Abstract
Abnormal embryological development of the pulmonary veins can manifest as either partial or total anomalous drainage into the systemic venous circulation. Echocardiography does not provide adequate information in all cases as the optimal visualization of anomalous structures is limited by the availability of acoustic window; also it is highly operator dependent. However, multidetector CT angiography, with its multiplanar reformatting and volume rendering techniques, offers precise information about the three-dimensional anatomy and spatial relationships of the cardiovascular structures. With advent of dual source CT scanners and use of advanced dose reduction techniques, this information can be obtained in a short time with minimal radiation dose. In this pictorial essay, we present the multidetector CT imaging findings of the spectrum of total and partial anomalous pulmonary venous connections, using a third-generation dual source CT scanner.
Introduction
Anomalous development of the pulmonary veins in the early embryonic life gives rise to a diverse gamut of congenital defects in the pulmonary venous connections. Failure of all pulmonary veins to drain into the left atrium (LA) is termed “total anomalous pulmonary venous connection” (TAPVC) while failure of at least one, but not all, pulmonary veins to connect with the LA is labelled “partial anomalous pulmonary venous connection” (PAPVC).
Conventionally, transthoracic echocardiography has been used as the initial imaging modality for evaluation of pulmonary venous anomalies. However, owing to its small field of view, it is not always possible to trace each pulmonary vein and follow it to its drainage in the pulmonary venous atrium. Exact delineation of the anatomy of the pulmonary venous connections along with evaluation of cardiac and extracardiac structures is imperative for proper surgical planning. Seale et al reported that failure to perform definitive repair of associated cardiac lesions and presence of pre- and post-operative pulmonary vein obstruction or stenosis were independent risk factors for death in these patients.1 This underlines the importance of accurate anatomical assessment prior to and post surgery.
Multidetector CT (MDCT) angiography is increasingly being used for the characterization of pulmonary venous drainage anomalies owing to its non-invasive nature, faster scan times and multiplanar reconstruction capabilities. MRI can provide anatomical as well as functional assessment along with demonstrating the effects on pulmonary vasculature and cardiac chambers. There is no associated radiation risks and non-contrast acquisitions are also possible. Although recently, many centers have started using MRI for the assessment of these patients, its widespread use is precluded by its limited availability, longer scan times and frequent need of sedation or general anesthesia in pediatric patients. With the advent of dual source CT scanners and use of advanced dose reduction techniques in the third generation scanners, images can be obtained in a very short time having excellent spatial resolution and with minimal radiation dose.
At All India Institute of Medical Sciences, New Delhi, India, all patients with congenital heart defects, who are surgical candidates, undergo CT angiographic examination prior to surgery. Echocardiography, apart from being operator-dependent, permits limited evaluation owing to inadequate acoustic windows. CT angiography permits a complete evaluation of the primary defect and associated cardiac and extracardiac anomalies, which may have a bearing on the surgical conduct and outcome, in these patients. This practice is also facilitated by the fact that the said examination can be performed with a submilliSievert radiation dose on the third generation dual source CT scanner.
