, Haigron P, de Crevoisier R, et al. . Segmentation of pelvic structures for planning CT using a geometrical shape model tuned by a multi-scale edge detector. Phys Med Biol 2014; 59: 1471–84. doi: 10.1088/0031-9155/59/6/1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li D, Liu L, Kapp DS, Xing L. Automatic liver contouring for radiotherapy treatment planning. Phys Med Biol 2015; 60: 7461–83. doi: 10.1088/0031-9155/60/19/7461 [DOI] [PubMed] [Google Scholar]
- 202.Chen A, Niermann KJ, Deeley MA, Dawant BM. Evaluation of multiple-atlas-based strategies for segmentation of the thyroid gland in head and neck CT images for IMRT. Phys Med Biol 2012; 57: 93–111. doi: 10.1088/0031-9155/57/1/93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chandra SS, Dowling JA, Greer PB, Martin J, Wratten C, Pichler P, et al. . Fast automated segmentation of multiple objects via spatially weighted shape learning. Phys Med Biol 2016; 61: 8070–84. doi: 10.1088/0031-9155/61/22/8070 [DOI] [PubMed] [Google Scholar]
- 204.Altman MB, Kavanaugh JA, Wooten HO, Green OL, DeWees TA, Gay H, et al. . A framework for automated contour quality assurance in radiation therapy including adaptive techniques. Phys Med Biol 2015; 60: 5199–209. doi: 10.1088/0031-9155/60/13/5199 [DOI] [PubMed] [Google Scholar]
- 205.Otto K. Real-time interactive treatment planning. Phys Med Biol 2014; 59: 4845–59. doi: 10.1088/0031-9155/59/17/4845 [DOI] [PubMed] [Google Scholar]
- 206.Ziegenhein P, Ph Kamerling C, Oelfke U. Interactive dose shaping part 1: a new paradigm for IMRT treatment planning. Phys Med Biol 2016; 61: 2457–70. doi: 10.1088/0031-9155/61/6/2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ph Kamerling C, Ziegenhein P, Sterzing F, Oelfke U. Interactive dose shaping part 2: proof of concept study for six prostate patients. Phys Med Biol 2016; 61: 2471–84. doi: 10.1088/0031-9155/61/6/2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Raaymakers BW, Lagendijk JJ, Overweg J, Kok JG, Raaijmakers AJ, Kerkhof EM, et al. . Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol 2009; 54: N229–N237. doi: 10.1088/0031-9155/54/12/N01 [DOI] [PubMed] [Google Scholar]
- 209.Mutic S, Dempsey JF. The ViewRay System: Magnetic Resonance–Guided and Controlled Radiotherapy. Semin Radiat Oncol 2014; 24: 196–9. doi: 10.1016/j.semradonc.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 210.Li N, Zarepisheh M, Uribe-Sanchez A, Moore K, Tian Z, Zhen X, et al. . Automatic treatment plan re-optimization for adaptive radiotherapy guided with the initial plan DVHs. Phys Med Biol 2013; 58: 8725–8738. doi: 10.1088/0031-9155/58/24/8725 [DOI] [PubMed] [Google Scholar]
- 211.Amit G, Purdie TG. Automated planning of breast radiotherapy using cone beam CT imaging. Med Phys 2015; 42: 770–779. doi: 10.1118/1.4905111 [DOI] [PubMed] [Google Scholar]
- 212.Court LE, Tishler RB, Petit J, Cormack R, Chin L. Automatic online adaptive radiation therapy techniques for targets with significant shape change: a feasibility study. Phys Med Biol 2006; 51: 2493–2501. doi: 10.1088/0031-9155/51/10/009 [DOI] [PubMed] [Google Scholar]
- 213.Li X, Quan EM, Li Y, Pan X, Zhou Y, Wang X, et al. . A fully automated method for CT-on-rails-guided online adaptive planning for prostate cancer intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2013; 86: 835–841. doi: 10.1016/j.ijrobp.2013.04.014 [DOI] [PubMed] [Google Scholar]
- 214.Acharya S, Fischer-Valuck BW, Kashani R, Parikh P, Yang D, Zhao T, et al. . Onlinemagnetic resonance image guided adaptive radiation therapy: first clinical applications. Int J Radiat Oncol Biol Phys 2016; 94: 394–403. doi: 10.1016/j.ijrobp.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 215.van de Sande MA, Creutzberg CL, van de Water S, Sharfo AW, Hoogeman MS. Which cervical and endometrial cancer patients will benefit most from intensity-modulated proton therapy? Radiother Oncol 2016; 120: 397–403. doi: 10.1016/j.radonc.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 216.Euchner J. Building aculture of innovation. Res Manag 2016; 59: 10–11. doi: 10.1080/08956308.2016.1232131 [DOI] [Google Scholar]