Relevant embryology
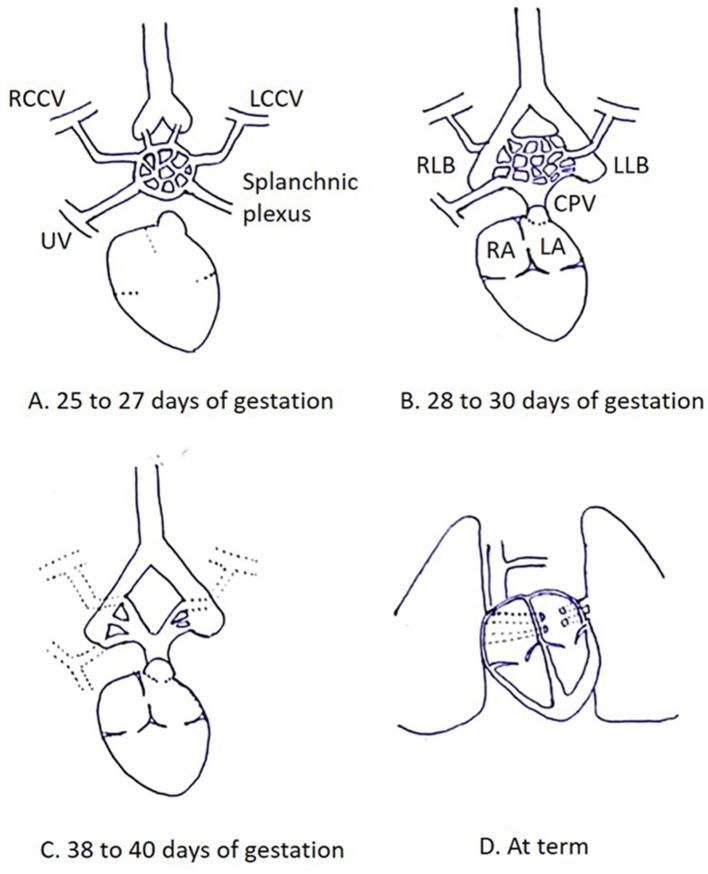
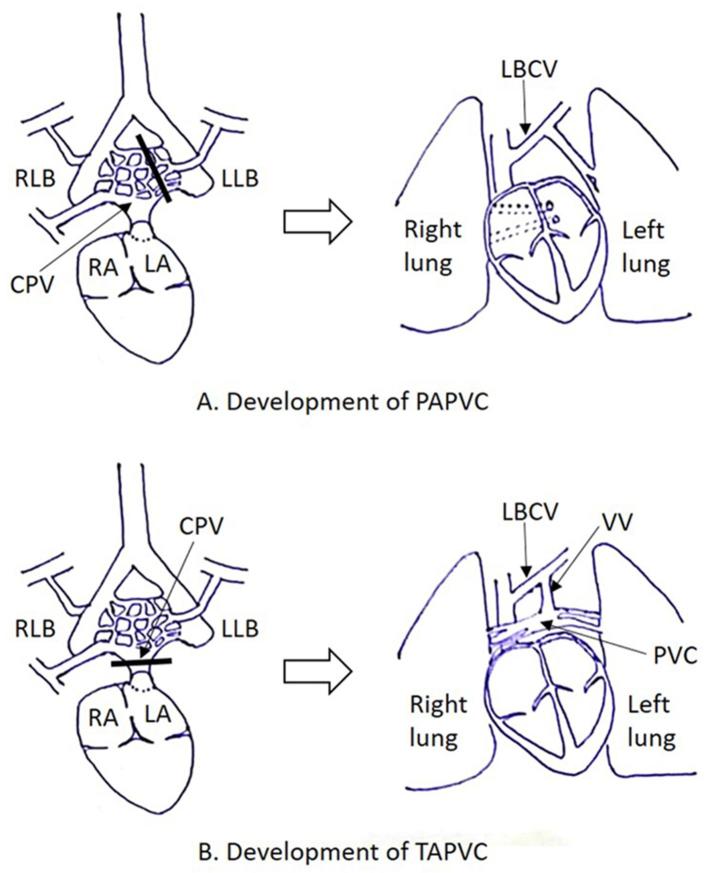
Pulmonary venous development is a complex process taking place through the early embryonic period. It is hypothesized that blood from the lung buds drains in to the splanchnic plexus, which in turn communicates with the cardinal and umbilicovitelline venous systems (25–27 days of gestation).2 An outpouching from the dorsal wall of the left atrium (LA) forms the common pulmonary vein (CPV) (27–29 days of gestation), which communicates with the part of splanchnic plexus draining blood from the primitive lung buds (28–30 days of gestation). The CPV ultimately gets incorporated within the dorsal wall of LA, giving rise to four pulmonary veins which connect directly and separately to the LA while connections with the cardinal and umbilicovitelline veins involute (Figure 1).3 Failure of these processes to occur properly gives rise to anomalies of pulmonary venous drainage (Figure 2).
Figure 1.
Normal development of pulmonary venous drainage. (A) Diagram illustrates lung buds draining in to the splanchnic plexus, which in turn communicates with the cardinal and umbilicovitelline venous systems. (B) An outpouching from the dorsal wall of the LA forms the CPV, which communicates with the part of splanchnic plexus draining blood from the primitive lung buds. (C) Connections with cardinal and umbilicovitelline veins involutes. (D) CPV ultimately gets incorporated within the dorsal wall of LA, giving rise to four pulmonary veins which connect directly and separately to the LA. CPV, common pulmonary vein; LA, left atrium; LCCV, left common cardinal vein; LLB, left lung bud; RA, right atrium; RCCV, right common cardinal vein; RLB, right lung bud; UV, umbilical vein.
Figure 2.
Embryology of anomalous pulmonary venous connections. (A) Partial anomalous pulmonary venous connection occurs when one or more of the pulmonary veins fail to establish connection with the CPV before connections with the splanchnic venous system have regressed. (B) Total anomalous pulmonary venous connection occurs when all the pulmonary veins fail to establish connection with the CPV before connections with the splanchnic venous system have regressed. CPV, common pulmonary vein; LA, left atrium; LCCV, left common cardinal vein; LLB, left lung bud; RA, right atrium; RCCV, right common cardinal vein; RLB, right lung bud; UV, umbilical vein.
MDCT angiography technique
All examinations were performed on a performed on a SOMATOM FORCE (Siemens Healthcare, Forchheim, Germany) CT scanner. The scanner is a third-generation dual source CT scanner with two X-ray tubes and corresponding detectors with an angular offset of 90°. They rotate simultaneously capturing image data in half the time as compared to single source scanners. The scanner has a temporal resolution of as low as 66 ms. Along with increasing the speed of acquisition, the use of advanced dose reduction techniques also help reduce the radiation dose by more than half.
Patient’s heart rate varied from 90 to 150 beats per minute; however, no form of heart rate control was required in any case. The pediatric patients were immobilized using a binder; no sedation was required owing to very short acquisition times. Non-ionic iodinated contrast (1.5–2.0 ml kg−1) was administered via a peripheral intravenous line using a dual head power injector at rates varying from 1.0 to 4.0 ml s−1. A “manual” bolus tracking method was used; the CT acquisition was manually triggered when visual estimate of optimal contrast opacification within the pulmonary vessels was achieved on the monitoring sequence.
Examinations were performed using the turbo high-pitch mode at a pitch of 3.2 (table feed 737 mm s−1), and using the following imaging parameters: tube voltage of 80 kVp with automated tube current modulation (CAREDose; Siemens Healthcare, Forchheim, Germany) and a reference tube current-time product of 270 mAs/rotation. The examinations were performed with prospective ECG-gating and the start of acquisition was synchronized to 60% of the R-R interval of the simulated ECG. Images were reconstructed using advanced model-based iterative reconstruction (strength level 3; Siemens Healthcare, Forchheim, Germany) with a medium soft tissue kernel (Bv40) and a slice thickness of 0.6 mm at an increment of 0.4 mm (field of view, 200 mm; pixel matrix, 512 × 512).
Axial sections were analyzed along with coronal and sagittal multiplanar reformats, followed by volume rendered and maximum intensity projection images on an external workstation (Multi Modality Workplace; Siemens Healthcare, Forchheim, Germany). All the scans performed for evaluation of anomalous pulmonary venous connections during the period of preparation of manuscript showed optimum opacification of the pulmonary venous structures and thus were diagnostic. No repeat scans were needed. The radiation dose estimate in these patients ranged from 0.55 to 0.97 mSvts (conversion factor, k = 0.021 mSv/[mGy · cm].
Total anomalous pulmonary venous connection
TAPVC accounts for 1–2% of all cases of major congenital heart diseases.4 In TAPVC, all the blood returning from the lungs drains into the systemic venous system. It results in neonatal cyanosis and can be fatal unless blood is shunted from the right sided circulation to the left side via an atrial septal defect or patent ductus arteriosus.5
Also pulmonary venous obstruction can occur in all types of TAPVC with the incidence being highest in the infracardiac variety and lowest in the cardiac type. Operative mortality is also observed to be higher in patients with obstructed systems.1
TAPVC is classified according to the point of drainage to the systemic venous system and thus can be supracardiac, cardiac, infracardiac or of mixed variety.
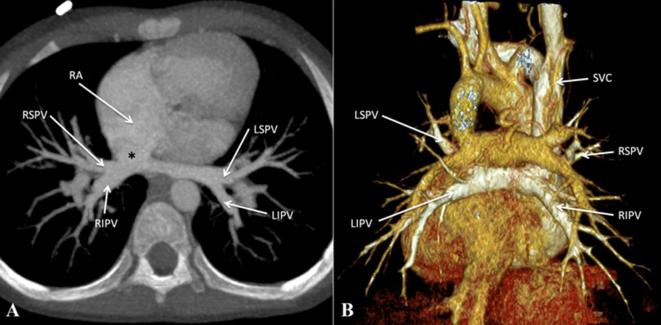
The supracardiac variety is the commonest type of TAPVC where the confluence of pulmonary veins commonly drain to the left brachiocephalic vein via a vertical vein (Figure 3).6 In other instances, drainage can be to the right or left superior vena cava or to the azygous venous system (Figure 4).7 In this variety, obstruction can occur at the origin of the vertical vein or at the site of its drainage into the left brachiocephalic vein. In the cardiac variety of TAPVC, the confluence of the pulmonary veins drains to the right atrium, either directly or through the coronary sinus (Figure 5). Obstruction can occur at the level of drainage into the coronary sinus.
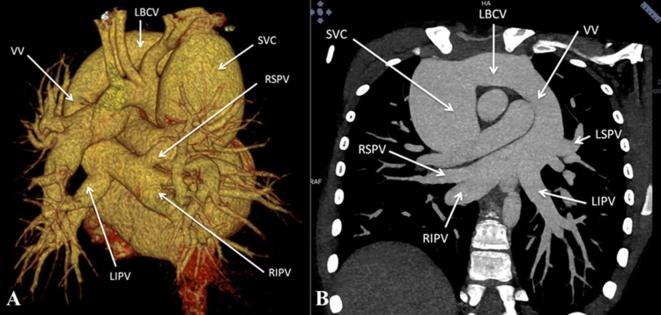
Figure 3.
Supracardiac total anomalous pulmonary venous connection. (A) Contrast-enhanced volume rendered CT image (posterior view) and (B) Contrast-enhanced CT MIP image, show drainage of all four pulmonary veins into the LBCV via a VV. LBCV, left brachio cephalic vein; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; MIP, maximum intensity projection; RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein; SVC, superior vena cava; VV, vertical vein.
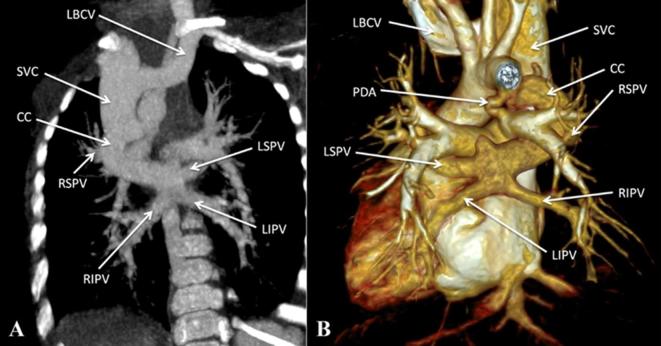
Figure 4.
Supracardiac total anomalous pulmonary venous connection. (A, B) Contrast-enhanced CT MIP image and volume rendered CT image (posterior view) respectively, show bilateral pulmonary venous drainage into a CC which drains into the SVC. CC, common channel; LBCV, left brachio cephalic vein; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; MIP, maximum intensity projection; PDA, patent ductus arteriosus; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; SVC, superior vena cava; VV, vertical vein.
Figure 5.
Cardiac total anomalous pulmonary venous connection. (A) Contrast-enhanced CT MIP image and (B) contrast-enhanced volume rendered CT image (posterior view) show bilateral pulmonary venous drainage directly into the RA, adjacent to the RA-SVC junction (asterisk). LBCV, left brachiocephalic vein; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; MIP, maximumintensity projection; PDA, patent ductus arteriosus; RA, right atrium; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; SVC, superior vena cava; VV, vertical vein.
In the infracardiac variety, the confluence of pulmonary veins connects to the infradiaphragmatic systemic veins. The pulmonary veins usually meet posterior to the LA and give rise to a descending vein. This vein descends through the esophageal hiatus in the diaphragm and connects commonly to the portal venous system (Figure 6). Less commonly, drainage occurs to the inferior vena cava, hepatic veins or ductus venosus. Obstruction is commonly seen in infracardiac variety of TAPVC and can occur where the vein travels through the diaphragm or at its junction with the portal system.
Figure 6.
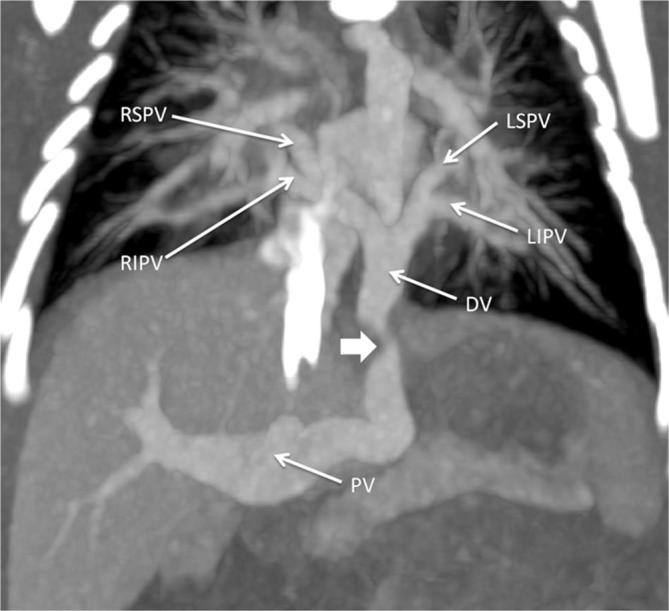
Infracardiac total anomalous pulmonary venous connection. Contrast-enhanced CT MIP image shows drainage of all four pulmonary veins into a common DV which ultimately drains into the PV, with the DV showing significant stenosis at the level of diaphragmatic hiatus (indicated by thick white arrow). DV, descending vein; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MIP, maximum intensity projection; PV, portal vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein .
Varying combinations of the above three varieties is seen in the mixed variety of TAPVC (Figure 7).
Figure 7.
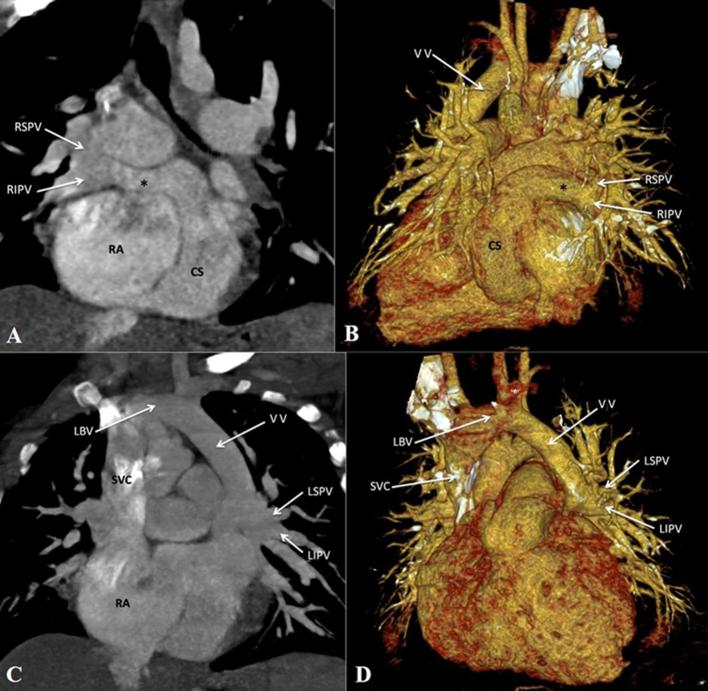
Mixed total anomalous pulmonary venous connection. (A, B) Contrast-enhanced CT MIP image and volume rendered CT image (posterior view) respectively, show drainage of right pulmonary veins to the dilated CS via a common channel (asterisk). (C, D) Contrast-enhanced CT MIP image and volume rendered image respectively, show the left pulmonary veins draining to the LBV via a VV. CS, coronary sinus; LBV, left brachio cephalic vein; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MIP, maximum intensity projection; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; SVC, superior vena cava; VV, vertical vein.
In cases of TAPVC, particularly mixed variety or cases associated with heterotaxy syndromes, echocardiography alone may not be adequate for diagnosis. MDCT angiography provides accurate anatomic delineation of the anomalous connections and associated cardiac and extracardiac anomalies.
Cor triatriatum
Defective resorption of the CPV into the LA with associated stenosis of the CPV orifice results in cor triatriatum. The condition is characterized by presence characterized by a fibromuscular membrane dividing the LA into two chambers (Figure 8). The posterosuperior chamber, representative of the CPV, receives the pulmonary venous drainage while the LA proper is anteroinferior and is connected with the LA appendage and the mitral valve. The two chambers are in communication through an opening in the membrane which may be small, large, multiple, central or eccentric.
Figure 8.
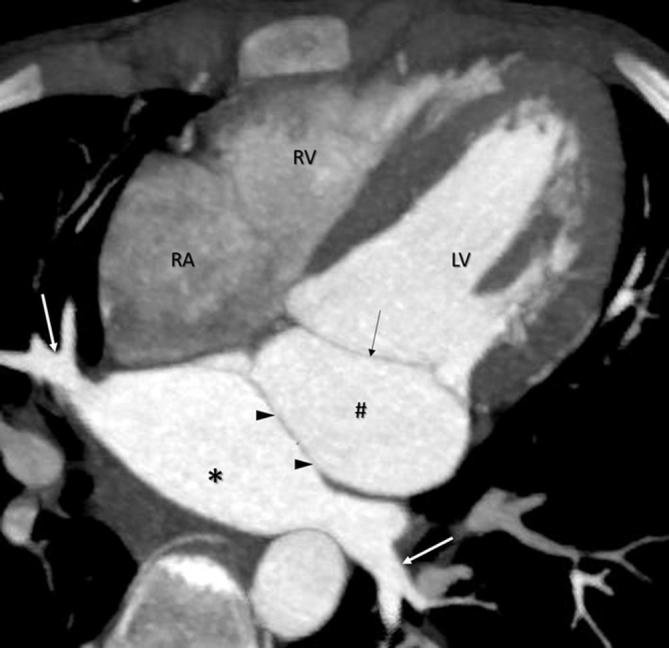
Cor triatriatum. Contrast enhanced CT MIP image, shows a membrane (arrowheads) dividing the left atrial cavity into an accessory chamber (indicated by asterisk), receiving the pulmonary veins (white arrows) and the LA proper (indicated by #) which communicates with the mitral valve (black arrow). RA, right atrium; RV, right ventricle; LV, left ventricle.
In symptomatic individuals, features of pulmonary venous hypertension predominate and include pulmonary edema and pulmonary hypertension. Severity of the clinical symptoms is strongly related to the degree of obstruction caused by the fibromuscular membrane and the number of involved pulmonary veins.
Partial anomalous pulmonary venous connection
PAPVC usually presents as an incidental finding on cross-sectional imaging as patients are usually asymptomatic or have mild symptoms. It has a reported prevalence of 0.4–0.7%.8, 9
In PAPVC, more than one, but not all, pulmonary veins have anomalous drainage. Most commonly involved vein is the right superior pulmonary vein which connects anomalously to the superior vena cava or to the right atrium (Figure 9). A sinus venosus atrial septal defect is a common association, seen in up to half of these patients, followed by presence of an ostium secundum atrial septal defect and a patent foramen ovale.10 (Figure 9). A few other commonly observed patterns include drainage of right or left pulmonary veins into the coronary sinus and left pulmonary veins connecting with the left brachiocephalic vein (Figure 10).
Figure 9.
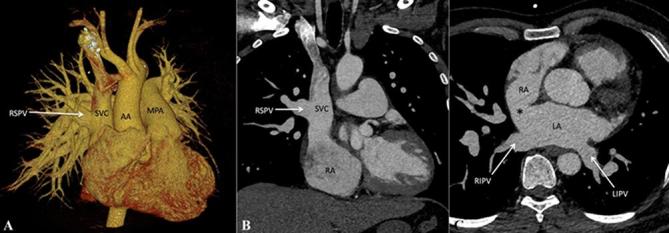
Partial anomalous pulmonary venous connection. (A) Contrast-enhanced volume rendered CT image (posterior view) and(B) Contrast-enhanced CT MIP image, show drainage of RSPV into the SVC while the other three pulmonary veins drain normally into the LA. C, Axial contrast-enhanced CT MIP image, shows associated sinus venosus atrial septal defect (indicated by asterisk). AA, ascending aorta; LA, left atrium; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MPA,main pulmonary artery; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; superior vena cava.
Figure 10.
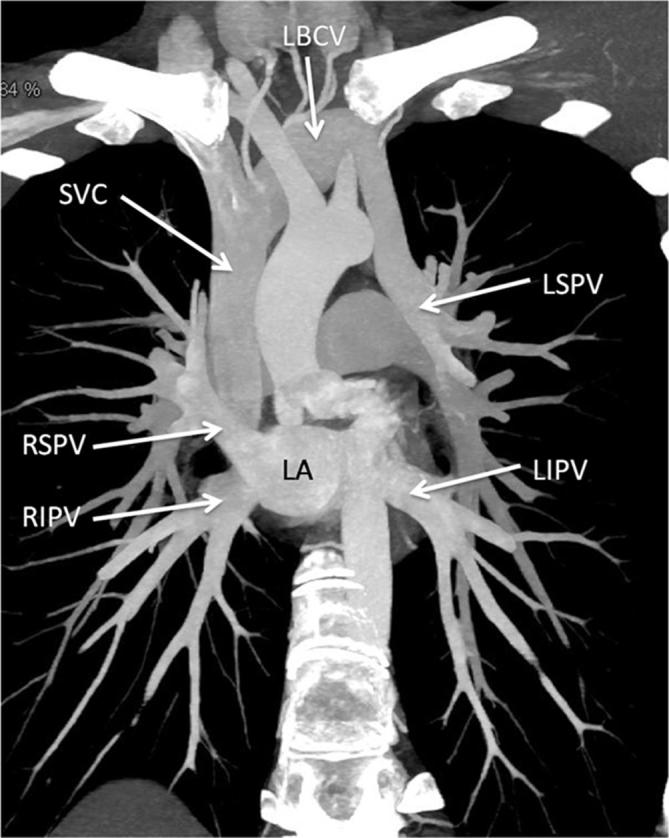
Partial anomalous pulmonary venous connection. Contrast-enhanced CT MIP image, shows connection of LSPV into the LBCV while the other three pulmonary veins drain normally into the LA. LA, left atrium; LBCV, left brachio cephalic vein; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; superior vena cava.
A specific subset of PAPVC involves connection of an anomalous vein draining all or part of the right lung into the supradiaphragmatic or infradiaphragmatic IVC (Figure 11), with occasional drainage observed into the hepatic veins, azygous venous system, portal vein, right atrium or coronary sinus. It forms a part of the “congenital pulmonary venolobar syndrome” or commonly known “Scimitar syndrome” where other associations include hypogenetic lung, pulmonary sequestrations, pulmonary arterial interruption, accessory diaphragm, diaphragmatic hernia and horseshoe lung.
Figure 11.
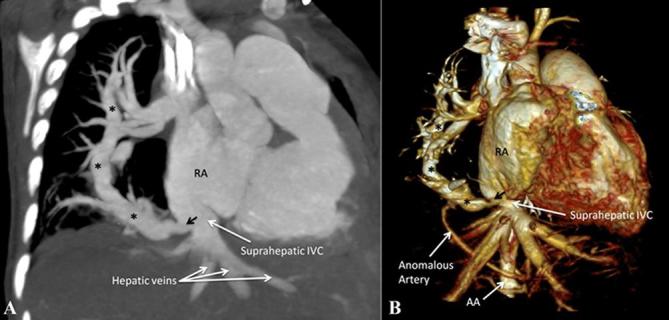
Scimitar syndrome. (A) Contrast-enhanced CT MIP image shows presence of an anomalous vein (indicated by asterisks) draining the entire right lung into the suprahepatic portion of IVC just below the right atrium. (B) Contrast-enhanced volume rendered CT image (right anterior oblique view) demonstrates the anomalous vein (indicated by asterisks) and its site of drainage. Note is also made of an anomalous systemic artery arising from the abdominal aorta supplying the right lung. AA, ascending aorta; IVC, inferior vena cava; MIP, maximum intensity projection; RA, right atrium.
In addition to the demonstration of anomalous connections and the interatrial communication, other associated findings secondary to right-sided volume overload, elevated pulmonary pressures and interatrial shunting, like right atrial and right ventricular enlargement along with flattening of interventricular septum are also frequently identified in cases of anomalous pulmonary venous drainage.
Conclusion
A wide gamut of pulmonary venous drainage abnormalities can be detected by imaging. Accurate differentiation and characterization of these anomalies is imperative for treatment planning. MDCT Angiography, owing to its non-invasive nature, multiplanar reconstruction capabilities, ability to obtain maximum intensity projection and volume rendered images, and rapidity of acquisition along with recent advancements such as dual source CT scanners and various dose reduction strategies, has become an important part of the diagnostic workup of anomalous pulmonary venous drainage.
Contributor Information
Niraj Nirmal Pandey, Email: nirajpandey2403@gmail.com.
Arun Sharma, Email: drarungautam@gmail.com.
Priya Jagia, Email: drpjagia@yahoo.com.
REFERENCES
- 1.Seale AN, Uemura H, Webber SA, Partridge J, Roughton M, Ho SY, et al. Total anomalous pulmonary venous connection: morphology and outcome from an international population-based study. Circulation 2010; 122: 2718–26. doi: 10.1161/CIRCULATIONAHA.110.940825 [DOI] [PubMed] [Google Scholar]
- 2.Latson LA, Prieto LR. Congenital and acquired pulmonary vein stenosis. Circulation 2007; 115: 103–8. doi: 10.1161/CIRCULATIONAHA.106.646166 [DOI] [PubMed] [Google Scholar]
- 3.Zylak CJ, Eyler WR, Spizarny DL, Stone CH. Developmental lung anomalies in the adult: radiologic-pathologic correlation. Radiographics 2002; 22: S25–S43. doi: 10.1148/radiographics.22.suppl_1.g02oc26s25 [DOI] [PubMed] [Google Scholar]
- 4.Ferguson EC, Krishnamurthy R, Oldham SA. Classic imaging signs of congenital cardiovascular abnormalities. Radiographics 2007; 27: 1323–34. doi: 10.1148/rg.275065148 [DOI] [PubMed] [Google Scholar]
- 5.Herlong JR, Jaggers JJ, Ungerleider RM. Congenital heart surgery nomenclature and database project: pulmonary venous anomalies. Ann Thorac Surg 2000; 69(4 Suppl): 56–69. doi: 10.1016/S0003-4975(99)01237-0 [DOI] [PubMed] [Google Scholar]
- 6.Karamlou T, Gurofsky R, Al Sukhni E, Coles JG, Williams WG, Caldarone CA, et al. Factors associated with mortality and reoperation in 377 children with total anomalous pulmonary venous connection. Circulation 2007; 115: 1591–8. doi: 10.1161/CIRCULATIONAHA.106.635441 [DOI] [PubMed] [Google Scholar]
- 7.White CS, Baffa JM, Haney PJ, Pace ME, Campbell AB. MR imaging of congenital anomalies of the thoracic veins. Radiographics 1997; 17: 595–608. doi: 10.1148/radiographics.17.3.9153699 [DOI] [PubMed] [Google Scholar]
- 8.Haramati LB, Moche IE, Rivera VT, Patel PV, Heyneman L, McAdams HP, et al. Computed tomography of partial anomalous pulmonary venous connection in adults. J Comput Assist Tomogr 2003; 27: 743–9. doi: 10.1097/00004728-200309000-00011 [DOI] [PubMed] [Google Scholar]
- 9.Dillman JR, Yarram SG, Hernandez RJ. Imaging of pulmonary venous developmental anomalies. AJR Am J Roentgenol 2009; 192: 1272–85. doi: 10.2214/AJR.08.1526 [DOI] [PubMed] [Google Scholar]
- 10.Ammash NM, Seward JB, Warnes CA, Connolly HM, O'Leary PW, Danielson GK. Partial anomalous pulmonary venous connection: diagnosis by transesophageal echocardiography. J Am Coll Cardiol 1997; 29: 1351–8. doi: 10.1016/S0735-1097(97)82758-1 [DOI] [PubMed] [Google Scholar]